Abstract
The advent of multicellular organisms, some 800 million years ago, necessitated the development of mechanisms for cell-to-cell synchronization and for the spread of signals across increasingly large cell populations [168, 185]. Many structures and mechanisms have evolved to achieve such functions [4, 15]. Among these mechanisms, one which is prominent in both the invertebrate and the vertebrate world, across the entire phylogenetic scale, involves the transmembrane flux of large cytosolic and extracellular molecules [4, 15, 65, 66, 69–71, 121, 128, 129, 147, 154, 163]. These fluxes, in turn, are dependent on the formation of specific channels that in all animal classes are made by tetra-span integral membrane proteins [65, 66, 69–71, 121, 128, 129, 147, 154, 163] (Fig. 1).
Keywords: Gap junctions, Connexons, Pannexons, Innexons, Membrane channels, Ca2+, ATP, Glutamate
Three junctional protein families form membrane channels permeable to large molecules
Early electrophysiological and electron microscopy studies converged in the realization that gap junctions, the membrane domains that concentrate intramembrane particles at sites of close membrane apposition, were the physical substrate of cell-to-cell communication in both invertebrate [59] and vertebrate tissues [139] (Fig. 1). The finding that similar drugs (the long-chain alcohols heptanol and octanol) and conditions (intracellular acidification) inhibited intercellular communication in both invertebrate and vertebrate systems [58, 85] was taken as further support that all gap junctions had a similar structure and function. Still, the different size of intramembrane particles, their different partition into the P- and the E-fracture faces of the cell membrane, and the different width of the gap space delineated by the two interacting membranes, suggested that the proteins making vertebrate gap junctions were different from those making the invertebrate structures [96]. These differences have functional consequences, as most elegantly demonstrated by co-culturing cell lines from different animal species. In these experiments, heterotypic coupling was shown between insect cells, as well as between different types of vertebrate cells, whereas virtually no coupling was observed between cells of phylogenetically distant species [48].
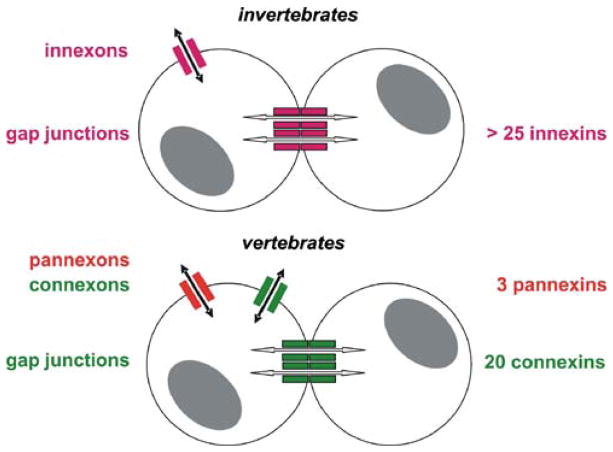
Fig. 1.
Innexins, pannexins, and connexins form different types of gap junctional and “hemi-channels.” Invertebrates express many different innexins (purple; 25 isoforms in C. elegans) that may form either gap junction channels for cell-to-cell coupling (open arrows) or innexon “hemi-channels” for the permeability of the nonjunctional membrane (solid arrows). Vertebrates express three different isoforms of pannexins (red) that form pannexon “hemi-channels” and 20 different connexin isoforms (green) that essentially form gap junction channels. A few connexin isoforms may also form connexon “hemi-channels”
There is no longer any question that vertebrate gap junction channels are made by various combinations of different connexin proteins [66, 69–71]. The 20–21 isoforms of this family in rodents and man differ in size, but share a similar membrane topography. Thus, all connexins feature four transmembrane domains connected by two extracellular loops, each comprising three highly conserved Cys residues, a cytoplasmic loop, and both N and C termini in the cytosol (Fig. 2). The difference in size of the different connexin isoforms is essentially due to a different length of the cytoplasmic loop and/or the C terminus [39, 64, 65, 69–71, 109]. Current nomenclature (Table 1) designates connexin proteins as nCxZ, where n is the species (e.g., h, m, r for human, mouse, rat, respectively), and Z is the predicted molecular weight, in kDa. The genes encoding connexin proteins are named according to subgroups, in the order of discovery (e.g., Gjb1 encodes Cx32, Gjb2 encodes Cx26, Gja1 encodes Cx43, etc).
Fig. 2.
Membrane topography of the proteins forming gap junctions and “hemi-channels.” All invertebrate innexins, vertebrate ortholog pannexins, and nonhomologous vertebrate connexins feature four transmembrane domains connected by two extracellular loops and one cytoplasmic loop, and have both N and C termini in the cytosol. Connexins possess 3 Cys residues (solid circles) in each of their extracellular loops. Pannexins and innexins feature only two such Cys residues per loop. Contrasting with both connexins and innexins, pannexin proteins are glycosylated (tree-like structure) on one of their extracellular loop. The gray areas schematize the two lipid layers
Table 1.
The family of human connexins
| Gene | Connexin | Chromosome | Accession number |
|---|---|---|---|
| GJA1 | Alpha 1, Cx43 | 6q22–q23 | BC026329 |
| GJA1P1 | Alpha 1 pseudogene | 5q21.3 | M65189 |
| GJA3 | Alpha3, Cx46 | 13q12.11 | AF075290 |
| GJA4 | Alpha 4, Cx37 | 1p35.1 | M96789 |
| GJA5 | Alpha 5, Cx40 | 6q21.1 | 121014 |
| GJA6P | Alpha 6 pseudogene | Xp22 | 100126825 |
| GJA8 | Alpha 8, Cx50 | 1q21.1 | U34802 |
| GJAp | Alpha 9, Cx59 | 1p34 | AF179597 |
| GJA10 | Alpha 10, Cx62 | 6q15–q16 | AF296766 |
| GJB1 | Beta 1, Cx32 | Xq13.1 | X04325 |
| GJB2 | Beta 2, Cx26 | 13q11–q12 | M86849 |
| GJB3 | Beta 3, Cx31 | 1p34 | BC012918 |
| GJB4 | Beta 4, Cx30.3 | 1p35–34 | 127534 |
| GJB5 | Beta 5, Cx31.1 | 1p34.3 | BC004379 |
| GJB6 | Beta 6, Cx30 | 13q12 | AJ005585 |
| GJB/ | Beta 7, Cx25 | 6q15 | AJ414563 |
| GJC1 | Gamma 1, Cx45 | 17q21.31 | U03493 |
| GJC2 | Gamma 2, Cx47 | 1q41–q42 | AF011643 |
| GJC3 | Gamma 3, Cx30.2 | 7q22.1 | AF503615 |
| GJD2 | Delta 2, Cx36 | 15q13.1 | AB037509 |
| GJD3 | Delta 3, Cx31.9 | 17q21.1 | AF514298 |
| GJD4 | Delta 4, Cx40.1 | 10p11.22 | AJ414564 |
| GJE1 | Epsilon 1, Cx23 | 6q24.1 | 349149 |
Attempts to identify the proteins making invertebrate gap junctions initially revealed the proteins Ogre, Passover, Uncoordinated, and Shaking B in Drosophila and Caenorhabditis (hence the original OPUS acronym to name these proteins) with no primary sequence homology to connexins [128, 129, 195]. Since that time, more than 25 other junctional proteins revealing significant similarities have been identified in C. elegans, and it is clear that many other forms are expressed in other invertebrate species [75, 128, 129, 195] (Table 2). Collectively, these proteins have been named innexins to stress their invertebrate distribution and their role, analogous to that of connexins, in the formation of gap junctions (Fig. 1). Strikingly, innexins also share with connexins a similar structure and membrane topography (Fig. 2), even though the two sets of proteins have no homology in their primary amino acid sequence [75, 128, 129, 195]. Notably, connexins display three conserved Cys within each of the two extracellular loops, whereas innexins only carry two such residues. Thus, it is curious in retrospect that hydra development was reported to be blocked by an antibody prepared against Cx32 [53], that other antibodies have detected connexin-like proteins in anemone [112] and other marine invertebrates [5], and that junctional proteins isolated from hepatopancreas of crayfish and lobster were reported to have a sequence similar to that of rat liver connexins [51].
Table 2.
A present view of the growing family of invertebrate innexins
| Gene | Innexin | Species | Accession number |
|---|---|---|---|
| LOC100168767 | Innexin | Acyrthosiphon pisum | 100168767 |
| LOC100168642 | Innexin | 100168642 | |
| LOC100162039 | Innexin | 100162039 | |
| LOC100167822 | Innexin | 100167822 | |
| LOC100163262 | Innexin-1 | 100163262 | |
| LOC100163012 | Innexin-1 | 100163012 | |
| LOC100163664 | Innexin 3 | 100163664 | |
| LOC100165879 | Innexin 3 | 100165879 | |
| AaeL_AAEL014847 | Innexin | Aedes aegypti | 5565409 |
| AaeL_AAEL014846 | Innexin | 5565408 | |
| AaeL_AAEL014227 | Innexin | 5579528 | |
| AaeL_AAEL011248 | Innexin | 5574614 | |
| AaeL_AAEL008588 | Innexin | 5570797 | |
| AaeL_AAEL006726 | Innexin | 5568297 | |
| AgaP_AGAP001476 | AGAP001476-PA | Anopheles gambiae | 1281698 |
| AgaP_AGAP001477 | AGAP001477-PA | 1281697 | |
| AgaP_AGAP001487 | AGAP001487-PA | 4577242 | |
| AgaP_AGAP001489 | AGAP001489-PA | 1281682 | |
| AgaP_AGAP004510 | AGAP004510-PA | 1274656 | |
| AgaP_AGAP006241 | AGAP006241-PA | 1276902 | |
| LOC725480 | Innexin-1 | Apis mellifera | 725480 |
| LOC724832 | Innexin-2 | 724832 | |
| LOC55165 | Innexin-3 | 551165 | |
| LOC552285 | Innexin-7 | 552285 | |
| LOC725553 | Innexin shaking B | 725553 | |
| LOC725513 | Innexin shaking B | 725513 | |
| LOC413472 | Innexin shaking B | 413472 | |
| LOC692687 | Innexin 2 | Bombyx mori | 692687 |
| Inx2 | Innexin 2 | 732853 | |
| LOC692656 | b- iInnexin | 692656 | |
| Bm1_26040 | Brugia malayi | 6100106 | |
| Bm1_40790 | Innexin, protein1, a | 6103042 | |
| Bm1_48735 | Innexin, protein1, a | 6104632 | |
| Bm1_40470 | Innexin unc-7 | 6102078 | |
| Bm1_34995 | Innexin unc-7 | 6101888 | |
| Bm1_51260 | Innexin family protein | 6105137 | |
| Bm1_48195 | Innexin family protein | 6104524 | |
| Bm1_33440 | Innexin family protein | 6101576 | |
| Bm1_32310 | Innexin family protein | 6101352 | |
| Bm1_31695 | Innexin family protein | 6101231 | |
| Bm1_19285 | Innexin family protein | 6098762 | |
| Bm1_05505 | Innexin family protein | 6096031 | |
| Bm1_35075 | Innexin, inx-3 | 6101904 | |
| Bm1_48730 | Innexin, inx-5 | 6104631 | |
| Bm1_05870 | Innexin, inx-7 | 6096103 | |
| Bm1_51095 | Innexin inx-10 | 6105104 | |
| Bm1_25215 | Innexin, inx-11 | 6099945 | |
| Bm1_21210 | Innexin inx-14 | 6099146 | |
| CBG14811 | CBG14811 | Caenorhabditis briggsae AF16 | 5640339 |
| CBG14794 | CBG14794 | 56403232 | |
| CBG06096 | CBG06096 | 5632895 | |
| CBG05256 | CBG05256 | 5641195 | |
| CBG22739 | GBC22739 | 5641111 | |
| CBG23507 | CBG23507 | 5636295 | |
| CBG08617 | CBG08617 | 5636183 | |
| CBG10939 | CBG10939 | 5640527 | |
| CBG19227 | CBG19227 | 5637409 | |
| CBG03987 | CBG03987 | 5627861 | |
| CBG17376 | CBG17376 | 5639502 | |
| CBG09460 | CBG09460 | 5636929 | |
| Inx-1 | INneXin | Caenorhabditis elegans | 180968 |
| Inx-3 | INneXin | 180866 | |
| Inx-4 | INneXin | 184979 | |
| Inx-5 | INneXin | 181086 | |
| Inx-6 | INneXin | 178231 | |
| Inx-7 | INneXin | 177364 | |
| Inx-8 | INneXin | 178101 | |
| Inx-9 | INneXin | 191694 | |
| Inx-10 | INneXin | 188580 | |
| Inx-11 | INneXin | 179710 | |
| Inx-12 | INneXin | 171944 | |
| Inx-13 | INneXin | 171943 | |
| Inx-14 | INneXin | 172488 | |
| Inx-15 | INneXin | 172004 | |
| Inx-16 | INneXin | 172005 | |
| Inx-17 | INneXin | 172006 | |
| Inx-18 | INneXin | 182801 | |
| Inx-19 | INneXin | 171805 | |
| Inx-20 | INneXin | 188818 | |
| Inx-21 | INneXin | 171930 | |
| Inx-22 | INneXin | 171929 | |
| Eat-5 | EATing | 172480 | |
| Unc-7 | UNCoordinated | 181608 | |
| Unc-9 | UNCoordinated | 181443 | |
| CsIV_sQgp2 | Innexin-like protein 1 | Campoletis sonorensis ichnovirus ichnovirus | 5075634 |
| CsIV_sQgp1 | Innexin-like protein 2 | 5075635 | |
| CpipJ-CPIJ018867 | Innexin | Culex quinquefasciatus | 6052895 |
| CpipJ-CPIJ000259 | Innexin | 6030978 | |
| CpipJ-CPIJ014809 | Innexin inx2 | 6047913 | |
| CpipJ-CPIJ012807 | Innexin inx3 | 6045586 | |
| CpipJ-CPIJ014745 | Innexin inx3 | 6048414 | |
| Dana\GF10895 | GF10895 | Drosophila ananassae | 6493762 |
| Dana\GF15974 | GF15974 | 6498775 | |
| Dana\GF15976 | GF15976 | 6498707 | |
| Dana\GF16006 | GF16006 | 6498807 | |
| Dana\GF17986 | GF16986 | 6500765 | |
| Dana\GF20329 | GF20329 | 6503039 | |
| Dana\GF20330 | GF20330 | 6503040 | |
| Dana\GF20357 | GF20357 | 6503065 | |
| Dana\GF20443 | GF20443 | 6503150 | |
| Dere\GG12050 | GG12050 | Drosophila erecta | 655099 |
| Dere\GG14053 | CG14053 | 654448 | |
| Dere\GG17574 | GG17574 | 6549828 | |
| Dere\GG17644 | GG17644 | 6551473 | |
| Dere\GG17645 | GG17645 | 6551556 | |
| Dere\GG18003 | CG18003 | 6550369 | |
| Dere\GG18075 | GG18075 | 6550410 | |
| Dere\GG19626 | GG19626 | 6551474 | |
| Dgrij\GH12627 | GH12627 | Drosophila grimshawi | 6565092 |
| Dgrij\GH16270 | GH16270 | 6557007 | |
| Dgri\GH17771 | GH17771 | 6569440 | |
| Dgrij\GH19423 | GH19423 | 6563397 | |
| Dgrij\GH24223 | GH24223 | 6565731 | |
| Dgrij\GH24273 | GH24273 | 6565708 | |
| Dgrij\GH24274 | GH24274 | 6565706 | |
| Dgrij\GH24686 | GH24686 | 6565709 | |
| Inx2 | Innexin 2 | Drosophila melanogaster | 31646 |
| Inx3 | Innexin 3 | 44266 | |
| Inx5 | Inx5 | 32901 | |
| Inx65 | Inx6 | 31645 | |
| Inx7 | Innexin 7 | 33027 | |
| Ogre | Optic ganglion reduced | 45382 | |
| Shak B | CG34358 | 33062 | |
| Zpg | Zero population growth | 251414 | |
| Dmoj\GI11038 | GI11038 | Drosophila mojavensis | 6585922 |
| Dmoj\GI13206 | GI13206 | 6582220 | |
| Dmoj\GI15107 | GI15107 | 6584021 | |
| Dmoj\GI15723 | GI15723 | 6584424 | |
| Dmoj\GI21580 | GI21580 | 6585028 | |
| Dmoj\GI21582 | GI21582 | 6585026 | |
| Dmoj\GI21669 | GI21669 | 6585029 | |
| Dmoj\GI24278 | GI24278 | 6572903 | |
| Dper\GL12912 | GL12912 | Drosophila persimilis | 6597239 |
| Dper\GL13242 | GL13242 | 6601691 | |
| Dper\GL14652 | GL14652 | 6597493 | |
| Dper\GL15919 | GL15919 | 6599427 | |
| Dper\GL15920 | GL15920 | 6599428 | |
| Dper\GL23353 | GL23353 | 6587198 | |
| Dper\GL26810 | GL26810 | 6599899 | |
| Dper\GL26811 | GL26811 | 6599892 | |
| Dper\GL26820 | GL26820 | 6599900 | |
| Dpse\GA10092 | GA10092 | Drosophila pseudoobscura | 4812514 |
| Dpse\GA13015 | GA13015 | 4801698 | |
| Dpse\GA14306 | GA14306 | 4814446 | |
| Dpse\GA15552 | GA15552 | 4815318 | |
| Dpse\GA18281 | GA18281 | 4814473 | |
| Dpse\GA20423 | GA20423 | 4815170 | |
| Dsec\GM12279 | GN12279 | Drosophila sechellia | 6612617 |
| Dsec\GM13833 | GM13833 | 6611035 | |
| Dsec\GM17485 | GM17485 | 6618950 | |
| Dsec\GM17487 | GM17487 | 6618948 | |
| Dsec\GM17498 | GM17498 | 6618951 | |
| Dsec\GM22657 | GM22675 | 6615159 | |
| Dsec\GM22683 | GM22683 | 6615104 | |
| Dsec\GM22765 | GM22765 | 6614954 | |
| Dsim\GDE13120 | GD13120 | Drosophila simulans | 6737031 |
| Dsim\GD15600 | GD15600 | 6726412 | |
| Dsim\GD16170 | GD16170 | 6725330 | |
| Dsim\Gd16171 | Gd16171 | 6725328 | |
| Dsim\GD16826 | GD16826 | 6725331 | |
| Dsim\GD17854 | GD17854 | 6740252 | |
| Dsim\GD18012 | GD18012 | 6729965 | |
| Dsim\GD24447 | GD24447 | 6740253 | |
| Dsim\GD24658 | GD24658 | 6739865 | |
| Dvir\GJ11982 | GJ11982 | Drosophila virilis | 6623709 |
| Dvir\GJ15253 | GJ15253 | 6635459 | |
| Dvir\GJ15793 | GJ15793 | 6634501 | |
| Dvir\GJ15802 | GJ15802 | 6634801 | |
| Dvir\GJ15803 | GJ15803 | 6634803 | |
| Dvir\GJ15819 | GJ15819 | 6634459 | |
| Dvir\GJ18913 | GJ18913 | 6631960 | |
| Dvir\GJ23057 | GJ23057 | 6631216 | |
| Dwil\GK12587 | GL12912 | Drosophila willistoni | 6645049 |
| Dwil\GK13974 | GK13974 | 6650386 | |
| Dwil\GK20048 | GK20048 | 6641471 | |
| Dwil\GK20049 | GK20049 | 6641472 | |
| Dwil\GK25052 | GK25052 | 6648670 | |
| Dwil\GK25094 | GK25094 | 6648701 | |
| Dwil\GK25295 | GK25295 | 6648282 | |
| Dwil\GK25296 | GK25296 | 6648280 | |
| Dwil\GK25636 | GK25636 | 6648283 | |
| Dyak\GE10490 | GE10490 | Drosophila yakuba | 6538133 |
| Dyak\GE15333 | GE15333 | 6526113 | |
| Dyak\GE15360 | GE15360 | 6526063 | |
| Dyak\GE15472 | GE15472 | 6525881 | |
| Dyak\GE15694 | GE15694 | 6525472 | |
| Dyak\GE17551 | GE17751 | 6525473 | |
| Dyak\GE17553 | GE17753 | 6525476 | |
| Dyak\GE20477 | GE20477 | 6533216 | |
| HfIV_sB5gp2 | Viral innexin-b5.1 | Hyoposoter fugitivus ichnovirus | 5076301 |
| HfIV_sB7gp2 | Innexin Vnx-b7 | 5141585 | |
| HfIV_sB17gp2 | Innexin Vnx-b17 | 5076289 | |
| HfIV_sB10gp1 | Viral innexin-bio.1 | 5076304 | |
| HfIV_sC3gp2 | Viral innexin-c3.1 | 5076321 | |
| HfIV_sC16gp1 | Innexin Vnx-c16 | 5076283 | |
| HfIV_sD5gp1 | Innexin Vnx-d5.1 | 5076286 | |
| HfIV_sD5gp1 | Innexin Vnx-d5.1 | 5076286 | |
| HfIV_sD5gp2 | Innexin Vnx-d5.2 | 5076285 | |
| HfIV_sG1gp1 | Viral innexin-g1.1 | 5076399 | |
| HfIV_sG1gp3 | Viral innexin-g1.2 | 5076401 | |
| HfIV_sG1gp4 | Viral innexin-g1.3 | 5076402 | |
| LOC100118632 | Innexin | Nasonia vitripennis | 100118632 |
| NEMVEDRAFT_vig218755 | Nematostella vectensis | 5502737 | |
| LOC656737 | Innexin | Tribolium castaneum | 656737 |
| LOC657243 | Innexin | 657243 | |
| LOC657166 | Innexin | 657166 | |
| LOC661739 | b-innexin | 661739 | |
| LOC656913 | Innexin-1 | 656913 | |
| LOC657081 | Innexin-7 | 657081 | |
| LOC657000 | Innexin-7 | 657000 | |
| Hme1 | Innexin | Hirudo medicinalis | 25264684 |
| Hme2 | Innexin | 2526687 | |
| Hme3 | Innexin | 77997501 | |
| Hme4 | Innexin | 77997503 | |
| Hme5 | Innexin | 77997505 | |
| Hme6 | Innexin | 77997507 | |
| Hme7 | Innexin | 77997509 | |
| Hme8 | Innexin | 77997511 | |
| Hme9 | Innexin | 77997513 | |
| Hme10 | Innexin | 77997515 | |
| Hme11 | Innexin | 77997517 | |
| Hme12 | Innexin | 77997519 | |
| Hvu1 | Innexin | Hydra vulgaris | 86769609 |
| Hvi2 | Innexin | Heliothis virescens | 48926842 |
| Sam1 | Innexin | Shistocerca americana | 10720059 |
| Sam | Innexin | 10720060 | |
| Sfr1 | Innexin | Spodoptera frugiperda | 37781375 |
| Tci3 | Innexin | Toxoptera citricida | 52630963 |
| Aca1 | Innexin | Aplysia californica | 54398898 |
| Aca2 | Innexin | 54398902 | |
| Aca3 | Innexin | 54398904 | |
| Aca4 | Innexin | 54398900 | |
| Aca5 | Innexin | 60550112 | |
| Aca6 | Innexin | 60550114 | |
| Aca7 | Innexin | 89118243 | |
| Aca8 | Innexin | 60550118 | |
| Cli1 | Innexin | Clione limacina | 8515128 |
| Cli2 | Innexin | 14210377 | |
| Cli3 | Innexin | 14210379 | |
| Cva1 | Innexin | Chaeptorus varipedatus | 15706257 |
| Dja1 | Innexin | Dugesia japonica | 86355173 |
| Dja2 | Innexin | 86355153 | |
| Dja3 | Innexin | 86355155 | |
| Dja4 | Innexin | 86355157 | |
| Dja5 | Innexin | 86355159 | |
| Dja6 | Innexin | 86355175 | |
| Dja7 | Innexin | 86355161 | |
| Dja8 | Innexin | 86355163 | |
| Dja9 | Innexin | 86355165 | |
| Dja10 | Innexin | 86355167 | |
| Dja11 | Innexin | 86355169 |
Sequencing of mammalian genomes has revealed a third family comprising only three genes that code for proteins with a primary sequence showing about 20% similarity to that of innexins [121, 154, 195]. On this basis, these proteins were thought to represent vertebrate homologs of the innexins, and were termed pannexins (Table 3) to encompass both invertebrate and vertebrate members [121, 154]. Like connexins and innexins, all three pannexins display N- and C-terminal domains within the cytoplasm, large extracellular and cytoplasmic loop domains, and four membrane spanning segments (Fig. 2). Like innexins but in contrast to connexins, pannexins contain two Cys residues in each extracellular loop [121, 154]. Furthermore, and in marked contrast with both innexins and connexins, pannexins display consensus sequences for glycosylation [13, 14, 125] (Fig. 2). The distribution of Pnx1 (Pnx1), the most studied form, is widespread and, in most types of cells and tissues, largely overlaps with that of connexins [17, 8]. While at least some phenotypes resulting from loss of specific connexin species are not compensated by pannexin changes [136, 146], suggesting a different function of the two protein families, in other cases, the effects of Pnx1 transfection mimicked that of Cx43, implicating a comparable/overlapping role of these two proteins [93].
Table 3.
The family of human pannexins
| Gene | Pannexin | Chromosome | Accession number |
|---|---|---|---|
| PNX1 | Pnx1 | 11q21 | 24145 |
| PNX2 | Pnx2 | 22q13–33 | 56666 |
| PNX3 | Pnx3 | 11q24.2 | 116337 |
Connexins and innexins form cell-to-cell channels at gap junctional regions of the cell membrane
Expression and deletion studies in a variety of systems have established that connexin hexamers, termed connexons, concentrate at gap junction domains of the cell membrane, where the intercellular space is reduced to a gap 2–3 nm wide. At these sites, the connexons of one cell align with, and strongly bind to the connexons of an adjacent cell, establishing a continuous intercellular hydrophilic pathway (Fig. 1) for the cell-to-cell exchange of multiple types of cytosolic molecules [64, 65, 69–71, 155]. The functional importance of this electrical and metabolic coupling is shown by a variety of striking and tissue-specific phenotypes that can be experimentally induced after overexpression or knock-out of individual connexin isoforms, as well as after the knock-in replacement of one isoform by another [92, 192]. It is further stressed by the identification of a number of diseases that are undoubtedly linked to connexin mutations [43, 57, 90, 94, 127, 143]. A variety of other diseases are thought to be due to altered amounts and/or function of these gap junction proteins [21, 108, 153].
Similarly, innexins oligomerize to form innexons that cluster at gap junctions of invertebrate cells (Fig. 1). Functional expression studies in paired Xenopus oocytes demonstrated that several, even though not all innexins also formed intercellular channels [8, 95, 166], and that at least some innexin mutations give rise to phenotypes expected for lack of gap junction-mediated intercellular communication [10, 25, 27, 37, 179]. In fact, it was on the basis of such dysfunctional phenotypes that the OPUS gene family was first identified.
In contrast, and in spite of an initial report [18], pannexons appear unable to form sizable amounts of cell-to-cell channels under most conditions [76, 146, 147, 163] (Fig. 1). This lack of formation of functional gap junctions is likely due to the glycosylation of the extracellular loops of pannexins [13, 14, 125], which, as mentioned above, is not observed for either connexins or innexins. Still, forced expression of pannexins in paired Xenopus oocytes increased the conductance of the junctional cell membrane to current carrying ions in a way that can only be accounted for by the formation of pannexin cell-to-cell channels [13].
Connexins, pannexins, and innexins can also form “hemi-channels” in nonjunctional regions of the cell membrane
The finding of nonjunctional permeability induced by expression of connexins, pannexins, and innexins, together with the biochemical, immunological, and functional studies showing that at least some connexon and innexon types may also inserted in nonjunctional regions of the cell membrane [42, 114, 123] challenged the classical views about junctional proteins, by indicating that, at least under certain conditions, some of these proteins may also form functional channels in domains of the cell membrane that are not involved in cell contact. Most strikingly, these channels were found to allow for the leakage of cytosolic molecules, notably ATP and glutamate, into the extracellular medium and to permit the reverse uptake into cells of large extracellular and membrane-impermeant tracers, notably propidium iodide and Lucifer Yellow, [61, 144, 147, 163, 171]. Given that this dye permeability is a major characteristic of connexin and innexin cell-to-cell channels, and that the bidirectional flux of large molecules across the membrane could be abolished by drugs known to block gap junction channels [73, 91, 98, 103, 134], it was proposed that the nonjunctional channels were half of a gap junction channel, made by a nonpaired connexon or innexon (Fig. 1). The subsequent identification of similar channels in cells (e.g., red blood cells) that do not express connexins or innexins but express pannexins [105], further indicated that these channels may also be made by unpaired pannexons (Fig. 1). For these reasons, these channels were referred to as hemichannels even though this is an obviously semantically improper term to indicate a fully functional and regulated structure [38, 147, 163]. Still, because the term has now gained usual acceptance, we refer to these structures, whether made by single connexons, innexons, or pannexons, as “hemi-channels”.
There is now undisputed evidence that Cx46 and Cx50 form such “hemi-channels” when expressed in Xenopus oocytes [165] and probably also in the lens [198], and it is widely believed that Cx43 can also form such structures in a variety of cell types [65, 99, 103, 144, 148, 163]. Other reports have proposed that this property is shared by several other connexin isoforms, including Cx23, Cx26, Cx30, Cx30.2, Cx 31.9,Cx32, Cx35, Cx36, Cx37, Cx41.8, Cx45, Cx45.6, Cx55.5, and Cx56 [20, 46, 60, 80, 118, 144, 151, 181, 182, 191]. However, for the reasons that shall be discussed below, it has proven difficult to unambiguously demonstrate the presence of functionally open and unpaired connexons made by these proteins [146, 163]. At least a few innexins [8, 27, 179] and one (Pnx1) of the three known pannexin proteins [18, 77, 105, 124, 180] also form functionally open innexons and pannexons, respectively (Fig. 1).
“Hemi-channels” formed of different proteins have both similar and distinct biophysical and regulatory characteristics
In keeping with their parallel evolution, similar structure, and membrane topography, most “hemi-channels” share a number of similar biophysical properties that are deemed diagnostic of a composition by one of the three aforementioned families of junctional properties. Thus, they generally feature a large unitary conductance (200–500 pS) [38, 65, 147, 163], although the unitary conductance of certain connexin “hemi-channels” is much lower (e.g., those attributed to either Cx31.9 or Cx36 are <20 pS) [20], a reversal potential near 0 mV when tested with standard internal and external solutions, indicating little selectivity for the major current carrying ions, and a sizable open probability at positive resting potentials (~20% at V≥40 mV) [38, 65, 147, 163]. Most “hemi-channels” show an in–out permeability to ATP and an out–in permeability to propidium iodide, Lucifer Yellow and 6 carboxyfluorescein [36, 65, 147, 163, 167]. Opening of all types of “hemi-channels” is induced by membrane depolarization (V≥40–60 mV). Connexin-based “hemi-channels” have been proposed to be activated by low divalent ion concentrations, which for Cx46 and Cx50 shift activation kinetics to lower voltages [164]. Such shift has not been demonstrated for other putative connexin “hemi-channels.” Cx43 “hemi-channels” have been reported to be opened under ischemic conditions [11, 33, 34, 84, 99, 135, 151], whereas Pnx1 “hemi-channels” have been demonstrated to be activated by a diverse set of experimental conditions, including mechanical stress, e.g., as provided by an osmotic shock [6, 7, 102, 134], strong depolarizations, and activation of purinergic receptors, including P2Y1r, P2Y2r, and P2X7r, by ATP and other agonists [26, 72, 87, 105, 122, 126, 173, 174, 189] (Fig. 3).
Fig. 3.
Conditions leading to the detection of functional “hemi-channels.” Functional connexon “hemi-channels” are revealed in the absence of extracellular [Ca2+] or [Mg2+], and upon supraphysiological depolarizations. Pannexon “hemi-channels” are activated in the presence of normal extracellular [Ca2+] and physiologically relevant membrane depolarizations. Many “hemi-channels” of different protein composition are activated by mechanical stress and membrane depolarization
Closure of a number of “hemi-channels” can be induced by several drugs, including carbenoxolone, α-glycyrrhetinic acid, flufenamic acid, mefloquine, and alkanols that also turn off most gap junction channels [14, 124, 169, 177]. The effective concentration of these drugs varies depending on the identity of the channel-forming protein, thereby possibly allowing for discrimination of which channel type is involved in ion or molecular flux. For example, Pnx1 channels appear to be 1,000- to 10,000-fold more sensitive to mefloquine than are Cx43 gap junction channels [36, 79] and less sensitive than connexons to uncoupling flufenamates [16]. Peptides corresponding to the extracellular loops of Pnx1 and Cx43 have also been reported to be effective in blocking both current flow and dye uptake through pannexons and connexons [73, 124, 190]. However, one of the peptides reported to most effectively block Cx32 “hemi-channels” corresponded to an intracellular epitope [40]. Furthermore, the specificity of these peptides has been challenged, as a result both of cross-inhibition of Pnx1 and Cx46 “hemi-channels” in Xenopus oocytes, and effective blockade by polyethylene glycols [133]. A more physiological regulation of connexons is seen under conditions of lowered intracellular pH [184, 197] or PKC and MAPK activation [8, 40, 49, 56], which are reported to close most connexin “hemi-channels”, and after exposure to either IL1β or TNFα, which is reported to open them [113, 137, 176]. The last finding is quite surprising, given that IL-1 β treatment has been shown in several studies to cause gap junctions to disappear [22, 44, 83, 111, 145]. Conversely, “hemi-channels” may be opened by FGF-1 [149]
The biophysical properties reported for connexin “hemi-channels” differ from those of the gap junction channels formed by the same connexin. Thus, while the unitary conductance of the main state of connexin “hemi-channels” is about twice that of the same connexons in gap junctions, and the substate conductance seen at high positive potentials is generally more than twice that of the corresponding gap junction channel. Voltage sensitivity is also different in “hemi-channels” and gap junction channels. In invertebrates, gap junctions tend to be sensitive to transmembrane (or “inside–outside”) potential, closing when either or both cells are depolarized [23, 157], whereas in mammals, gap junctions are closed by the potential across the gap junction (nonzero transjunctional voltage), each connexin having a specific sensitivity, as shown by Boltzmann parameters [65, 161]. Moreover, a fraction of the junctional conductance is usually preserved in gap junction channels even at the highest voltages. This minimal conductance ranges from virtually zero to almost unity for different connexins and represents occupancy of a channel substate. The predominant form of voltage dependence seen in pannexons and connexons “hemi-channels” is conspicuously different from the gating seen in either innexin or connexin gap junctions. Thus, “hemi-channels” open in response to membrane depolarization above about +40 mV, a gating which is attributed to the so-called “loop gate” that is exposed to the transmembrane field in unpaired connexons [65]. For Pnx1, current activation increases with voltage without attenuation, whereas for connexons, there is a decrease in current at the highest voltages, due to the gating to a substate caused by the transjunctional voltage sensor. The gating to substate and its absence in a C-terminally GFP-tagged Cx43, has provided the strongest evidence to date that Cx43 connexons can open in mammalian cells, albeit under nonphysiological conditions [32].
A major difference between pannexons and connexons is that pannexons have been shown to open under physiological conditions, while most connexons have not. Notably, whereas pannexin channels can be opened at normal resting potential and in normal extracellular Ca2+ solutions, by mechanical stretch and by P2 receptor stimulation, connexons have only been demonstrated to open under supra- or pathophysiological conditions (no extracellular Ca2+ or Mg2+, depolarization exceeding +40 mV). Although both pannexons and connexons have been reported to be affected by raising intracellular [Ca2+]i levels, the reality of this regulation is still debated [106].
The identity of the protein forming “hemi-channels” is disputed
Given that innexins, connexins, and pannexins can all form “hemi-channels” which share several common features, including permeability characteristics and sensitivity to blocking drugs, the question arises of which of these protein species actually establishes the functional membrane conductance and permeability. A definitive answer to this question is complicated by the fact that pannexins and connexins have largely overlapping distributions in vertebrates. Also, it is conceivable that, with evolution, different cell types have acquired different types of “hemi-channels” to fulfill a specific role in a specific environment, so that the situation in one cell system might not be applicable to another.
Data from the initial experiments were taken as an indication that “hemi-channels” were made by connexons, mostly because gap junction channels and connexon channels share a conductance for multiple ions, a permeability to large hydrophilic molecules and a sensitivity to the same set of drugs [56, 163, 188]. Furthermore, the conductance of “hemi-channels” was about twice as high as that of gap junction channels made by the same connexin, as would be anticipated if the two connexons joined in series in a gap junction were dissociated from each other. The most compelling evidence for Cx43 “hemi-channels” comes from HeLa cells overexpressing Cx43, in which currents were evoked by membrane depolarization above +20 mV, single channel conductance was about twice that of Cx43 gap junction channels, conductance was minimally affected by extracellular Ca2+ levels, but no channel activity was observed at membrane potentials ≤0 mV [32]. Moreover, the “hemi-channel” formed by Cx43-GFP featured no residual conductance, consistent with properties of gap junction channels formed by this construct, and a N-terminal GFP-Cx43 fusion protein was found not to form “hemi-channels,” again consistent with lack of formation of functional gap junction channels by this construct [32].
The major concern regarding the assignment of connexons as functional “hemi-channels” is that the lack of opening of connexons at negative resting potentials is not consistent with the dye uptake observed under such conditions in many cell types [38, 147, 163]. This finding, as well as a variety of expression studies, analysis of cells lacking connexins, and experiments conducted under conditions expected to close gap junction channels have clearly documented that proteins other than connexins can form “hemi-channels” in vertebrate cells [38, 104, 146, 163]. For example, in different cell types, the uptake of extracellular and membrane-impermeant tracers occurs under conditions (presence of extracellular Ca2+, limited depolarization) in which open connexon “hemi-channels” cannot be revealed by electrophysiology [32, 38, 146, 163]. These considerations raise the issue of whether most of the literature published on Cx43 “hemi-channels” is actually reporting other Ca2+-dependent phenomena, such as transport, vesicular uptake and release, or other pathways.
The demonstration that pannexins by themselves form “hemi-channels” was provided by expression of Pnx1 in single oocytes, which lead to the appearance of large currents activating at membrane potentials above −20 mV [16]. These currents were both voltage- and time-dependent, being larger and inactivating more rapidly at larger depolarization. The co-expression of Pnx1 and Pnx2 elicited currents that were larger than those induced by Pnx1 alone, whereas Pnx2 by itself did not yield currents. Because activation was delayed in the co-expression experiments, it was hypothesized that pannexins 1 and 2 also form heteromeric nonjunctional channels [16]. By contrast, Pnx3 neither formed channels itself, nor modified those formed by the other pannexins [16].
It is therefore likely that in a number of cases, the “hemi-channels” attributed to Cx43 were actually due to pannexin activation. One example involves the selection of the J774 macrophage cell line for resistance to ATP-induced cell killing by repeated exposure of the cells to 10 mM ATP. Resistant clones were found to be deficient in Cx43, from which it was inferred that the so-called P2Z receptor or permeabilization pore induced by high concentrations of ATP was the Cx43 “hemi-channel” [12]. This hypothesis was disproved by cloning of the P2X7 receptor [175], by studies showing that the permeabilization pore was present in macrophages lacking Cx43 [3], that J774 cells co-express Cx43 and P2X7 receptors [52], and by experiments in which Cx43 transfection did not induce the permeabilization pore in BHK cells [62]. The demonstration that Pnx1 is found in retinal horizontal cells similarly raises the possibility that the large conductance “hemi-channel” currents recorded from these cells might actually be carried through pannexin channels [45], rather than through connexin “hemi-channels” [42, 155], since the high conductance of pannexons would be expected to provide substantially more ephaptic current at the restricted horizontal-photoreceptor contact than would retinal connexons.
Two independent groups have recently identified Pnx1 as a part of a complex that provides membrane permeabilization to large molecules, following the activation of the P2X7 receptor by ATP or analogues of the endogenous nucleotide [105, 124, 196]. Thus, initial activation of the P2X7r selective cation channels allows for the permeation of molecules up to 900 Da [50, 116]. Two hypotheses have been proposed to account for this change of the ionotropic receptor. The first involves the dilation of the P2X7 cation channel itself [19, 28, 178], while the second involves the recruitment of a protein, most likely Pnx1, forming a lytic pore [81, 150, 175]. Interestingly, the resulting membrane permeabilization, which allows for a nonselective influx of large molecular weight probes [105, 124], does not correlate with the activation of caspase1, nor with the processing and release of IL-1β [124], even though blockade of Pnx1 “hemi-channels” with mimetic peptides prevents both the uptake of the tracer YoPro and caspase1 activation [124]. At any rate, the apparent coupling of Pnx1 activation to that of P2Z/P2X7 receptors, raises the issue of whether pannexin channels can be activated by other receptors. Indeed, the metabotropic P2Y1 and P2Y2 receptors can also activate Pnx1 opening [106]. In addition, uptake of the YoPro tracer can be induced by other P2X receptors, including P2X2 [24] as well as P2X4 and P2X5, presumably via a change of the channel pore [Surprenant, personal communication].
The junctional roles of connexin and innexin channels is unquestionable
A common role of the intercellular channels made by connexins and innexins at gap junctions of vertebrate and invertebrate tissues is to establish direct communication between cells in contact, i.e., to allow for direct and bidirectional exchange of current-carrying ions and other cytosolic, membrane-impermeant molecules [64, 65, 69–71, 109, 158, 192]. Several specific functions have been experimentally demonstrated to be due to these communications, and many more are attributed to gap junctions on the basis of strong, still circumstantial evidence [109, 158]. The in vivo importance of these functions is stressed by the many phenotypes that have been reported after deletion, overexpression, or replacement of specific connexin isoforms in mice [159, 192], as well as by the increasing number of human diseases that have been linked to either connexin mutations or pathogenic single nucleotide polymorphisms [2, 30, 68, 108, 140, 193]. While the role of innexins has been less intensively investigated, the direct intercellular communications mediated by these proteins have been shown to be essential for synapse establishment in the retina and the CNS [158, 159, 192].
More recently, other roles of connexins, that may be independent of the establishment of cell-to-cell channels, have been revealed. Thus, it is now clear that gap junction proteins interact with other membrane and cytosolic proteins and signaling pathways, and may profoundly affect the expression of multiple genes [64, 69–71, 78, 162]. These novel roles call for a reevaluation of the mechanism of some of the previous cell alterations shown to be dependent on gap junction proteins. Whether nonjunctional connexons may also contribute remains to be shown, given that connexin “hemi-channels” can be functionally demonstrated only under supraphysiological or pathological conditions.
The junctional roles of pannexin channels is questionable
There is presently little evidence that pannexins provide direct intercellular communication through gap junctions. Injection of pannexin RNAs indicated that Pnx1, but not Pnx2 and Pnx3, formed gap junctional channels in pairs of Xenopus oocytes, that were not very voltage sensitive [16, 18]. Furthermore, the conductance of these channels was about 15% smaller when Pnx1 was co-expressed with Pnx2, which was interpreted as reflecting the formation of heterotypic Pnx1–Pnx2 channels [16, 18]. However, gap junctional communication has so far been reported only after pannexins had been exogenously expressed [18], raising the question of whether the endogenous levels of the proteins, if not their structure and notably their glycosylation, prevent the series assembly of 2 pannexons into a full gap junction channel [13, 14, 125]. Future studies should address this issue and test whether pannexins could, for example, contribute to the synchronization of neurons in the inferior olive and hippocampus, where synchronized oscillations are generated. Indeed, while these oscillations were altered after loss of Cx36, the predominant neuronal connexin, the high frequency oscillations of the neuronal networks was surprisingly unchanged [41, 47, 158], suggesting a compensation by another cell-to-cell communication mechanism. Pannexons, however, seem an unlikely candidate because the large single channel conductance would provide very strong coupling even if only a few channels were functional.
Pannexin “hemi-channels” mediate paracrine cell-to-cell communication
In contrast, several functions dependent on indirect cell-to-cell communication might use large “hemi-channels” for the release of ATP [87], glutamate [194], and epoxyeicosatrienoic acid (EET) [81]. While the mechanism of this release has been repeatedly attributed to Cx43 “hemi-channels,” the discovery that pannexons are permeable to ATP under physiological conditions [104] establishes that channels made of pannexins may be involved in such release, that is required for different cell functions.
Calcium waves
Two distinct pathways responsible for the intercellular propagation of calcium waves have been identified. One involves the diffusion of calcium-mobilizing second messengers (e.g., Ca2+, IP3, cADP-ribose) from the cytosol of one cell to that of another, through the gap junctional channels made by either connexins or innexins [147, 152]. The other is an extracellular pathway, which involves the diffusion of molecules acting on cell surface membrane receptors [147, 152]. In several cell types, this pathway involves the release of ATP through membrane channels, allowing for the activation of ATP-sensitive purinergic receptors (P2Rs), a family comprised by metabotropic P2Y and ionotropic P2X receptors, on both the very same cell that released ATP (autocrine pathway) and/or the neighboring cells (paracrine pathway) [147]. The latter, extracellular pathway operates in many cell systems [47, 67, 119] and, at least in some of these cases, is dependent on ATP release and on the interaction of the nucleotide with membrane receptors [62]. Many mechanisms could account for the release of cytosolic ATP across the cell membrane, including several types of channels (volume-activated anion channels, VDAC, pore forming P2X7r). Previous studies concluded that Cx43 “hemi-channels” were instrumental to this end between cultured astrocytes exposed to low divalent cation solutions, because drugs blocking gap junctions prevented the intercellular propagation and amplification of the calcium waves [35, 170, 172]. However, further studies showing that amplification of intercellular calcium waves was still present in cultures of astrocytes from Cx43-null mice, and absent in those from P2X7r-null mice [173], have since demonstrated that the poreforming P2X7 receptor is the most likely candidate to mediate ATP release. More recently, Pnx1 has been shown to be part of the P2X7r complex, providing both a site for ATP release [104, 105], and a mechanism for amplifying the extent to which intercellular calcium waves spread between astrocytes [147].
Vasodilation
It has long been known that erythrocytes release ATP following shear stress and hypoxia [11, 160]. However, it is only recently that the mechanism involved in such release has been shown to depend on Pnx1 “hemi-channels” [104]. Thus, erythrocytes express channels with a unitary conductance (400–500 pS), voltage dependence and mechanosensitivity of “hemi-channels,” and express Pnx1, but no connexins. Pnx1 “hemi-channels” were shown to be permeable to ATP and to large molecular weight dyes, such as carboxyfluorescein. ATP release from erythrocytes was potentiated by high K+ and osmotic shock, and prevented by carbenoxolone at concentrations lower than those usually required to block connexin gap junction channels. The physiological contribution of Pnx1 was proposed to be related to the local control of blood flow, whereby the ATP released from oxygen-deprived or shear-stressed red blood cells would stimulate purinergic receptors on nearby endothelial cells, initiating the intercellular propagation of a calcium wave. In turn, the elevation of intracellular calcium would induce the release of NO onto the vascular smooth muscle, leading to vasodilation and to a consequent increase in perfusion. The ATP released from erythrocytes could also feedback activating the P2X7 receptors of these same cells, that normally mediate the release of EET through Pnx1 and CFTR channels, given that both carbenoxolone and the CFTR inhibitors glibenclamide and niflumic acid prevented basal and ATP-evoked release of EET [81]. Since EET is an important player in the regulation of vasomotion in several vessels [66], pannexons stand out as crucial channels in this modulation.
Taste sensation
Gustatory receptor cells do not display the exocytic machinery involved in neurotransmitter release of most other neuronal cells, and hence cannot release ATP molecules via a vesicular-dependent pathway, yet they are able to convey information about the quality of tastants, via afferent fibers. The taste buds involved in the perception of sweetness and bitterness contains sensory neurons equipped with G-coupled receptors which are involved in the transduction of information, while the buds involved in the perception of saltiness and sourness transduce the information through ion channels [110]. Recent studies have indicated that ATP and serotonin (5-HT) are key mediators in the transduction mechanism of sweet–bitter taste buds, via the ATP release that takes place after tastant presentation. In the extracellular medium, ATP stimulates P2 receptors of the presynaptic taste neurons, leading to the release of 5-HT from these cells. A previous report had implicated Cx43 “hemi-channels” in this mechanism, mostly because conditions known to block gap junction channels, also blocked the outward ATP currents, and in spite of the fact that other conditions acting on gap junctions (e.g., niflumic acid, carbenoxolone, quinine) had no effect on the ATP flux [142]. Blocking conditions included the use of connexin mimetic peptides, which have since been shown to block more efficiently Pnx1 “hemi-channels” than connexin “hemi-channels” [190]. A recent study now indicates that the ATP release takes place through Pnx1 channels of receptor neurons [77]. Furthermore, immunocytochemistry shows that Cx43 is not expressed in receptor cells but in the nearby epithelial cells of taste buds [77]. Thus, it is likely that pannexons are the privileged route for the ATP release which modulates taste sensation.
Pannexin “hemi-channels” mediate intracellular signaling
There is also increasing, still circumstantial evidence for several intracellular functions of pannexons.
Immune response
Pnx1 was recently proposed to be involved in the immune response, by associating with the P2X7 receptor signaling cascade to form the inflammosome [88, 124]. This complex of cytosolic proteins induces the activation of caspase1, which is necessary for the processing of interleukin IL-1β and IL-18, two important components of the inflammatory response. Activation of P2X7 receptors by ATP is essential for IL-1β release [50, 86, 126], and appears to be dependent on functional Pnx1 “hemi-channels” [105, 124]. Pnx1 is also necessary for the Toll-like receptor-independent formation of inflammosomes comprising cryopirin [88] which, in turn, is required for the activation of caspase1. It has also been suggested that Pnx1 may trigger the activation of the cryopyrin inflammosome, by mediating the passage of bacterial products from endosomes into the cytosol [88].
