Abstract
Relationships between increased adiposity and fat-soluble vitamin storage and metabolism are poorly understood. To examine these associations, 6% or 21% dietary fat was fed to rats for 11 weeks and tissue vitamin A storage determined. Two levels of supplemental vitamin A were administered. At the end of the tenth week, 3,4-didehydroretinol (DR) was administered orally, and its kinetics were followed for 1 week in serum and tissues. Model-based compartmental analysis was applied to these data. Kidney total retinol (R) concentrations were elevated in rats fed 6% compared with 21% dietary fat (n = 24/group). The fractional transfer coefficient (FTC) describing the movement of tracer from plasma to extravascular stores was two times higher in the 6% compared with the 21% fat group. Consistent with the elevated renal R in 6% fat fed rats, there was a 2-fold increase in the FTC representing tracer distribution from plasma to kidney in the 6% compared with 21% fat group. Taken together with a fat main effect on renal vitamin A, our data support the evidence that faster turnover of kidney R may help set the mechanism governing vitamin A tissue distribution during deficiency. Rats fed 21% versus 6% dietary fat conserved hepatic R more efficiently.
Keywords: kinetic analysis; retinol; Windows version of Simulation, Analysis, and Modeling software; adipose tissue
Given the reduced circulating vitamin A reported in obese adults (1, 2) and the need for clinically relevant biomarkers, it is increasingly important to understand how fat-soluble vitamin metabolism is affected by increased adiposity (3, 4). Furthermore, conflicting reports of increased serum retinol-binding protein 4 [RBP4 or RBP; the circulating protein transporter for retinol (R)] in obese humans (5, 6), highlight the relevance of these questions. Considering the reported expansion of intravascular volume in obese adults (7) and the storage of vitamin A in adipose tissue (8), an important clinical question arises: do vitamin A requirements differ in obese versus lean individuals? If so, what are the driving mechanisms responsible for these differences? How nutritional status is affected in obese individuals is an emerging research area (4, 9).
While it is well established that dietary fat promotes the absorption of fat-soluble vitamins (10), it is less clear what effect increased adiposity has on storage and metabolism of these vitamins. One hypothesis is that enlarged fat pads store more vitamin A per tissue compared with fat pads from leaner mammals (11). Alternatively, increased adiposity presents a greater metabolic need and, thus, vitamin A requirements differ between lean and obese (12, 13). Early experiments in laboratory rats suggested that chylomicra deliver vitamin A to extrahepatic tissues in a postprandial bolus (14). More recently, rats fed no vitamin A had minimal adipose storage of vitamin A in six adipose depots compared with the adipose of rats fed excessive levels of dietary vitamin A (15). In subsequent work, these adipose depots represented a vitamin A store that is tapped during vitamin A restriction (16). Because rats in slight negative hepatic vitamin A balance, as evidenced by total liver vitamin A decreasing 9 nmol/day, were predicted to have half of whole body vitamin A mass in extrahepatic tissues (17), we asked whether increasing dietary fat and altering body adiposity would affect whole body partitioning of vitamin A.
Using 3,4-didehydroretinol (DR), a vitamin A analog, as a tracer, our aims were 3-fold: first, to characterize the effect of higher versus normal dietary fat on tissue storage of vitamin A in rats. We hypothesized, based on liver stellate cell lipid droplet retinoid concentration not being affected by dietary triglyceride level (18), that higher versus lower dietary fat would not alter storage of vitamin A in extrahepatic tissues (19). Second, we determined whether vitamin A tracer tissue distribution, representing recently ingested R, differed by dietary fat level. Third, we compared kinetic rate constants in rats fed normal versus higher dietary fat. We explored how varying dietary fat differentially affects how a recently administered vitamin A analog turns over in plasma and is distributed to tissues in vitamin A-deficient rats.
MATERIALS AND METHODS
Animals and diet
Weanling (21 d) male outbred Sprague-Dawley rats (Charles River, Kingston, NY) were housed individually in corn cob bedding. Upon arrival, rats were randomized to ad libidum feeding of a vitamin A free, 6% or 21% fat purified diet. The 6% and 21% fat diets (TD.04175 and TD.04176; Harlan-Teklad, Madison, WI) contained (in g per kg diet), respectively, casein (200, 240); dl-methionine (3, 3.6); sucrose (280, 280); corn starch [215.0436, not added (NA)]; maltodextrin (150, 155.1147); cellulose (50, 50); soybean oil (55, 55); Primex shortening (NA, 160); AIN-93G-MX TD 94046 mineral mix (35, 42); calcium phosphate (3.2, 3.75); vitamin mix without choline, A, D, E (5, 6); vitamin D3 (500,000 U/g; 0.0044, 0.0053); dl-α-tocopheryl acetate (500 IU/g; 0.242, 0.29); choline dihydrogen citrate (3.5, 4.2); and TBHQ (0.01, 0.04). All rats were housed in a 12-h light/dark cycle, at 22°C and 40–55% humidity, with free access to water. Seven days after arrival, a dose of 17 (5.6 μg) or 70 (23 μg) nmol retinyl acetate dissolved in corn oil (92 μL; 80 mg fat) was pipetted into each rat's mouth every morning, 2 to 3 h after the beginning of the light cycle. These studies were approved by the University of Wisconsin-Madison Research Animal Resources Center in compliance with federal legislations on the care of laboratory animals as documented in the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals.
Kinetic study and blood sampling
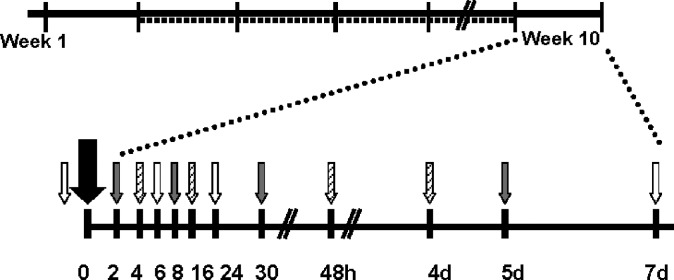
Rats (n = 48, 45.4 ± 3.6 g) were randomized to 6% (n = 24) or 21% fat purified diet (n = 24) ad libitum for 77 days. On day 70, all rats were dosed with 3,4-didehydroretinyl acetate (20–33) (DRAc; 0.7 μmol). In vivo, DRAc is metabolized to DR and can be distinguished from R by HPLC. After the DRAc dose, blood (1 ml) was sampled at baseline and then 2, 4, 6, 8, 16, 24, 30, 48, 96, 120, and 168 h (n = 4/treatment group/time) via a blind stick to the jugular vein. Blood was collected serially no more than three times from each rat to remove <10% of each rat's total blood volume in 1 week's time. Blood was processed as described previously (34). One group of rats was in the baseline, 6, and 24 h blood draws ending with the 168 h terminal blood draw (Fig. 1). Livers, perirenal, and epididymal fat pads were collected at 96, 120, and 168 h (n = 4/treatment group) and their wet weights recorded. Kidneys and lungs were also collected from all rats at these times. All tissues were stored at −80°C until analysis.
Fig. 1.
Upon arrival, rats (n = 48) were randomized to a vitamin A-free 6% or 21% fat diet (solid line). At the beginning of week 2, rats were dosed with either 17 or 70 nmol retinyl acetate daily (dashed line) until week 10. At the beginning of week 10, all rats were orally administered 700 nmol 3,4-didehydroretinyl acetate (big black arrow). Rats were randomized to 1 of 3 blood draw collection groups (gray, white, and diagonal line patterned small arrows).
Serum and liver analysis for DR and R by HPLC
Serum samples were analyzed for DR and R using a 200 μl protocol reported previously (31). Liver analysis (35) included DR and R esters. Briefly, two portions of liver (0.5 g each) from different locations were weighed. Anhydrous sodium sulfate (3 g) was added to the liver and ground together. Internal standard (3.4 nmol retinyl butyrate) was added to the liver homogenate to calculate extraction efficiency. The liver tissue was extracted with dichloromethane (50 ml). An aliquot (5 ml) was evaporated under argon. The residue was redissolved in 50:50 methanol/dichloroethane (100 μl); an aliquot (50 μl) was injected (717plus Autosampler, Waters Corp., Milford, MA) onto a Resolve® C18 reverse-phase column (3.9 × 300 mm, 5 μm; Waters). Two solvents were run in a gradient (1525 Binary HPLC pump, Waters) to elute DR and R esters: A, 92.5:7.5 (v/v) acetonitrile/water; B, 85:10:5 acetonitrile/methanol/dichloroethane both with 0.05% triethylamine (0.375 g triethylamine /l). A linear gradient was run from solvent A to B from 0–3 min, and held at 100% B until 26 min at 2 ml/min. For each sample, chromatograms were generated at 325 and 350 nm (996 Photodiode Array Detector, