Abstract
Maternal periodontitis has emerged as a putative risk factor for preterm births in humans. The periodontitis-associated dental biofilm is thought to serve as an important source of oral bacteria and related virulence factors that hematogenously disseminate and affect the fetoplacental unit; however the underlying biological mechanisms are yet to be fully elucidated. This study hypothesized that an oral infection with the human periodontal pathogens Campylobacter rectus and Porphyromonas gingivalis is able to induce fetal growth restriction, placental inflammation and enhance Toll-like receptors type 4 (TLR4) expression in a murine pregnancy model. Female Balb/C mice (n=40) were orally infected with C. rectus and/or P. gingivalis over a 16-week period and mated once per week. Pregnant mice were sacrificed at embryonic day (E) 16.5 and placentas were collected and analyzed for TLR4 mRNA levels and qualitative protein expression by real time PCR and immunofluorescence. TLR4 mRNA expression was found to be increased in C. rectus-infected group (1.98±0.886 fold difference, P<0.01, ANOVA) compared to controls. Microscopic analysis of murine placentas showed enhanced immunofluorescence of TLR4 in trophoblasts, mainly in the placental labyrinth layer. Also, combined oral infection with C. rectus and P. gingivalis significantly reduced the overall fecundity compared to controls (16.7% vs. 75%, infected vs. non-infected mice respectively, P=0.03, Kaplan-Meier). The results supported an enhanced placental TLR4 expression after oral infection with periodontal pathogens. The TLR4 pathway has been implicated in the pathogenesis of preterm births; therefore the abnormal regulation of placental TLR4 may give new insights into how maternal periodontitis and periodontal pathogens might be linked to placental inflammation and preterm birth pathogenesis.
Keywords: Mice, Periodontitis, Preterm Birth, Campylobacter rectus, Porphyromonas gingivalis, Toll-like receptors, Placenta
INTRODUCTION
Preterm birth (birth at ≤37 completed gestational weeks) is the major cause of neonatal mortality/morbidity in the world, accounting for up to 75–85% of the early neonatal deaths as well as to high rates of short-term (low birth weight) and long-term adverse sequelae (hearing/learning problems and cerebral palsy) [1;2]. The pathogenesis of preterm birth is thought to be multi-factorial, possibly initiated by multiple mechanisms including infection, uteroplacental ischemia, hemorrhage, stress and other immunologically mediated processes [3]; however, the development of a pro-inflammatory condition is a common effector pathway that centralizes all multiple risk factors [4]. In particular, uterine infections may account for 25–40% of preterm births and they are strongly linked with a pro-inflammatory systemic state. For example, uterine infections are known to upregulate the production of local pro-inflammatory cytokines, metalloproteinases and prostaglandins that lead to membrane weakening, early membrane rupture and uterine contraction initiation [5]. Uterine infections usually take advantage of ascending mechanisms, which are originated from vaginal infections (i.e. Neisseria gonorrhoeae or Ureaplasma urealyticum) that lead to intrauterine cavity access, decidua colonization, localized inflammation onset (or chorioamnionitis), intraamniotic infection and ultimately fetal infection [6].
Nonetheless, other sources of infection including the oral cavity have been proposed to facilitate the hematogenous transmission of pathogens that affect normal pregnancy development [7]. In particular, periodontal diseases (gingivitis and periodontitis) are part of the most common chronic infections affecting up to 50% of humans [8] and has been found to be an independent putative risk factor for pregnancy-related complications such as preterm births, low birth weight and preeclampsia, after adjusting for other known obstetric risk factors [9;10]. Periodontitis is initiated when specific microorganisms accumulate between the teeth and gums, forming bacterial biofilms commonly known as dental plaque. The body reacts to dental biofilms by activating the oral mucosal inflammatory response that –in some susceptible patients- is unsuccessful in controlling the infection. With time, the inflammatory response remains chronic and ultimately leads to periodontal connective tissue resorption (alveolar bone loss) and tooth loss [11]. During prolonged periodontal inflammation, periodontal pathogens and related virulence factors invade periodontal tissues, enter the blood stream by means of transient bacteremias [12] and disseminate throughout different systemic organs. In fact, important periodontal pathogens have been detected in human placentas of women with preeclampsia [13] and in the amniotic fluid of pregnant women with a diagnosis of premature labor [14] or premature labor with intact membranes [15;16].
Fetal exposure to periodontal pathogens from maternal oral biofilms has also been demonstrated in umbilical cord blood samples from preterm births by detecting maternal immunoglobulin G (IgG) as well as fetal immunoglobulin M (IgM) to one or more specific oral pathogens. In particular, mothers with a low IgG response to P. gingivalis combined with a high fetal IgM response to C. rectus showed the highest rate of preterm deliveries (66.7%) among 812 deliveries from a cohort study of pregnant mothers (adjusted OR 10.3; P<0.0001) [17], suggesting that P. gingivalis and C. rectus could act as fetal infectious agents eliciting complications during pregnancy. C. rectus is an Gram negative anaerobe and motile bacterium unique to the oral cavity that is phylogenetically related to H. pylori and is associated with ulceration of the periodontal attachment apparatus [18]. Interestingly, other Campylobacter species are known to be a significant causal agent of sheep and cattle abortion due to a marked tropism for placental tissues [19]. In animal models, C jejuni and C. fetus infections result in impaired development and fetal growth restriction (FGR) [20]. We have previously reported that a subcutaneous infection with C. rectus in pregnant mice disseminates to placental tissues and induces FGR [21;22], placental inflammation and structural alterations. [23]. Likewise, animal experiments using Porphyromonas gingivalis in a subcutaneous infection model have shown increased maternal inflammatory serum markers (Interleukin-6 and tumor necrosis factor alpha TNF-α), and increased fetal biochemical markers of placental inflammation (prostaglandin E2) in murine amniotic fluid [24;25].
Placental infection and subsequent inflammation have been associated with preterm labor, so the biological pathways related to early inflammatory responses are likely to mediate pathogenesis. Toll-like receptors (TLRs) are pattern recognition receptors that play a key role in the innate inflammatory response [26] and have been proposed to play important roles in pregnancy maintenance, placental immune protection and delivery initiation [27]. To date, a total of 10 human and 12 murine TLRs have been described. In general, TLRs can be categorized into two main groups based on their ligands: the first group consists of TLR1, 2, 4, and 6 which recognize bacterial molecules such as lipopolysaccharide (LPS), lipoteichoic acid and peptidoglycan. The second group consists of TLR3, 7, 8, and 9 that recognize pathogen-associated nucleic acids patterns [28]. Here in we focused on TLR4 which is selectively activated by Gram negative LPS, in conjunction with CD14 [29]. Since P. gingivalis and C. rectus are Gram negative periodontal pathogens, the main objective of this study was to determine whether an oral infection with C. rectus and C. rectus/P. gingivalis combined infection could affect fetal growth, fecundity and induce placental inflammation along with enhanced expression of TLR4 in a timed-pregnancy murine model.
METHODS
Timed-pregnancy murine model
Balb/C mice were obtained at 6–8 weeks of age and maintained on a 12-h light/dark cycle (0700 to 1900 light) and a constant temperature of 25°C, receiving distilled water and food ad libitum. To facilitate bacterial colonization, all female groups were changed to a soft chow enriched with a dextrose solution (30%) as plaque-promoting diet during infection period. For mating purposes, females were age-matched when 20 weeks old, and males were randomly assigned to experimental groups and remained the same until the end. Female pregnancies were confirmed by the presence of a vaginal plug plus significant weight changes (>1.5 grams gain in a week). Mice were infected daily over a 16-week period and mated once/week. When pregnant, female mice were sacrificed at embryonic day (E) 16.5 and placental tissues were collected and analyzed. All procedures were in accordance with animal guidelines and were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Bacterial strains and inoculum preparation
C. rectus 314 and P. gingivalis A7436 aliquots were maintained in Wilkins Chalgren anaerobic broth medium (WC broth; DSMZ, Braunschweig, Germany) containing 10% skim milk at −80°C. C. rectus aliquots were reconstituted on PRAS ETSA plates (Enriched Tryptic Soy Agar from Anaerobe Systems, Morgan Hill CA) and P. gingivalis aliquots on Anaerobic Reducible Blood Agar (from Remel, Lenexa KA). For experiments, bacteria were anaerobically grown under 5% CO2, 10% H2–85% N2 atmosphere at 37°C for 4–6 days. Bacterial suspensions were prepared from primary cultures at their log phase of growth, and concentrations were determined by spectrophotometry (Cecil Instruments, Cambridge, UK) with a measured optical density at 600 nm (C. rectus) and 660 nm (P. gingivalis) corresponding to 1×109 bacteria/ml respectively. Finally, all oral preparations were adjusted accordingly to keep the same concentration during oral infection experiments.
Oral infection
The oral infection model of experimental periodontitis has been described elsewhere [30]. This model involves a pretreatment phase with antibiotics to suppress the oral flora to permit the colonization of exogenously applied human oral bacterial strains and the use of carboxymethylcellulose (CMC) as a carrier to facilitate bacterial colonization. Briefly, before experiment baseline 40 female Balb/C mice were pretreated for 4 days with Kanamycin/Ampicillin (50mg/kg-25mg/kg) followed by a 3-day antibiotic wash out period, and then randomly assigned to experimental groups (Table 1 and Figure 1). Mice were topically infected in the oral cavity with 1×1010 live bacterial units/100uL in a phosphate-buffered saline (PBS) and 2% CMC preparation on a daily basis. Controls included a blank group (same diet without oral infection) and a negative control group (CMC application without bacteria).
Table 1. Experimental groups.
All groups were given ad libitum soft chow enriched with a dextrose solution (30%) as plaque-promoting diet during 16 weeks of experimentation. CMC=carboxymethylcellulose; CFU=colony forming units.
| Experimental Group | N | Description |
|---|---|---|
| Blank control | 8 | High-glucose soft chow |
| Negative control | 8 | High-glucose soft chow CMC topical application |
| Campylobacter rectus infection | 12 | High-glucose soft chow CMC + C. rectus (1×1010 CPU) |
| Campylobacter rectus and porphyromonas gingivalls infection | 12 | High-glucose soft chow CMC + C. rectus (1×1010 CFU) CMC + P. gingivalis (1×1010 CFU) |
Figure 1. Experiment timeline.
Female (3-month old) Balb/C mice were pretreated for 4 days with Kanamycin/Ampicillin (50mg/kg-25mg/kg) and then randomly assigned to experimental groups. Mice were infected daily over a 16-week period and mated once/week. When pregnant, mice were sacrificed at embryonic day (E)16.5 and placental tissues were collected and analyzed for TLR4 expression by immunofluorescence (confocal microscopy) followed by RT-PCR. Cr=C. rectus; Pg=P. gingivalis; CMC=carboxymethylcellulose.
Placental histology and Immunofluorescence
Placentas were fixed in 4% paraformaldehyde, bisected sagittally, processed and embedded in paraffin. Sections (~5 μm) were stained using hematoxylin/eosin (H&E) for structural analysis; other sections were processed for immunofluorescence. Briefly, tissue antigens were rescued with Safeclear® (Fisher Protocol, Fair Lawn NJ) for 20 minutes and washed under serial ethanol concentrations. After washing in 0.2% Triton/PBS, sections were incubated in 10% non-immune goat serum and bovine serum albumin in PBS for 1 hour. Rabbit anti-TLR4 monoclonal antibody (Zymed, Invitrogen, Carlsbad CA) and mouse anti-mouse Cytokeratin 7 monoclonal antibody (RCK105 from Abcam, Cambridge, UK) were incubated overnight on a 1:50 concentration. Cytokeratin 7 was chosen as a trophoblast marker following the recommendations of the workshop report on cell culture models of trophoblasts [31]. After vigorous washing, secondary biotinylated antibodies were applied for 1 hour (Alexa Fluor 568 goat anti-rabbit IgG and Alexa Fluor 488 goat anti–mouse IgG from Molecular Probes, Invitrogen, Carlsbad CA) for 1h. Sections were washed in 0.2% Triton/PBS, mounted and coverslipped with Vectashield (Vector labs, Burlingame CA). All stained sections were analyzed and photographed under confocal microscopy (LSM5, Carl Zeiss, Thornwood NY).
Quantitative RT-PCR for TLR4
Total RNA was isolated from all placental tissues (n=135) with the use of the RNeasy Mini Kit (Qiagen). cDNA from 2 μg of total RNA was synthesized using the Omniscript Kit (Qiagen) and random decamer primers. Real-time PCR was performed with 1 μL cDNA, TaqMan Universal PCR mix, and 20X primer (Mm00445273_m1 from Applied Biosystems, Foster City, CA), in a 7000 Sequence Detection System (ABI Prism, Applied Biosystems). Reactions were performed in duplicates and in two independent times. The Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene was used as an endogenous control (housekeeping gene). Results were evaluated using the delta-delta Ct method, where delta Ct was calculated as (TLR4 Ct) (GAPDH Ct), and the relative quantity of TLR4 mRNA expression was calculated by the delta–delta Ct as 2−[(infected sample delta Ct)−(control sample delta Ct)].
Statistical analysis
A sample size of 8 mice per group was calculated [power (1-β) of >0.90% with alpha-error threshold of (α)=0.05)] based on our previous results on fetal growth restriction after C. rectus systemic infection [22]. Categorical variables (number of pregnancies and number of fetuses/resorptions per group) were summarized using frequencies and percentages, and continuous variables (fetal weight/length and mRNA levels) were described using means and standard errors. Distributions of fetuses/resorptions were compared using the Chi-square test. Mean fetal weight and length values as well as mRNA levels were compared using Analysis of Variance (ANOVA). To determine whether oral infection correlated with FGR and resorption induction, a general linear regression model (GLM procedure) was used to control for clustering within litters per group. Also, resorptions were considered as zero (value=0) to account for their impact on the average litter weight and length. Kaplan-Meier estimation analysis was used to evaluate cumulative pregnancy events at 16 weeks. Cox proportional hazards ratios were used to determine the risk of no pregnancies over time after infection. If no significant differences were observed between the control groups (blank and negative), then controls were regrouped as non-infected group for analysis. The threshold for statistical significance was set at a P-value less than 0.05. All analyses were performed using SAS v.9.2. (SAS Institute, Cary, NC).
RESULTS
Oral infection with C. rectus and P. gingivalis induced growth restricted fetuses and more fetal resorptions
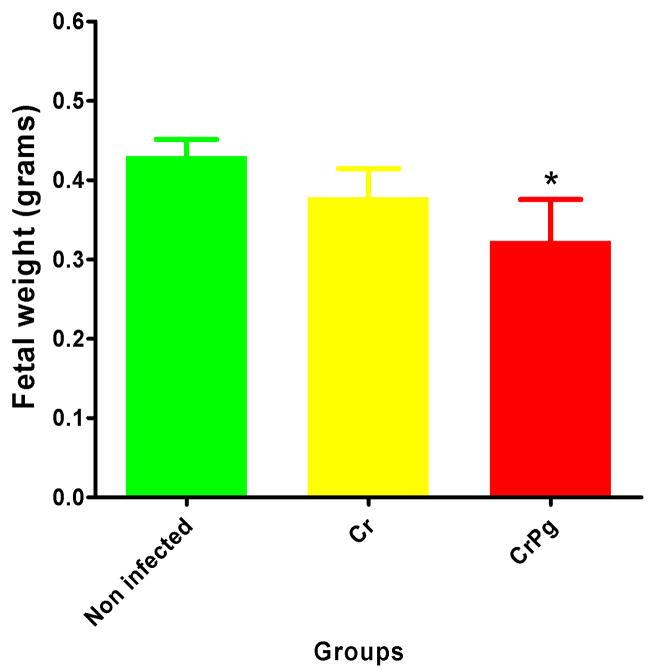
The murine pregnancy events and fetal outcomes after oral infection with periodontal pathogens are depicted in Table 2. The number of mice which became pregnant during the 16 week infection/mating protocol were significantly different among all groups (P<0.05, Chi-square test) as well as in the distribution of fetuses and resorptions (P<0.01). On average, fetuses from infected mice were smaller and lighter, although fetal lengths were not statistically different among all groups (P=0.08, ANOVA). When control groups were compared, no significant differences were observed for number of fetuses/resorptions [27(96.4%) vs. 41(80.4%), P=0.27, Chi-square], and for fetal weight values [0.47±0.012 vs. 0.39±0.035, P=0.87, ANOVA, blank and negative groups respectively]. However, when fetal weight values from infected mice were compared to those from non-infected mice (regrouped controls), a statistically significant decrease was observed for the C. rectus and P. gingivalis infected group (P<0.05, ANOVA, Figure 2). Overall, the general linear regression model which adjusted for clustering within litters was non-significantly associated with FGR induction (P=0.28, GLM procedure).
Table 2. Pregnancy events and fetal outcomes after oral infection.
Distribution of pregnancies, number of fetuses and resorptions were significantly different among groups (P<0.01, Chi square). Fetuses from infected mice were smaller and lighter on average, but fetal lengths were not different among all groups (P=0.08, ANOVA). CMC=carboxymethylcellulose; CFU=colony forming units.
| Experimental Group | N | Pregnancy events [n (%)] |
Fetuses [n %)] |
Resorptions [n (%)] |
Fetal length (mm) (mean±SE) |
Fetal weight (grams) (mean±SE) |
|---|---|---|---|---|---|---|
| Non-infected | 16 | 11(68.8) | 68(86.1) | 11(13.9) | 1.28±0.060 | 0.43±0.021 |
| C. rectus | 12 | 7(58.3) | 55(73.3) | 20(26.7) | 1.09±0.104 | 0.37±0.036 |
| C. rectus and P. gingivalis | 12 | 2(16.7) | 12(70.6) | 5(29.4) | 1.02±0.265 | 0.32±0.052* |
Figure 2. Oral infection induces low weight fetuses.
Averaged fetal weight values from infected mice were smaller when compared to those from non-infected mice. Columns and bars represent means and standard errors. Cr=C. rectus; Pg=P. gingivalis; *P<0.05, ANOVA.
Murine fecundity was affected after oral infection
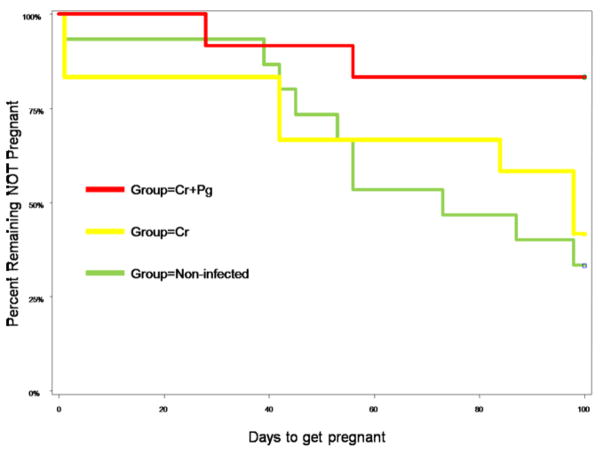
Murine fecundity was significantly different among experimental groups (Table 2 and Figure 3). 68.8% of non-infected mice got pregnant; however fecundity decreased to 58.3% in the C. rectus infected group and only 16.7% of mice receiving C. rectus and P. gingivalis combined infection got pregnant. When cumulative pregnancies events were estimated under Kaplan Meier analysis, it was found that the C. rectus and P. gingivalis group had the lowest fecundity rate (Hazard Ratio 0.19[0.041–0.856], P=0.03) compared to C. rectus group (HR 0.87[0.310–2.154], P=0.68) and to non-infected mice.
Figure 3. Oral infection affects murine fecundity.
Kaplan Meier Analysis - C. rectus and P. gingivalis infected mice had the lowest fecundity rate (16.7%, HR 0.19[0.041–0.856], P=0.03, Kaplan-Meier) compared to C. rectus (58.3%, Hazard Ratio 0.87[0.310–2.154], P=0.68) and to non-infected mice.
Oral infection induced placental inflammation and TLR4 expression
Figure 4 presents representative histological findings on murine placentas. In the control placental tissues, there were scattered inflammatory cells present in the maternal decidua of some samples (not shown). In contrast, areas of focal necrosis and increased inflammatory cell infiltrate were apparent in placentas from infected mice (Figure 4A). Under confocal microscopy, the qualitatively analysis of murine placentas from infected mice showed enhanced TLR4 immunofluorescence, particularly more evident in labyrinth trophoblasts (Figures 4B–F).
Figure 4. Oral infection induced inflammation and TLR4 expression in murine placentas.
Histological and immunofluorescence analysis of murine placentas. 4A: Representative image depicting extensive junctional zone (JZ) along with an increased inflammatory cell infiltrate (arrowheads) in placentas from infected mice. Decidua (D); Bar=50μm. 4B–4C: immunofluorescence of a placenta from the C. rectus and C. rectus/P. gingivalis infected groups respectively. Images represent 3 merged channels (Rhodamine, FITC and DIC) depicting trophoblasts expressing TLR4 from both groups. 4D–E: images depict the expression of Cytokeratin 7 (FITC-green) and TLR4 (Rhodamine-red) in labyrinth trophoblasts from a different C. rectus-infected section; 4F: image illustrates co-localization of Cytokeratin 7 and TLR4 suggesting enhanced expression of TLR4 by labyrinth trophoblasts. 4G-I: images correspond to fluorescence negative controls (no primary antibodies used) for Cytokeratin 7 and TLR4 respectively. Rhod=Rhodamine (red); FITC= Fluorescein isothiocyanat (green); White bars=20μm.
Oral infection increased placental TLR4 mRNA expression
In agreement with the histological observations, mRNA expression of TLR4 receptors was found to be significantly increased in the C. rectus group (1.98±0.886 fold difference, P<0.01 ANOVA) as shown in Figure 5. Even though the TLR4 expression was also higher in the C. rectus and P. gingivalis group, no significant differences were observed when compared to non-infected mice (1.29±0.871, P=0.06).
Figure 5. Oral infection with periodontal pathogens increased TLR4 mRNA expression in murine placentas.
After normalization, TLR4 mRNA levels were significantly increased in C. rectus group placentas (1.98±0.886 fold difference, P<0.01). Although TLR4 mRNA levels were also higher in the combined infection group, no significant differences were observed when compared to non-infected mice (1.29±0.871, P=0.06). Cr=C. rectus; Pg=P. gingivalis; *P<0.01, ANOVA).
DISCUSSION
This study sought to determine the effect of C. rectus and C. rectus/P. gingivalis oral infection on fetal growth restriction, fecundity and the placental TLR4 expression in a murine model of pregnancy. Previous experimental murine models of infection have examined the role of bacteria and/or isolated virulence factors (i.e. LPS) on preterm birth pathogenesis, reporting that C3HeB/FeJ mice develop up to 71% preterm births after heat-killed E. coli intrauterine injection [32]. However not many animal models of infection have addressed the pathogenic role of live oral bacterial infection on preterm births. Experiments with live periodontal bacteria have used subcutaneously-implanted stainless steel coiled chambers as an infection model to study a chronic distant infection in mice and rabbits, as it induces placental inflammation and growth restriction phenotypes (C. rectus and P. gingivalis) [33]. The periodontal pathogen Fusobacterium nucleatum has also been used for intravenous injections in mice, showing subsequent placental inflammation and increased fetal resorptions and stillbirths [34]. Systemic bacterial dissemination of periodontal pathogens has been evidenced in blood, liver, uteri and individual placentas of growth-restricted fetuses [21–25,33]. This report provides evidence that the mixed oral infection model with C. rectus and P. gingivalis induces FGR and resorptions together with histological evidence of placental inflammation with areas of focal necrosis, enhanced TLR expression and impaired fecundity.
The oral infection model did not significantly correlate with FGR induction, conversely to our previous observations using the chamber model of infection [22]. This finding might be explained in part by the murine immune system ability to fight the oral infection, decreasing bacterial systemic dissemination/exposure protecting the developing fetuses. Particularly, Baker et al. reported that Balb/C mice are able to produce high titers of P. gingivalis-specific IgG using the same model of oral infection [35] and we have previously reported that fetal and placental growth may be unaffected in heat killed-P. gingivalis pre-immunized rabbits [36]. In addition, the presence of C. rectus or P. gingivalis DNA was not detected in placentas coming from the oral infection model (data not shown). Nonetheless, overall infected groups had relatively less number of live fetuses, more resorptions and more growth restriction on average (Table 2 and Figure 2). Moreover, fecundity was significantly affected in infected groups (Figure 3). Only 16.7% of mice infected with C. rectus and P. gingivalis were able to get pregnant and had smaller litter sizes, situation that was not totally unanticipated as our previous observations on P. gingivalis infected animals suggested an impairment of fecundity ([37], unpublished observations). Therefore, we hypothesize that the difference in fetal restriction outcomes may be related to the mucosal immune system clearance at the portal of entry and the consequent low bacterial exposure at the placental level.
Even so, we found a placental inflammatory phenotype along with an enhanced TLR4 expression in murine placentas which is suggestive of an active inflammatory response to bacterial exposure. Histologically, placentas from the infected groups showed apparent chronic inflammation (Figure 4A), and the double-staining immunofluorescence analysis showed that TLR4 expression was notably confined to trophoblasts in the labyrinth layer (Figures 4B–F). Furthermore, placental TLR4 mRNA levels showed to be significantly increased almost two-fold in the C. rectus-infected group and mildly increased (30%) in the double infection group approaching statistical significance (Figure 5, P=0.06). This finding might be explained by the limited number of placentas (n=12) available for analysis since only 17% (2 out of 12) mice got pregnant in the combined infection group.
TLRs are highly involved in responding to inflammatory processes in the presence or absence of infection in several tissues. In particular, TLRs are thought to be critical players of the innate immune response during pregnancy, which have significant implications for the success or failure in both early and late gestation [38]. Toll-like receptors expression has been described in the human placenta, mostly at the dominant cell type: the trophoblast [39–41]. Trophoblasts are the first cells to differentiate from the outer layer of the blastocyst and are believed to participate during endometrial implantation and placental development [42]. Trophoblasts have also been proposed to coordinate the immune response during both processes by regulating regulate immune cells migration (macrophages and NK cells) to the endometrial implantation site through TLRs activation and chemokines production [43–45]. Furthermore, abnormalities in decidual TLRs expression or function have been linked to abnormal placentation, inflammation, and adverse pregnancy outcomes [46]. Interestingly, our data suggest that murine placentas develop pro-inflammatory features after oral infection with periodontal pathogens, where placental trophoblasts notably express more TLR4 as compared to placentas from non-infected mice (Figures 4C–H), and these observations were also consistent with the placental mRNA levels (Figure 5). In fact, TLR4 receptors have been shown to mediate the murine placental inflammatory response and fetal death to F. nucleatum, and mice deficient for TLR-4 show protection against bacterial and LPS-induced preterm birth [47]. Moreover, the selective antagonism of TLR-4 inhibits inflammation and preterm uterine contractility in a nonhuman LPS infection model in Rhesus monkeys [48].
The scope of this report was limited to determine TLR4 expression in response to oral Gram negative bacteria as proof of principle; however it is possible that other type of TLRs participate during the placental immune responses. For example, C. rectus offers a wide array of virulence factors including surface layer (S-layer) proteins [49], cytolethal distending toxin (CDT) [50], GroEL-like proteins [51] and lipopolysaccharide (LPS)[52]. In addition, P. gingivalis also possess a plethora of virulence factors including capsule, fimbriae, proteases (Gingipains) and LPS [53]. Therefore, either C. rectus or P. gingivalis might be able to induce placental inflammation via different virulence factors that could potentially be sensed by different TLRs including TLR-2 (peptidoglycans or fimbriae), TLR4 (LPS) or TLR-5 (flagellin).
In conclusion, previous models used to mimic chronic infections in mice result in dissemination of live oral bacteria to the placental tissues and impair fetal growth. However, this mixed oral infection model combines two commensal and critical pathogens that in combination are strongly associated with periodontal disease in humans [54]. Therefore, it is plausible to speculate that chronic systemic exposure to oral bacteria and related virulence factors may affect early pregnancy via pro-inflammatory mechanisms and such hypotheses warrant further investigations [55]. Further research is also needed to characterize the placental TLR response to oral pathogens in humans to elucidate and validate pathogenic mechanisms.
Acknowledgments
We thank Dr. Robert Bagnell and Victoria J. Madden for imaging assistance and Dr. David Barrow for management support. This work was supported by the National Institutes of Health grant 5U01DE014577.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Report of a WHO Expert Committee. The prevention of perinatal mortality and morbidity. World Health Organ Tech Rep Ser. 1970;457:1–60. [PubMed] [Google Scholar]
- 2.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985 Jan 10;312(2):82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006 Dec;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994 Sep 30;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 5.Shoji T, Yoshida S, Mitsunari M, Miyake N, Tsukihara S, Iwabe T, Harada T, Terakawa N. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro- and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol. 2007 Oct;75(2):82–90. doi: 10.1016/j.jri.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997 Mar;11(1):135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 7.Hill GB. Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol. 1998 Jul;3(1):222–32. doi: 10.1902/annals.1998.3.1.222. [DOI] [PubMed] [Google Scholar]
- 8.Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 9.Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006 Jan;107(1):29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 10.Contreras A, Herrera JA, Soto JE, Arce RM, Jaramillo A, Botero JE. Periodontitis Is Associated With Preeclampsia in Pregnant Women. J Periodontol. 2006 Feb;77(2):182–8. doi: 10.1902/jop.2006.050020. [DOI] [PubMed] [Google Scholar]
- 11.Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32 Suppl 6:57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 12.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006 Jun;33(6):401–7. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 13.Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007 Apr;78(4):670–6. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- 14.Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, Martinez M, Gamonal J. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007 Jul;78(7):1249–55. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 15.Chaim W, Mazor M. Intraamniotic infection with fusobacteria. Arch Gynecol Obstet. 1992;251(1):1–7. doi: 10.1007/BF02718272. [DOI] [PubMed] [Google Scholar]
- 16.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004 Aug;21(6):319–23. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 17.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL, Jr, Beck JD, Offenbacher S. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001 Dec;6(1):175–82. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- 18.Rams TE, Feik D, Slots J. Campylobacter rectus in human periodontitis. Oral Microbiol Immunol. 1993 Aug;8(4):230–5. doi: 10.1111/j.1399-302x.1993.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara N, Takakura S, Saito T, Iinuma Y, Ichiyama S. A case of perinatal sepsis by Campylobacter fetus subsp. fetus infection successfully treated with carbapenem--case report and literature review. J Infect. 2006 Nov;53(5):e199–e202. doi: 10.1016/j.jinf.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan AM, Dore CJ, Coid CR. Campylobacters and impaired fetal development in mice. J Med Microbiol. 1988 Jan;25(1):7–12. doi: 10.1099/00222615-25-1-7. [DOI] [PubMed] [Google Scholar]
- 21.Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal Campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J Periodontol. 2005 Nov;76(11 Suppl):2133–43. doi: 10.1902/jop.2005.76.11-S.2133. [DOI] [PubMed] [Google Scholar]
- 22.Yeo A, Smith MA, Lin D, Riche EL, Moore A, Elter J, Offenbacher S. Campylobacter rectus mediates growth restriction in pregnant mice. J Periodontol. 2005 Apr;76(4):551–7. doi: 10.1902/jop.2005.76.4.551. [DOI] [PubMed] [Google Scholar]
