Abstract
Objectives
To compare post-acute intermediate care in an inpatient nurse-led unit with conventional post-acute care on general medical wards of an acute hospital and to examine the model of care in a nurse-led unit.
Design
Randomised controlled trial with six month follow up.
Setting
Urban teaching hospital and surrounding area, including nine community hospitals.
Participants
238 patients accepted for admission to nurse-led unit.
Interventions
Care in nurse-led unit or usual post-acute care.
Main outcome measures
Patients' length of stay, functional status, subsequent move to more dependent living arrangement.
Results
Inpatient length of stay was significantly longer in the nurse-led unit than in general medical wards (14.3 days longer (95% confidence interval 7.8 to 20.7)), but this difference became non-significant when transfers to community hospitals were included in the measure of initial length of stay (4.5 days longer (−3.6 to 12.5)). No differences were observed in mortality, functional status, or living arrangements at any time. Patients in the nurse-led unit received significantly fewer minor medical investigations and, after controlling for length of stay, significantly fewer major reviews, tests, or drug changes.
Conclusions
The nurse-led unit seemed to be a safe alternative to conventional management, but a full accounting of such units' place in the local continuum of care and the costs associated with acute hospitals managing post-acute patients is needed if nurse-led units are to become an effective part of the government's recent commitment to intermediate care.
Introduction
Meeting the post-acute needs of frail or dependent elderly people has long challenged the NHS. Older patients take longer, on average, than younger patients to regain stability after acute illness and cannot always be transferred quickly from hospital.1 Adults with disability or conditions such as alcoholism may also require extended convalescence and careful discharge planning.2 The therapeutic needs of “difficult to discharge” patients combine with increased numbers needing hospitalisation in winter to create persistent demand for acute general medical beds in hospitals.3 Recognising this, the Department of Health has made intermediate care a priority in its new investment plan.4
Intermediate care encompasses a range of services intended to reduce avoidable hospital admission or readmission and to improve transition from hospital to home.5 One model is the post-acute nurse-led unit for patients deemed medically stable but not ready for discharge.6 In Britain nurse-led units were introduced as nursing development units.7,8 They received additional funding and employed senior nursing staff at practitioner level. However, results from experiments with these units have been inconsistent,9–12 and the validity of some of the studies has been questioned.6,13
The formation of post-acute nursing development units was strongly associated with the concept of therapeutic nursing. The hypothesis underlying this approach is that, by transferring appropriate patients to a low technology setting where nurses rather than doctors manage recuperation and can encourage self care, patients' clinical outcomes will improve and hospital length of stay will be reduced.7,8,14,15 Although the concept is controversial, it has attracted much interest. The nursing development model has been adopted by numerous NHS trusts, but to date its effectiveness in routine practice has not been reported.16
In this article, we summarise findings from an evaluation of a nurse-led unit providing post-acute intermediate care. After a pilot study demonstrated feasibility and gave promising results, we conducted a randomised controlled trial to compare the nurse-led unit with usual care in terms of length of stay, patient function, and discharge to a more dependent living arrangement.17 This trial was supported by follow up at six months in order to examine patients' use of services and outcomes beyond first discharge from the acute trust. We also conducted an economic evaluation and a qualitative study to examine perceptions of care and the unit's acceptability to patients, their families, and health professionals and performed sub-analyses of the content and quality of nursing care. In this paper we report the main results of the inpatient trial and follow up.
Patients and methods
The evaluation took place between July 1997 and September 2000, and the study methods were approved by the local research ethics committee.
Patients
From November 1997 all patients referred to the nurse-led unit of Southampton University Hospitals Trust for post-acute care were considered for entry to the trial. We continued to recruit patients until we reached our target sample size (April 1999). Patients were referred from other general medicine wards of the hospital and assessed for admission to the unit. The admission criteria were that patients must be at least 16 years old, medically stable (that is, not requiring tests or investigations) for at least 24 hours, must consent to transfer to the unit, and must have the doctor's referral and patient's resuscitation status recorded in their medical notes. Eligible patients were randomised to care on the nurse-led unit or to usual post-acute care in the hospital with the stipulation that they should not be transferred to the nurse-led unit.
Patients who entered the trial or suitable proxies were approached for their written consent to be telephoned for a brief interview six months after randomisation and to agree that their general practitioner could, if willing, provide the research team with information about their use of services during the six months' follow up. Because funding for follow up was granted six months after the inpatient trial had begun, we sought consent for some patients by post, with telephone follow up. We approached all other patients in hospital after their assessment for transfer to the nurse-led unit. Patients were able to agree to one aspect of data collection and refuse consent for another.
The nurse-led unit
The unit opened in January 1997 as an alternative to usual post-acute care on general medical wards. It had 10 beds and 22 nursing staff (ratio of 3:2 for qualified to unqualified nurses, no special training required). It is part of a teaching hospital trust in Southampton (population about 300 000) and is located near the main hospital site in a smaller setting emphasising outpatient care. The main site has stroke rehabilitation and elderly care units. The area is also served by a community healthcare trust with nine small hospitals and by two local authorities providing social services.
The nurse-led unit has a policy of open visiting. Nurses dress informally to promote a non-clinical atmosphere. On a patient's referral from the medical directorate's acute wards, the unit's staff assess the patient, determine whether admission is suitable, plan treatments, deliver care, and arrange discharge. There is no formal medical involvement; if staff have concerns, they can request a medical review at any time. Doctors can be called to the ward in an emergency, or nurses can send a patient back to the main site for medical attention via the accident and emergency department. A dedicated physiotherapist visits three times a week, and other ancillary services are available on request.
Randomisation
A randomised consent design was used for the trial.18 We adopted this approach after the pilot analysis identified ethical and scientific difficulties with conventional methods of randomisation and consent, which were tried initially.17 The randomised consent design has been recommended in cases where new treatment is compared with the best available standard treatment, as in the control condition for this study, and where standard consent procedures for randomised controlled trials can lead to unnecessary distress and confusion.19 The nurse-led unit's patient population fitted this criterion, as patients were mostly elderly and still unwell. Moreover, a high proportion of recipients of the nurse-led service were cognitively impaired (17/48 (35%) of those in the pilot17). Their relatives or doctors were willing to decide on transfer, but many were reluctant to make proxy decisions about inclusion in a randomised controlled trial. To exclude such patients when they were major recipients of the service would have been unethical20 and could have seriously compromised validity by producing a non-representative study sample.
Allocations to the two treatment arms were computerised and stratified by referring ward to ensure equal access to the nurse-led unit. They were then placed in sealed opaque envelopes that were held by research nurses. When a bed became available on the nurse-led unit, a researcher assessed the next case on a waiting list of referred patients. If the patient was eligible, the next envelope for that ward was opened. If the allocation was to the nurse-led unit, the patient or carer was asked for written consent to transfer (this was standard practice before the trial started) and, at the request of the local research ethics committee, given the chance to refuse collection of trial data.
Outcomes
Inpatient trial
Primary outcomes of the inpatient trial were length of stay, discharge to a higher level of support, and change in physical functioning (measured by the Barthel Index) between baseline and first discharge from the hospital trust. We collected data on length of stay and discharge destination, including inpatient mortality, from the trust's patient administration system and abstracted information on patients' functional status at randomisation and discharge from their medical notes. The patient administration system and medical notes also provided a record of patients' demographic characteristics, main diagnoses on admission (from ICD codes), and the number of major and minor medical reviews, tests and investigations, therapy sessions, changes to medication, and new complications during the post-acute period.
Follow up
We reassessed patients' physical functioning and move to a more dependent living arrangement at six months during a brief structured telephone interview with patients or carers. We also reassessed length of inpatient stay in three ways: from randomisation to first discharge “home” (that is, including immediate transfers to community hospital), from first discharge home to six months after randomisation (that is, readmission days), and from randomisation to six months later (that is, total length of stay). We obtained data on length of stay from the patient administration system and cross checked these against patients' case notes. We measured patients' perceived quality of life at six months using the reintegration to normal living scale.21 We recorded any deaths after hospital discharge from the patient administration system and confirmed these at follow up. We obtained information on use of primary care services directly from the patients' general practices and district nurses. During the telephone interviews, we asked patients whether they received home care, “meals on wheels,” or other social services.
Sample size
We determined sample size for the primary outcomes of the inpatient trial and calculated that 200 patients in total would be required to have 80% power in a 5% test if the true difference in length of stay were 8 days (with standard deviation 20 days), that 210 patients would be needed to detect a 10 point difference in Barthel Index (SD 20 points) with 95% power, and that 220 patients be needed to detect a reduction from 55% to 35% in the rate of patients discharged to a higher level of support with 85%. We therefore chose a sample size of 240 in total to allow for refusal, dropout, and uncertainty about the true size of differences on which power calculations were based.
Statistical analysis
We compared binary outcome variables of the treatment and control arms of the trial in a logistic regression model, controlling for the stratifying variable, the hospital ward from which patients were referred. Results are presented as controlled odds ratios with 95% confidence intervals. We estimated differences in mean lengths of stay and Barthel scores, with their associated 95% confidence intervals, using regression models controlled for referring ward. In our analysis of Barthel scores we also controlled for functional status at entry to the trial. Median values are also presented for these quantitative variables. Where data were not normally distributed we performed uncontrolled Mann-Whitney U tests (results not shown because conclusions were unchanged).
We compared mean number of new complications, medical reviews, tests, changes to medication, and therapy sessions while in the hospital trust in Poisson regression models, controlling for referring ward and including length of stay as an exposure variable. These models excluded two subjects in each group who had zero length of stay. The Poisson models produced the ratio of mean numbers per patient per day between treated and control groups, with associated 95% confidence intervals, while we estimated ratios of means per patient from models that excluded length of stay.
We repeated analyses with all controlled models using patient's sex as a covariate (results not shown because conclusions were unaffected). Patients were included in their allocated group for analysis irrespective of any subsequent treatment received.
Statistical analyses of follow up data proceeded along similar lines. In our analyses of primary and community district nursing care we controlled for hospital length of stay.
Results
Patients' characteristics
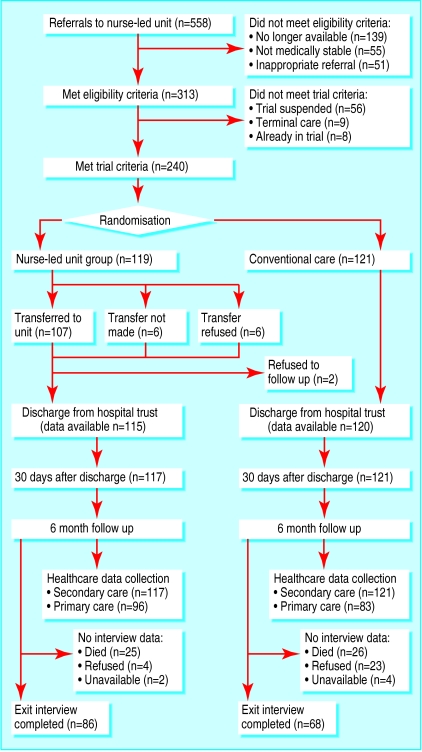
A total of 240 patients were randomised, and the final inpatient sample was 238 because medical records were missing for two patients (see figure). Of the 119 patients randomised to the nurse-led unit, six refused the offer of transfer and another six were not transferred because of unexpected deterioration or unexpectedly quick discharge from hospital. At follow up, we obtained data on use of secondary and primary care services for 238 and 179 patients respectively and interviewed 154 patients or carers. More of the patients allocated conventional care declined to participate in the follow up (23/121 (19%) v 4/117 (3.5%)), but we found no important differences between those who consented and those who refused.
Table 1 shows the patients' baseline characteristics. Patients were generally elderly but not extremely old. Most lived alone in the community, and many were frail or cognitively impaired. Social needs were most commonly noted as the reason for referral to the nurse-led unit (65 cases), but no reason was given for 91 patients.
Table 1.
Characteristics of hospital inpatients at randomisation to post-acute care in nurse-led unit or conventional care. Figures are numbers (percentages) of patients unless stated otherwise
| Characteristic | Nurse-led unit (n=119) | Conventional care (n=121) |
|---|---|---|
| Age (years): | ||
| Mean (SD) | 72.2 (8.50) | 69.3 (9.73) |
| Range | 37-91 | 31-81 |
| Sex: | ||
| Male | 61 (51) | 48 (39) |
| Female | 58 (49) | 73 (60) |
| Living arrangements before hospital admission: | ||
| Institutional care | 8 (7) | 7 (6) |
| Living alone | 73/117 (62) | 70/121 (58) |
| Receiving home nursing services if not in nursing home | 10/83 (12) | 7/82 (9) |
| Receiving social services if not in residential care | 29/76 (38) | 25/77 (32) |
| Medical diagnosis at admission: | ||
| Respiratory: | 21 (18) | 22 (18) |
| Exacerbation of chronic problem | 21 | 21 |
| Cardiovascular: | 33 (29) | 37 (31) |
| Stroke | 12 | 18 |
| Unstable angina or myocardial infarction | 4 | 4 |
| Chronic heart disease | 17 | 15 |
| Gastrointestinal: | 14 (12) | 6 (5) |
| Ulcer | 9 | 2 |
| Other | 5 | 4 |
| Symptoms or signs*: | 16 (14) | 13 (11) |
| Syncope or collapse | 6 | 6 |
| Other*: | 30 (26) | 41 (34) |
| Urinary tract infection | 4 | 4 |
| Physical function at admission (Barthel index†): | ||
| Mean (SD) | 63.5 (23.5) | 60.3 (22.8) |
| Range | 0-100 | 0-100 |
| Reason for referral to nurse-led unit: | ||
| Social | 31 (26) | 34 (28) |
| Rehabilitation | 24 (20) | 27 (22) |
| Other | 17 (14) | 16 (13) |
| None given | 47 (36) | 44 (36) |
| Cognitively impaired at randomisation | 39/114 (34) | 32/119 (27) |
These two categories are extremely varied, and only the most common diagnoses in each group are given.
100 point scale with higher values indicating better function.
Length of stay
Table 2 gives details of patients' length of inpatient stay, including readmission days. Patients in the nurse-led unit stayed in the hospital trust more than 14 days longer, on average, than those managed conventionally. Six of the 107 patients in the nurse-led unit (6%) were transferred to community hospitals for post-acute convalescence compared with 30/113 (27%) patients in the control group (difference −21.0% (95% confidence interval −32.8% to −10.3%), P=0.000). Taking these transfers into account (that is, length of stay until first discharge home), we found the difference between groups in initial length of post-acute hospitalisation dropped to 4.5 days and was no longer significant. Over the entire study period, differences in total patient length of stay continued to diminish.
Table 2.
Lengths of hospital stay and subsequent readmissions of hospital inpatients given post-acute care in nurse-led unit or conventional care
| Nurse-led unit (n=117) | Conventional care (n=121) | Difference in means (95% CI)
|
|||
|---|---|---|---|---|---|
| Uncontrolled | Controlled for referring ward | ||||
| Initial length of stay in hospital trust (days)*: | |||||
| Mean | 32.5 | 18.2 | 14.3 (7.8 to 20.7) | 14.5 (8.2 to 20.9) | |
| Median (range) | 26 (1-183) | 11 (0-104) | |||
| Length of stay until first discharge home (days)†: | |||||
| Mean | 33.4 | 28.9 | 4.5 (−3.6 to 12.5) | 4.8 (−3.1 to 12.8) | |
| Median (range) | 27 (1-183) | 18 (0-184) | |||
| Readmissions (days)‡: | |||||
| Mean | 7.7 | 10.6 | −2.8 (−8.0 to 2.4) | −2.9 (−8.1 to 2.4) | |
| Median (range) | 0 (0-97) | 0 (0-162) | |||
| Total time in hospital (days)§: | |||||
| Mean | 41.1 | 39.5 | 1.7 (−7.4 to 10.7) | 1.9 (−7.1 to 11.0) | |
| Median (range) | 32 (1-183) | 31 (0-184) | |||
From randomisation to post-acute care to first discharge from trust.
“Home” signifies any discharge destination that is not a hospital (such as residential care), so length of stay in hospital trust plus community hospitals.
Between first discharge home and 6 months' follow up.
From randomisation to 6 months' follow up.
Patient outcomes
As tables 3 and 4 show, patients were neither better nor worse off if cared for in the nurse-led unit. Patients in the nurse-led unit were somewhat more likely than those in the control group to have been discharged to a higher level of support, especially when the discharge destinations for patients initially transferred to community hospital were included in the calculation, but these differences were not significant (table 3). After controlling for Barthel score at baseline, we found no difference between the groups' functional status at discharge or after six months (table 4). Mortality at six months' follow up was substantial in both groups and virtually identical, as was the patients' perceived quality of life. Patients in the nurse-led unit had noticeably, though not quite significantly, lower readmission rates than control patients (12% v 20% at 1 month, 32% v 41% at 6 months).
Table 3.
Outcomes for hospital inpatients given post-acute care in nurse-led unit or conventional care. Values are numbers (percentages) of patients unless stated otherwise
| Nurse-led unit | Conventional care | Odds ratio (95% CI)
|
|||
|---|---|---|---|---|---|
| Uncontrolled | Controlled for referring ward | ||||
| Change to higher level of support in living arrangements: | |||||
| At first discharge* | 30/99 (30) | 17/77 (22) | 1.54 (0.77 to 3.05) | 1.82 (0.88 to 3.77) | |
| At first discharge home† | 29/104 (28) | 19/106 (18) | 1.77 (0.92 to 3.41) | 1.95 (0.98 to 3.88) | |
| By 6 months | 29/75 (39) | 21/61 (34) | 1.20 (0.59 to 2.43) | 1.17 (0.57 to 2.42) | |
| New move to institutional care: | |||||
| At first discharge* | 19/93 (20) | 12/75 (16) | 1.35 (0.61 to 2.99) | 1.55 (0.67 to 3.55) | |
| At first discharge home† | 21/98 (21) | 15/102 (15) | 1.58 (0.76 to 3.28) | 1.73 (0.81 to 3.70) | |
| By 6 months | 20/70 (29) | 14/59 (24) | 1.29 (0.58 to 2.84) | 1.30 (0.58 to 2.92) | |
| Mortality: | |||||
| By first discharge* | 9/117 (8) | 8/121 (7) | 1.18 (0.44 to 3.16) | 1.22 (0.44 to 3.37) | |
| By 6 months | 25/117 (21) | 26/121 (22) | 0.99 (0.53 to 1.85) | 1.00 (0.53 to 1.88) | |
| Readmission: | |||||
| Within 30 days of first discharge* | 12/101 (12) | 16/80 (20) | 0.54 (0.24 to 1.22) | 0.50 (0.22 to 1.14) | |
| Within 6 months | 37/117 (32) | 50/121 (41) | 0.66 (0.37 to 1.10) | 0.64 (0.37 to 1.10) | |
Among subjects not transferred to another hospital.
“Home” signifies any discharge destination that is not a hospital (such as residential care).
Table 4.
Physical function and reintegration to normal life of hospital inpatients given post-acute care in nurse-led unit or conventional care
| Nurse-led unit | Conventional care | Difference in means (95% CI)
|
|||
|---|---|---|---|---|---|
| Uncontrolled | Controlled for referring ward | ||||
| Physical function (Barthel index*): | |||||
| At first discharge: | (n=104) | (n=108) | |||
| Mean | 74.8 | 70.0 | 4.8 (−1.5 to 11.1) | 2.5 (−1.5 to 6.5) | |
| Median (range) | 85 (0-100) | 75 (0-100) | |||
| At 6 months: | (n=82) | (n=66) | |||
| Mean | 74.0 | 68.5 | 1.1 (−0.7 to 2.8) | 0.8 (−0.7 to 2.3) | |
| Median (range) | 80 (0-100) | 75 (0-100) | |||
| Quality of life at 6 months (Wood-Dauphinee score†): | (n=59) | (n=50) | |||
| Mean | 16.3 | 15.4 | 0.9 (−0.7 to 2.4) | 0.9 (−0.7 to 2.4) | |
| Median (range) | 17 (4-22) | 16 (7-22) | |||
100 point scale with higher values indicating better function. Controlled model includes baseline Barthel index.
22 point scale with higher values indicating better reintegration. Scale completed during interviews with patients only, not with carer proxies.
Processes of care
Table 5 compares processes of care in the two groups. As expected, patients in the nurse-led unit had significantly fewer minor medical reviews, both overall and per patient per day. They also had significantly fewer major medical reviews, tests and investigations, changes to medication, and number of new complications in hospital per patient per day. The patients in the nurse-led unit received significantly more physiotherapy and occupational therapy sessions than the control patients, but when we adjusted the analyses for the longer lengths of stay in the nurse-led unit the differences were no longer significant. Nurses transferred 22/103 patients (21% (95% confidence interval 14% to 31%)) from the nurse-led unit back to acute medical wards during their initial post-acute stay. After hospital discharge, we found no differences in the number or type of contacts between patients and general practitioners, practice nurses, or district nurses. We also found no difference between groups in their propensity to receive social services (70/86 (81%) of patients in the nurse-led unit who were interviewed v 56/68 (82%) of control patients).
Table 5.
Indicators of processes of care for hospital inpatients given post-acute care in nurse-led unit or conventional care
| Events | Mean No of events/patient
|
Mean No of events/patient/day
|
|||||
|---|---|---|---|---|---|---|---|
| Nurse-led unit | Conventional care | Mean ratio (95% CI)* | Nurse-led unit | Conventional care | Mean ratio (95% CI)* | ||
| In hospital† | |||||||
| Minor medical reviews | 4.81 (n=115) | 11.11 (n=119) | 0.44 (0.39 to 0.48) | 0.148 (n=115) | 0.600 (n=119) | 0.24 (0.22 to 0.26) | |
| Major medical reviews | 1.59 (n=115) | 1.22 (n=119) | 1.29 (1.04 to 1.61) | 0.049 (n=115) | 0.066 (n=119) | 0.71 (0.56 to 0.89) | |
| Tests or investigations | 4.97 (n=115) | 5.28 (n=119) | 0.95 (0.85 to 1.07) | 0.152 (n=115) | 0.285 (n=119) | 0.58 (0.51 to 0.65) | |
| Changes to medication | 2.38 (n=115) | 2.09 (n=119) | 1.13 (0.96 to 1.35) | 0.073 (n=115) | 0.113 (n=119) | 0.64 (0.54 to 0.77) | |
| Physiotherapy sessions | 2.28 (n=113) | 1.13 (n=119) | 2.04 (1.65 to 2.51) | 0.070 (n=113) | 0.061 (n=119) | 1.16 (0.93 to 0.44) | |
| Occupational therapy sessions | 2.35 (n=113) | 1.48 (n=118) | 1.58 (1.30 to 1.91) | 0.073 (n=113) | 0.082 (n=118) | 0.91 (0.74 to 1.12) | |
| New complications | 0.63 (n=117) | 0.60 (n=119) | 1.06 (0.77 to 1.48) | 0.019 (n=117) | 0.032 (n=119) | 0.61 (0.44 to 0.86) | |
| After hospital‡ | |||||||
| GP visits in surgery | 1.70 (n=96) | 1.90 (n=80) | 0.93 (0.74 to 1.16) | NA | NA | NA | |
| GP visits at home | 2.76 (n=96) | 2.53 (n=80) | 1.06 (0.88 to 1.28) | NA | NA | NA | |
| District nurse visits | 4.24 (n=96) | 4.94 (n=80) | 0.78 (0.68 to 0.89) | NA | NA | NA | |
GP=general practitioner. NA=not appropriate.
Controlled for referring ward.
Excluding patients with zero length of stay before first discharge from hospital trust.
Including patients with zero length of stay before first discharge from hospital trust.
Discussion
The trial reported here was part of a multi-method study investigating the effectiveness and acceptability of an inpatient nurse-led unit providing post-acute intermediate care. With the exception of large and significant differences in initial inpatient length of stay, with the patients in the nurse-led unit remaining longer, we found no differences in primary outcomes. Moreover, when we included transfers to community hospital in the measurement of initial length of stay, the difference between groups was no longer significant. This is an important finding, not reported elsewhere in the literature. In effect, the beds being unblocked by the nurse-led unit are those in community hospitals.