Ischemia and cell death
Oxygen and glucose deprivation (OGD) induces neuronal necrosis due to imbalance of intracellular ionic concentrations [63, 99, 101, 199]. Large 500 pS conductance events characteristic of Pnx1 “hemi-channels” but not of the channels that could potentially be made by co-expressed connexins, was activated in freshly isolated mouse hippocampal pyramidal neurons following OGD [180]. The OGD-induced currents were blocked by carbenoxolone and La3+, but not by the rat P2X7 receptor antagonist brilliant blue G. Moreover, after microinjection of calcein green into hipocampal neurons in situ, the efflux of the large fluorescein tracer was only observed following OGD, which was also prevented by carbenoxolone [180]. Although the study does not demonstrate the mechanism whereby OGD induced Pnx1 activity, it was suggested that pannexons contributed to the anoxic depolarization, a frequently observed phenomenon in ischemic conditions, which results in neuronal death [180]. Recently, Pnx1 has also been implicated in the leakage of calcium from the endoplasmic reticulum [187], which is another event promoting neuronal necrosis following CNS ischemia [180].
The co-expression of Pnx1 and P2X7 receptors induced zeiosis of Xenopus oocytes following activation of the receptor by extracellular ATP, a phenomenon which was not observed when oocytes were injected either with Pnx1 mRNA alone or with both Pnx1 and the metabotropic purinergic P2Y receptor [105, 106]. These observations suggest that although activation of P2 receptors can induce Pnx1 currents, it is the specific signaling through the P2X7 receptor that leads to cell death.
Recent evidence shows that conditions of oxidative stress, which are expected to favor apoptosis in different cell systems, result in the loss of “hemi-channel” currents, variably attributed to connexons or pannexons [88, 124, 135, 138, 152, 156, 169].
Growth control and tumorigenesis
Several studies have provided evidence that the degree of cell coupling as well as the level of connexin expression are reduced in many types of tumor cells and that there is a negative correlation between tumor grade and connexin function and/or expression [89, 97, 131]. Because forced expression of connexins in transformed cells reduces the neoplastic phenotype, it has been proposed that connexins display a tumor suppressor role, by a mechanism likely to be independent of cell coupling and rather related to altered cellular distribution of connexin-binding partners that display transcriptional activity, such as Src, β-catenin and NOV [1, 54, 55, 89, 100 120], and/or to changes in the expression of multiple connexin-dependent genes [29, 78, 97, 115, 162]. Whether and how some of these mechanisms are also dependent on nonjunctional connexons remains to be fully established [141]. Still, clones of glioma C6 cells stably expressing either a myc- or eGFP-tagged Pnx1 had a reduced proliferation and motility compared to cells expressing only eGFP, suggesting a tumor-suppressive role of pannexons [93]. This role was confirmed in vivo, inasmuch as mice injected with Pnx1-expressing C6 cells developed smaller tumors than mice injected with eGFP-expressing C6 cells [93]. Because dye coupling mediated by Cx43 was increased and cell morphology altered in the Pnx1-transfected glioma cells, it was proposed that the tumor suppression action of Pnx1 involved both gap junctional and non gap junctional mediated mechanisms [93].
Calcium homeostasis
Control of the steady-state levels of cytosolic calcium involves a dynamic equilibrium between the active uptake of the cation into the endoplasmic reticulum (ER), which is mediated by the ATP-dependent Ca2+ pumps of the SERCA family, and the passive diffusion of Ca2+ from the ER into the cytosol. Several candidates have been proposed to account for the latter passive diffusion, including the reversal of SERCA, translocon channels, IP3 or ryanodine receptors, Bcl2, Pnx1 and Ca2+ ionophore-like channels [31, 107, 117, 130, 183, 186, 187, 200]. Interestingly, transfection of eGFP-Pnx1 in two cell lines expressing endogenous Pnx1 transcripts lead to the accumulation of the tagged-Pnx1 in the ER, which was associated with a reduced thapsigargin-induced Ca2+ release and with a higher Ca2+ efflux [187]. Conversely, downregulation of endogenous Pnx1 by siRNA strategy, decreased the efflux rate of Ca2+ from the ER [187]. Although these results are consistent with the idea that Pnx1 may provide leak channels and therefore contribute to intracellular calcium homeostasis, several open questions still remain. For instance, it would be expected that Pnx1 be tightly regulated by ER Ca2+ levels, such that Pnx1 channels would open when these levels were high and close when these levels decrease. Such regulation seems unlikely given that the calcium concentration is much higher in the ER (mM range) than in the cytosol (nM range), and that pannexons are opened by micromolar Ca2+ concentrations [106]. At any rate, the Pnx1-mediated leakage of ER Ca2+ could represent a key event in triggering the aforementioned neuronal necrosis following CNS ischemia [187] and tumor suppression [93].
“Hemi-channel” proteins modulate gene expression
Microarray analysis of the transcriptome has revealed that the expression of a wide variety of genes is significantly perturbed after loss of individual connexins [29, 78, 115]. Most of these changes can be to a large degree predicted assuming that in the wild-type tissues, the expression of a connexin gene is functionally linked to that of multiple other genes [78]. Thus, when the coordination profiles of individual genes are compared, the striking likeness of certain gene pairs contrasts with the dissimilarity of others. This approach has revealed that Cx43 and Pnx1 expression is very similarly interlinked to that of all other genes (Fig. 4), over the entire range of coordination values. This striking similarity predicts that an up- or a downregulation of either the Cx43 or the Pnx1 gene would equally affect the cell transcriptome, thus probably resulting in a similar phenotype. The data further suggests that, at the transcriptome level, the overexpression of the Cx43 gene may compensate for the underexpression of the Pnx1 gene, and vice versa. However, this compensation could not be expected to correct those phenotypes resulting from altered direct cell-to-cell communication, given the quite limited efficacy of pannexons to form functional gap junction channels.
Fig. 4.
Cx43 and Pnx1 control the expression of a similar set of genes. Log–log plots of Pearson’s coefficients for Gja1 (X axis), Panx1, KIf16, and Nfx1 (Y axis) with all other genes. The red plot indicates that almost all genes are similarly coordinated with that of the two junctional proteins (overlap of the coordination profiles—OVL=93.2). By contrast, the blue plot shows that almost all genes exhibit opposite coordination for Gja1 and KIf16 (Kruppel-like factor 16; OVL=−94.7). Moreover, the green plot indicates that for other genes there is no association of coordinated expression between Gja1 and the other gene (here, Nfx1, nuclear transcription factor X-box binding 1; OVL=−0.4)
The present and the future
The discovery that connexin, pannexin, and innexin proteins may form “hemi-channels” in nonjunctional regions of the cell membrane, in addition to paired innexons and connexons forming the classical cell-to-cell channels at gap junctions, has opened a new field in the area of junctional proteins. First, these findings provide a clear view of some of the mechanisms that mediate paracrine intercellular signaling. Second, they provide innovative insights into the regulation of several other cell functions, for which the occurrence of “hemi-channels” provides a plausible if, in most cases, still putative mechanism. Future work should now expose these novel ideas to direct experimental testing. Even if many of the current speculations we have now about “hemi-channels” would turn out to be erroneous concepts, we are certainly entering a time of exciting, new love romance with junctional proteins [132; Bennett MV, talk at the last International Gap Junction Conference, Helsingør, Denmark, August 2007].
Romance, should not detract from the need of a rigorous and critical assessment of the data, notably with regard to the nature of the proteins making “hemi-channels” in different tissues. As repeatedly stressed in this review, this is still an area of hot debate. Many other questions remain to be addressed. For example, can pannexins form gap junction channels in vivo, e.g., under conditions preventing the glycosylation of their extracellular loops? What is the function, if any, of Pnx2 and Pnx3? Are pannexins also forming functional “hemi-channels” in the membranes of cell organelles? What are their physiological functions in vivo ? Do they play any role in the pathogenic mechanisms that cause human diseases ? If so, can we act on “hemi-channels” to correct these dysfunctions ? A nonambiguous answer to most of these questions implies that we have the experimental tools (drugs, antibodies, peptides, siRNAs, knockout pannexin models, and transgenic animals overexpressing selected pannexins in a cell specific manner) to specifically interfere with the levels and/or the function of selected types of “hemi-channels.” It also requires that cell and animal models lacking or overexpressing “hemi-channels” be available for a direct testing of their function. Normal tissues and cells spontaneously lacking detectable “hemi-channel” function, in spite of expression of Cx36, Pnx1, and the P2X7 receptor, have been recently reported [146], and a mouse null for Pnx1 is said to be viable, possibly without any obvious phenotype (H. Monyer, R. Bruzzone, personal communication). These models should be helpful in approaching some of the many open questions raised by the physiologically paradoxical plasmalemmal “hemi-channels”.
Acknowledgments
Work of our teams is supported by grants from the National Institute of Health (HD32573, NS41282, NS52245), the Swiss National Science Foundation (310000–122430), the Juvenile Diabetes Research Foundation (1-2007-158), Novo Nordisk, and the European Union (FP7-222980).
References
- 1.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleksic B, Ishihara R, Takahashi N, Maeno N, Ji X, Saito S, Inada T, Ozaki N. Gap junction coding genes and schizophrenia: a genetic association study. J Hum Genet. 2007;52:498–501. doi: 10.1007/s10038-007-0142-5. [DOI] [PubMed] [Google Scholar]
- 3.Alves LA, Coutinho-Silva R, Persechini PM, Spray DC, Savino W, Campos de Carvalho AC. Are there functional gap junctions or junctional hemichannels in macrophages? Blood. 1996;88:328–334. [PubMed] [Google Scholar]
- 4.Ashcroft FM. Ion channels and disease. Academic press; San Diego USA: 2000. p. 481. [Google Scholar]
- 5.Azanza MJ, Pes N, Pérez-Bruzón RN, Aisa J, Raso M, Junquera C, Lahoz JM, Maestú C, Martínez-Ciriano C, Pérez-Castejón C, Vera-Gil A, Del Moral A. Localization of connexins in neurons and glia cells of the Helix aspersa suboesophageal brain ganglia by immunocytochemistry. Histol Histopathol. 2007;22:497–504. doi: 10.14670/HH-22.497. [DOI] [PubMed] [Google Scholar]
- 6.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bao L, Samuels S, Locovei S, Macagno ER, Muller KJ, Dahl G. Innexins form two types of channels. FEBS Lett. 2007;581:5703–5708. doi: 10.1016/j.febslet.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao X, Lee SC, Reuss L, Altenberg GA. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci U S A. 2007;104:4919–4924. doi: 10.1073/pnas.0603154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci. 2002;115:1859–1867. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- 11.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Beyer EC, Steinberg TH. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991;266:7971–7974. [PubMed] [Google Scholar]
- 13.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 14.Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes. 2008;15:119–132. doi: 10.1080/15419060802013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bröer S, Wagner CA. Membrane transporter diseases. Kluwer Academic; New York USA: 2003. p. 404. [Google Scholar]
- 16.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 17.Bruzzone R, Dermietzel R. Structure and function of gap junctions in the developing brain. 2006;326:239–248. doi: 10.1007/s00441-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buisman HP, Steinberg TH, Fischbarg J, Silverstein SC, Vogelzang SA, Ince C, Ypey DL, Leijh PC. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci USA. 1988;85:7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc Natl Acad Sci USA. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadjichristos CE, Kwak BR. Connexins: new genes in atherosclerosis. Ann Med. 2007;39:402–411. doi: 10.1080/07853890701436757. [DOI] [PubMed] [Google Scholar]
- 22.Chanson M, Derouette JP, Roth I, Foglia B, Scerri I, Dudez T, Kwak BR. Gap junctional communication in tissue inflammation and repair. Biochim Biophys Acta. 2005;1711:197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Chanson M, Roy C, Spray DC. Voltage-dependent gap junctional conductance in hepatopancreatic cells of Procambarus clarkii. Am J Physiol. 1994;266:C569–C577. doi: 10.1152/ajpcell.1994.266.2.C569. [DOI] [PubMed] [Google Scholar]
- 24.Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Liu Q, Ge Q, Xie J, Wang ZW. UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol. 2007;17:1334–1339. doi: 10.1016/j.cub.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 28.Cockcroft S, Gomperts BD. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C. Connexin30 deficiency causes instrastrial fluid–blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci USA. 2007;104:6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collings A, Islam MS, Juonala M, Rontu R, Kähönen M, Hutri-Kähönen N, Laitinen T, Marniemi J, Viikari JS, Raitakari OT, Lehtimäki TJ. Associations between connexin37 gene polymorphism and markers of subclinical atherosclerosis: the Cardiovascular Risk in Young Finns study. Atherosclerosis. 2007;195:379–384. doi: 10.1016/j.atherosclerosis.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Combettes L, Dumont M, Berthon B, Erlinger S, Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988;263:2299–2303. [PubMed] [Google Scholar]
- 32.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contreras JE, Sánchez HA, Véliz LP, Bukauskas FF, Bennett MV, Sáez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtin KD, Zhang Z, Wyman RJ. Gap junction proteins are not interchangeable in development of neural function in the Drosophila visual system. J Cell Sci. 2002;115:3379–3388. doi: 10.1242/jcs.115.17.3379. [DOI] [PubMed] [Google Scholar]
- 38.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 39.de Boer TP, van der Heyden MA. Xenopus connexins: how frogs bridge the gap. Differentiation. 2005;73:330–340. doi: 10.1111/j.1432-0436.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 40.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;11(25):34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Zeeuw CI, Chorev E, Devor A, Manor Y, Van Der Giessen RS, De Jeu MT, Hoogenraad CC, Bijman J, Ruigrok TJ, French P, Jaarsma D, Kistler WM, Meier C, Petrasch-Parwez E, Dermietzel R, Sohl G, Gueldenagel M, Willecke K, Yarom Y. Deformation of network connectivity in the inferior olive of connexin 36-deficient mice is compensated by morphological and electrophysiological changes at the single neuron level. J Neurosci. 2003;23:4700–4711. doi: 10.1523/JNEUROSCI.23-11-04700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobrowolski R, Sommershof A, Willecke K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- 44.Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI. Expression of pannexin family of proteins in the retina. FEBS Lett. 2006;580:2178–2182. doi: 10.1016/j.febslet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 47.Enkvist MO, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem. 1992;59:519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- 48.Epstein ML, Gilula NB. A study of communication specificity between cells in culture. J Cell Biol. 1977;75:769–787. doi: 10.1083/jcb.75.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 51.Finbow ME, Buultjens TE, Lane NJ, Shuttleworth J, Pitts JD. Isolation and characterisation of arthropod gap junctions. EMBO J. 1984;3:2271–2278. doi: 10.1002/j.1460-2075.1984.tb02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fortes FS, Pecora IL, Persechini PM, Hurtado S, Costa V, Coutinho-Silva R, Braga MB, Silva-Filho FC, Bisaggio RC, De Farias FP, Scemes E, De Carvalho AC, Goldenberg RC. Modulation of intercellular communication in macrophages: possible interactions between GAP junctions and P2 receptors. J Cell Sci. 2004;117:4717–4726. doi: 10.1242/jcs.01345. [DOI] [PubMed] [Google Scholar]
- 53.Fraser SE, Green CR, Bode HR, Gilula NB. Selective disruption of gap junctional communication interferes with a patterning process in hydra. Science. 1987;237:49–55. doi: 10.1126/science.3037697. [DOI] [PubMed] [Google Scholar]
- 54.Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanism of connexin-mediated growth suppression. J Biol Chem. 2004;279:36943–36950. doi: 10.1074/jbc.M403952200. [DOI] [PubMed] [Google Scholar]
- 55.Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, Winterhager E. Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem. 2004;279:36931–36942. doi: 10.1074/jbc.M404073200. [DOI] [PubMed] [Google Scholar]
- 56.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemi-channels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 58.Giaume C, Spira ME, Korn H. Uncoupling of invertebrate electrotonic synapses by carbon dioxide. Neurosci Lett. 1980;17:197–202. doi: 10.1016/0304-3940(80)90084-1. [DOI] [PubMed] [Google Scholar]
- 59.Gilula NB, Satir P. Septate and gap junctions in molluscan gill epithelium. J Cell Biol. 1971;51:869–872. doi: 10.1083/jcb.51.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González D, Gómez-Hernández JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. 2006;20:2329–2338. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- 61.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 62.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 64.Harris A, Locke D. Connexin biology: the role of gap junction in disease. The Humana; Totowa, NJ, USA: 2008. [Google Scholar]
- 65.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 66.Harrison DG, Cai H. Endothelial control of vasomotion and nitric oxide production. Cardiol Clin. 2003;21:289–302. doi: 10.1016/s0733-8651(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 67.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hempelmann A, Heils A, Sander T. Confirmatory evidence for an association of the connexin-36 gene with juvenile myoclonic epilepsy. Epilepsy Res. 2006;71:223–228. doi: 10.1016/j.eplepsyres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 69.Hervé JC. The connexins, part II. Biochim Biophys Acta. 2005;1711:97–246. doi: 10.1016/j.bbamem.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Hervé JC. The connexins, part I. Biochim Biophys Acta. 2004;1662:1–172. doi: 10.1016/j.bbamem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Hervé JC. The connexins, part III. Biochem Biophys Acta. 2005;1719:1–160. doi: 10.1016/j.bbamem.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Hickman SE, Semrad CE, Silverstein SC. P2Z purinoceptors. Ciba Found Symp. 1996;198:71–83. doi: 10.1002/9780470514900.ch4. [DOI] [PubMed] [Google Scholar]
- 73.Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 74.Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 75.Hua VB, Chang AB, Tchieu JH, Kumar NM, Nielsen PA, Saier MH. Sequence and phylogenetic analyses of 4 TMS junctional proteins of animals: connexins, innexins, claudins and occludins. J Membr Biol. 2003;194:59–76. doi: 10.1007/s00232-003-2026-8. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 77.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iacobas DA, Iacobas S, Spray DC. Connexin-dependent transcellular transcriptomic networks in mouse brain. Prog Biophys Mol Biol. 2007;94:169–185. doi: 10.1016/j.pbiomolbio.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Iglesias R, Locovei S, Roque AP, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor–Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iovine MK, Gumpert AM, Falk MM, Mendelson TC. Cx23, a connexin with only four extracellular-loop cysteines, forms functional gap junction channels and hemichannels. FEBS Lett. 2008;582:165–170. doi: 10.1016/j.febslet.2007.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- 84.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 85.Johnston MF, Simon SA, Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980;286:498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- 86.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K + release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 87.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol. 2007;94:245–264. doi: 10.1016/j.pbiomolbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Kleopa KA, Scherer SS. Molecular genetics of X-linked Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:107–122. doi: 10.1385/nmm:8:1-2:107. [DOI] [PubMed] [Google Scholar]
- 91.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 92.Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol. 2004;83:647–654. doi: 10.1078/0171-9335-00422. [DOI] [PubMed] [Google Scholar]
- 93.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 94.Laird DW. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J Biol Chem. 2008;283:2997–3001. doi: 10.1074/jbc.R700041200. [DOI] [PubMed] [Google Scholar]
- 95.Landesman Y, White TW, Starich TA, Shaw JE, Goodenough DA, Paul DL. Innexin-3 forms connexin-like intercellular channels. J Cell Sci. 1999;112:2391–2396. doi: 10.1242/jcs.112.14.2391. [DOI] [PubMed] [Google Scholar]
- 96.Larsen WJ. Structural diversity of gap junctions. A review. Tissue Cell. 1977;9:373–394. doi: 10.1016/0040-8166(77)90001-5. [DOI] [PubMed] [Google Scholar]
- 97.Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12:225–256. doi: 10.1615/critrevoncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 98.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin R, Martyn KD, Guyette CV, Lau AF, Warn-Cramer BJ. v-Src tyrosine phosphorylation of connexin43: regulation of gap junction communication and effects on cell tranformation. Cell Commun Adhes. 2006;13:199–216. doi: 10.1080/15419060600848516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 102.Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- 103.Liu TF, Li HY, Atkinson MM, Johnson RG. Comparison of lucifer yellow leakage and cell-to-cell transfer following intracellular injection in normal and antisense Novikoff cells under treatment with low extracellular Ca2+ Meth Find Exp Clin Pharmacol. 1996;18:493–497. [PubMed] [Google Scholar]
- 104.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 107.Lomax RB, Camello C, Van Coppenolle F, Petersen OH, Tepikin AV. Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. Second messenger-activated channels and translocons. J Biol Chem. 2002;277:26479–26485. doi: 10.1074/jbc.M201845200. [DOI] [PubMed] [Google Scholar]
- 108.Mas C, Taske N, Deutsch S, Guipponi M, Thomas P, Covanis A, Friis M, Kjeldsen MJ, Pizzolato GP, Villemure JG, Buresi C, Rees M, Malafosse A, Gardiner M, Antonarakis SE, Meda P. Association of the connexin36 gene with juvenile myoclonic epilepsy. J Med Genet. 2004;41:e93–e98. doi: 10.1136/jmg.2003.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meda P, Spray DC. Gap junction function. Adv Mol Cell Biol. 2000;30:263–322. [Google Scholar]
- 110.Medler K. Signaling mechanisms controlling taste cell function. Crit Rev Eukaryot Gene Expr. 2008;18:125–137. doi: 10.1615/critreveukargeneexpr.v18.i2.20. [DOI] [PubMed] [Google Scholar]
- 111.Même W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- 112.Mire P, Nasse J, Venable-Thibodeaux S. Gap junctional communication in the vibration-sensitive response of sea anemones. Hear Res. 2000;144:109–123. doi: 10.1016/s0378-5955(00)00047-2. [DOI] [PubMed] [Google Scholar]
- 113.Morita M, Saruta C, Kozuka N, Okubo Y, Itakura M, Takahashi M, Kudo Y. Dual regulation of astrocyte gap junction hemi-channels by growth factors and a pro-inflammatory cytokine via the mitogen-activated protein kinase cascade. Glia. 2007;55:508–515. doi: 10.1002/glia.20471. [DOI] [PubMed] [Google Scholar]
- 114.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Naus CC, Bond SL, Bechberger JF, Rushlow W. Identification of genes differentially expressed in C6 glioma cells transfected with connexin43. Brain Res Brain Res Rev. 2000;32:259–266. doi: 10.1016/s0165-0173(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 116.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 117.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol. 2008;586:2445–2461. doi: 10.1113/jphysiol.2008.150805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 120.Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007;313:4083–4090. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Panchin YV. Evolution of gap junction proteins—the pannexin alternative. J Exp Biol. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 122.Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 123.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 126.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 127.Petersen MB, Willems PJ. Non-syndromic, autosomal-recessive deafness. Clin Genet. 2006;69:371–392. doi: 10.1111/j.1399-0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 128.Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 129.Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 130.Pinton P, Ferrari D, Magalhães P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr Med Chem. 2007;14:2288–2303. doi: 10.2174/092986707781696564. [DOI] [PubMed] [Google Scholar]
- 132.Prochnow N, Dermietzel R. Connexons and cell adhesion: a romantic phase. Histochem Cell Biol. 2008;130:71–77. doi: 10.1007/s00418-008-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qu Y, Dahl G. Accessibility of Cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys J. 2004;86:1502–1509. doi: 10.1016/S0006-3495(04)74218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS ONE. 2007;2:e712. doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ravier MA, Güldenagel M, Charollais A, Gjinovci A, Caille D, Söhl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 137.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Richard G. Connexin disorders of the skin. Clin Dermatol. 2005;23:23–32. doi: 10.1016/j.clindermatol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 141.Rodríguez-Sinovas A, Cabestrero A, López D, Torre I, Morente M, Abellán A, Miró E, Ruiz-Meana M, García-Dorado D. The modulatory effects of connexin 43 on cell death/ survival beyond cell coupling. Prog Biophys Mol Biol. 2007;94:219–232. doi: 10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 142.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sabag AD, Dagan O, Avraham KB. Connexins in hearing loss: a comprehensive overview. J Basic Clin Physiol Pharmacol. 2005;16:101–116. doi: 10.1515/jbcpp.2005.16.2-3.101. [DOI] [PubMed] [Google Scholar]
- 144.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scemes E. Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia. 2008;56:145–153. doi: 10.1002/glia.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scemes E, Bavamian S, Charollais A, Spray DC, Meda P. Lack of “hemichannel” activity in insulin-producing cells. Cell Commun Adhes. 2008;15:143–154. doi: 10.1080/15419060802014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schalper KA, Palacios-Prado N, Orellana JA, Sáez JC. Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes. 2008;15:207–218. doi: 10.1080/15419060802014198. [DOI] [PubMed] [Google Scholar]
- 149.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martínez AD, Sáez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell. 2008;19:3501–3513. doi: 10.1091/mbc.E07-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol. 1999;277:C766–C776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- 151.Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 152.Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 153.Severs NJ, Dupont E, Thomas N, Kaba R, Rothery S, Jain R, Sharpey K, Fry CH. Alterations in cardiac connexin expression in cardiomyopathies. Adv Cardiol. 2006;42:228–242. doi: 10.1159/000092572. [DOI] [PubMed] [Google Scholar]
- 154.Shestopalov VI, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2008;65:376–394. doi: 10.1007/s00018-007-7200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 55.5. J Comp Neurol. 2007;501:765–779. doi: 10.1002/cne.21282. [DOI] [PubMed] [Google Scholar]
- 156.Shintani-Ishida K, Uemura K, Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714–H1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- 157.Socolar SJ, Politoff AL. Uncoupling cell junctions in a glandular epithelium by depolarizing current. Science. 1971;172:492–494. doi: 10.1126/science.172.3982.492. [DOI] [PubMed] [Google Scholar]
- 158.Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 159.Söhl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev. 2004;47:245–259. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 160.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 161.Spray DC, Harris AL, Bennett MV. Voltage dependence of junctional conductance in early amphibian embryos. Science. 1979;204:432–434. doi: 10.1126/science.312530. [DOI] [PubMed] [Google Scholar]
- 162.Spray DC, Iacobas DA. Organizational principles of the connexin-related brain transcriptome. J Membr Biol. 2007;218:39–47. doi: 10.1007/s00232-007-9049-5. [DOI] [PubMed] [Google Scholar]
- 163.Spray DC, Ye ZC, Ramson BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 164.Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J Gen Physiol. 2006;127:67–75. doi: 10.1085/jgp.200509397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stebbings LA, Todman MG, Phelan P, Bacon JP, Davies JA. Two Drosophila innexins are expressed in overlapping domains and cooperate to form gap-junction channels. Mol Biol Cell. 2000;11:2459–2470. doi: 10.1091/mbc.11.7.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4− permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 168.Stoka AM. Phylogeny and evolution of chemical communication: an endocrine approach. J Mol Endocrinol. 1999;22:207–225. doi: 10.1677/jme.0.0220207. [DOI] [PubMed] [Google Scholar]
- 169.Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–2210. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- 170.Stout C, Charles A. Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia. 2003;43:265–273. doi: 10.1002/glia.10257. [DOI] [PubMed] [Google Scholar]
- 171.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 172.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 173.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia. 2004;48:217–229. doi: 10.1002/glia.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 176.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemi-channels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 177.Tao L, Harris AL. 2-aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol. 2007;71:570–579. doi: 10.1124/mol.106.027508. [DOI] [PubMed] [Google Scholar]
- 178.Tatham PE, Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990;95:459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, Ephrussi A, Wood CG, Lehmann R, Fuller MT. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–2539. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- 180.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 181.Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- 182.Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J. 2006;91:2142–2154. doi: 10.1529/biophysj.106.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toyoshima C, Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature. 2002;418:605–611. doi: 10.1038/nature00944. [DOI] [PubMed] [Google Scholar]
- 184.Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trinkaus JP. The forces that shape the embryo. 2. Prentice-Hall Inc; Englewood USA: 1984. Cells into organs; p. 543. [Google Scholar]
- 186.Van Coppenolle F, Vanden Abeele F, Slomianny C, Flourakis M, Hesketh J, Dewailly E, Prevarskaya N. Ribosome–translocon complex mediates calcium leakage from endoplasmic reticulum stores. J Cell Sci. 2004;117:4135–4142. doi: 10.1242/jcs.01274. [DOI] [PubMed] [Google Scholar]
- 187.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006;174:535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Verselis VK, Trexler EB, Bukauskas FF. Connexin hemichannels and cell-cell channels: comparison of properties. Braz J Med Biol Res. 2000;33:379–389. doi: 10.1590/s0100-879x2000000400003. [DOI] [PubMed] [Google Scholar]
- 189.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 191.Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 193.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 194.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yen MR, Saier MH. Gap junctional proteins of animals: the innexin/pannexin superfamily. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Young MT, Pelegrin P, Surprenant A. Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol. 2007;71:92–100. doi: 10.1124/mol.106.030163. [DOI] [PubMed] [Google Scholar]
- 197.Yu J, Bippes CA, Hand GM, Muller DJ, Sosinsky GE. Aminosulfonate modulated pH-induced conformational changes in connexin26 hemichannels. J Biol Chem. 2007;282:8895–8904. doi: 10.1074/jbc.M609317200. [DOI] [PubMed] [Google Scholar]
- 198.Zampighi GA. Distribution of connexin50 channels and hemichannels in lens fibers: a structural approach. Cell Commun Adhes. 2003;10:265–270. doi: 10.1080/cac.10.4-6.265.270. [DOI] [PubMed] [Google Scholar]
- 199.Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. Role for nitric oxide in permeability of hippocampal neuronal hemi-channels during oxygen glucose deprivation. J Neurosci Res. 2008;86:2281–2291. doi: 10.1002/jnr.21675. [DOI] [PubMed] [Google Scholar]
- 200.Zimniak P, Little JM, Radominska A, Oelberg DG, Anwer MS, Lester R. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry. 1991;30:8598–8604. doi: 10.1021/bi00099a015. [DOI] [PubMed] [Google Scholar]