- 23.Bobetsis YA, Barros SP, Lin DM, Weidman JR, Dolinoy DC, Jirtle RL, Boggess KA, Beck JD, Offenbacher S. Bacterial infection promotes DNA hypermethylation. J Dent Res. 2007 Feb;86(2):169–74. doi: 10.1177/154405910708600212. [DOI] [PubMed] [Google Scholar]
- 24.Lin D, Smith MA, Elter J, Champagne C, Downey CL, Beck J, Offenbacher S. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 2003 Sep;71(9):5163–8. doi: 10.1128/IAI.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin D, Smith MA, Champagne C, Elter J, Beck J, Offenbacher S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect Immun. 2003 Sep;71(9):5156–62. doi: 10.1128/IAI.71.9.5156-5162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006 Sep;84(9):712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 27.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007 Nov;114(11):1326–34. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 28.Brikos C, O’Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol. 2008;(183):21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 29.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006 Aug;84(4):333–41. doi: 10.1111/j.1440-1711.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 30.Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, Papapanou PN, Schmidt AM. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003 Aug 1;23(8):1405–11. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 31.Frank HG, Genbacev O, Blaschitz A, Chen CP, Clarson L, Evain-Brion D, Gardner L, Malek A, Morrish D, Loke YW, Tarrade A. Cell culture models of human trophoblast--primary culture of trophoblast--a workshop report. Placenta. 2000 Mar;21 Suppl A:S120–S122. doi: 10.1053/plac.1999.0528. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005 Apr;12(3):145–55. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005 Feb;192(2):554–7. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004 Apr;72(4):2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994 Dec;39(12):1035–40. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 36.Boggess KA, Price WA, Preisser JS, Moise KJ, Jr, Offenbacher S. Insulin-like growth factor and interleukin-1beta levels and subsequent fetal size in response to chronic Porphyromonas gingivalis exposure in the pregnant rabbit. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 2):1219–23. doi: 10.1016/j.ajog.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Collins JG, Windley HW, III, Arnold RR, Offenbacher S. Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect Immun. 1994 Oct;62(10):4356–61. doi: 10.1128/iai.62.10.4356-4361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez JM, Xu H, Ofori E, Elovitz MA. Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am J Obstet Gynecol. 2007 Sep;197(3):296. doi: 10.1016/j.ajog.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005 Aug;26(7):540–7. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002 Sep;107(1):145–51. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum Pathol. 2004 Jan;35(1):47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64(3):126–30. doi: 10.1159/000101734. [DOI] [PubMed] [Google Scholar]
- 43.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005 Dec 15;175(12):8096–104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 44.Mor G. Inflammation and Pregnancy: The Role of Toll-like Receptors in Trophoblast-Immune Interaction. Ann N Y Acad Sci. 2008 Apr;1127:121–8. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 45.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25(5):375–88. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 46.Krikun G, Lockwood CJ, Abrahams VM, Mor G, Paidas M, Guller S. Expression of Toll-like receptors in the human decidua. Histol Histopathol. 2007 Aug;22(8):847–54. doi: 10.14670/HH-22.847. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007 Aug 15;179(4):2501–8. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 48.ms Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008 Feb;15(2):121–7. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson SA. Campylobacter surface-layers (S-layers) and immune evasion. Ann Periodontol. 2002 Dec;7(1):43–53. doi: 10.1902/annals.2002.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun M, Kuhnert P, Nicolet J, Burnens AP, Frey J. Cloning and characterization of two bistructural S-layer-RTX proteins from Campylobacter rectus. J Bacteriol. 1999 Apr;181(8):2501–6. doi: 10.1128/jb.181.8.2501-2506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinode D, Yoshioka M, Tanabe S, Miki O, Masuda K, Nakamura R. The GroEL-like protein from Campylobacter rectus: immunological characterization and interleukin-6 and -8 induction in human gingival fibroblast. FEMS Microbiol Lett. 1998 Oct 1;167(1):1–6. doi: 10.1111/j.1574-6968.1998.tb13199.x. [DOI] [PubMed] [Google Scholar]
- 52.Ogura N, Matsuda U, Tanaka F, Shibata Y, Takiguchi H, Abiko Y. In vitro senescence enhances IL-6 production in human gingival fibroblasts induced by lipopolysaccharide from Campylobacter rectus. Mech Ageing Dev. 1996 May 24;87(1):47–59. doi: 10.1016/0047-6374(96)01701-0. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien-Simpson NM, Veith PD, Dashper SG, Reynolds EC. Antigens of bacteria associated with periodontitis. Periodontol 2000. 2004;35:101–34. doi: 10.1111/j.0906-6713.2004.003559.x. [DOI] [PubMed] [Google Scholar]
- 54.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007 Oct;78(10):1911–25. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 55.Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003 Feb;101(2):227–31. doi: 10.1016/s0029-7844(02)02314-1. [DOI] [PubMed] [Google Scholar]