Study limitations
At the level of nurse-led units, our study's sample size is one. However, in its staffing and funding, the unit we examined was more typical of normal NHS provision than nursing development units evaluated in the past. We would argue that all innovations in intermediate care must be locally sensitive and contingent on existing services as well as pressing needs. What is generalisable is the patient population, the pressures on general medical beds, and the low technology setting on a separate ward.
The use of a randomised consent design was somewhat unusual and might have resulted in a high refusal rate among the patient allocated to the nurse-led unit. This would have diluted the effects that could be observed. In the event, refusal rates were considerably lower than those reported elsewhere for such designs.22
There are other limitations to this evaluation. Firstly, we chose primary outcomes that are well validated and consistently used in studies of post-acute care, but these may not be optimal measures of effectiveness. Specifically, the Barthel index offers little in relation to patients' own goals for recovery but rather narrowly equates functional status with the need for formal support.
Secondly, we derived the Barthel scores at admission and discharge from patients' medical notes, but at six months we derived them from patients' or carers' reports. Although randomisation should ensure an unbiased comparison between groups, the judgments of capability might have differed between professional and lay assessors.
Thirdly, given that transfers to community hospital emerged as an important factor, it would have been useful if we had examined styles of service delivery, perhaps with the process indicators from the inpatient portion of the trial. However, this was beyond the scope of the trial.
Fourthly, not all localities feature community hospital alternatives to acute hospital care. In such areas, patterns of treatment, including the effectiveness of a nurse-led unit, may differ from what we observed.
Finally, a fully rounded view of the unit's effects can be gained only by integrating results from the economic, qualitative, and process studies with those presented here.
Implications of study
Our finding regarding length of stay has implications for the development of intermediate care. It suggests that if acute hospital trusts became responsible for post-acute transitional care, community hospital trusts would find resources released to develop strategies to avoid patient admissions. This may constitute an overall benefit for the NHS, but a whole-systems assessment of responsibilities along the full continuum of care is needed. For example, acute trusts may identify organisational benefits to maintaining an in-house transfer destination for those patients whose pre-admission status predicts complex discharge arrangements or slower than average recovery time. This could be especially true if, as in many inner cities, they lack the option of community beds. Clearly, however, there are also costs; hospital days are more expensive in acute than community trusts.
The clinical outcomes we observed suggest that concerns about patient safety in the absence of routine medical involvement are unfounded. Nor did we see any shifts in service demand towards primary care. However, our results cannot support claims made for the therapeutic benefits of a separate nurse-led unit above and beyond usual nursing care. There were some indications that readmission rates might have been lower for the patients discharged from the nurse-led unit, the value of which should not be minimised. If true, lower readmission rates would mean less disruption in the recovery process for a potentially large set of predominantly elderly patients, which would have related cost savings. However, this finding was only suggestive and should be treated with caution. The outcomes of functional status, dependency of living arrangement, and mortality were no better for patients cared for in the nurse-led unit than for control patients. Other randomised controlled evaluations of nurse-led units have also failed to show effectiveness in either organisational or patient outcomes.6,12,13
Conclusions
At a time when the government has committed an additional £900m a year over a five year period to intermediate care, our central finding is that nurse-led post-acute care in hospital is no worse, but also no better, than usual care and follow up. To make the most of the new resources, the place of acute hospitals in the provision of intermediate care will need to be examined closely.
What is already known on this topic
Nursing development units were the first nurse-led intermediate care units, designed to support patients who are medically stable but not ready for discharge
Although evaluations of such units have attracted considerable interest, the evidence for their effectiveness is not clear and their generalisability to routine practice is open to question
What this study adds
Nurse-led units are safe and do not shift service demands from hospital to community—instead, they seem to unblock beds in community hospitals
To make the most of additional resources allocated to intermediate care, a whole-systems assessment of local responsibilities along the full continuum of care will be needed
Figure.
Flow of patients through trial
Acknowledgments
We thank the managers, clinicians, and patients involved with the nurse-led unit at Southampton University Hospitals Trust. Members of the Southampton NLU Evaluation Team were AS, BW, RMP, RW, JW, JIB, Peter Lees, Julie Pearce, Karen Postle, Lisa Sheron, and Jerry Warr.
Footnotes
Funding: The study was supported by a University of Southampton research studentship for BW, based in the School of Nursing and Midwifery, and by a grant from the NHSE R&D Directorate South and West (D/10/11.97/Steiner).
Competing interests: None declared
References
- 1.Audit Commission. The coming of age: improving care services for older people. London: Audit Commission; 1997. [Google Scholar]
- 2.Lipley N. Nurse-led elderly care wards to free acute beds. Nurs Stand. 2000;14:5. [Google Scholar]
- 3.Audit Commission. The way to go home: rehabilitation and remedial services for older people. London: Audit Commission; 2000. [Google Scholar]
- 4.Department of Health. The NHS plan: a plan for investment, a plan for reform. London: Stationery Office; 2000. [Google Scholar]
- 5.Steiner A. Intermediate care: a good thing? Age Ageing (in press). [DOI] [PubMed]
- 6.Steiner A. Intermediate care: conceptual framework and review of the literature. London: King's Fund; 1997. [Google Scholar]
- 7.Pearson A, editor. Primary nursing in the Burford and Oxford nursing development units. London: Chapman and Hall; 1988. [Google Scholar]
- 8.Evans A, Griffiths P. The development of a nursing-led in-patient service. London: King's Fund; 1994. [Google Scholar]
- 9.Hall LE, Alfano GJ, Rifkin E, Levine HS. Longitudinal effects of an experimental nursing process (final report). New York: Loeb Center for Nursing and Rehabilitation; 1975. [Google Scholar]
- 10.Pearson A, Punton S, Durant I. Nursing beds: an evaluation of the effects of therapeutic nursing. Harrow: Scutari Press; 1992. [Google Scholar]
- 11.Griffiths P, Evans A. Evaluation of a nursing-led in-patient service. London: King's Fund; 1995. [Google Scholar]
- 12.Griffiths P, Wilson-Barnett J, Richardson G, Spilsbury K, Miller F, Harris R. The effectiveness of intermediate care in a nursing-led in-patient unit. Int J Nurs Stud. 2000;37:153–161. doi: 10.1016/s0020-7489(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths P, Wilson-Barnett J. The effectiveness of ‘nursing beds’: a review of the literature. J Adv Nurs. 2000;27:1184–1192. doi: 10.1046/j.1365-2648.1998.00655.x. [DOI] [PubMed] [Google Scholar]
- 14.Alfano GJ, Hall LE. The Loeb center for nursing and rehabilitation: a professional approach to nursing practice. Nurs Clin North Am. 1969;4:487–493. [PubMed] [Google Scholar]
- 15.Pearson A. The clinical nursing unit. London: William Heinemann Medical Books; 1983. [Google Scholar]
- 16.Vaughan B, Lathlean J. Intermediate care: models in practice. London: King's Fund; 1999. [Google Scholar]
- 17.Walsh B, Pickering RM, Brooking JI. A randomized controlled trial of nurse-led inpatient care for post-acute medical patients: a pilot study. J Clin Effectiveness Nurs. 1999;3:88–90. [Google Scholar]
- 18.Pocock SJ. Clinical trials: a practical approach. Chichester: John Wiley and Sons; 1983. [Google Scholar]
- 19.Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979;300:1242–1245. doi: 10.1056/NEJM197905313002203. [DOI] [PubMed] [Google Scholar]
- 20.Resau LS. Obtaining informed consent in Alzheimer's research. J Neurosci Nurs. 1995;27:57–60. doi: 10.1097/01376517-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wood-Dauphinee SL, Williams JI. Reintegration to normal living as a proxy to quality of life. J Chronic Disability. 1987;40:491–499. doi: 10.1016/0021-9681(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Altman DG, Whitehead J, Parmar MKB, Stenning SP, Fayers PM, Machin D. Randomised consent designs in cancer clinical trials. Eur J Cancer. 1995;31A:1934–1944. doi: 10.1016/0959-8049(95)00470-x. [DOI] [PubMed] [Google Scholar]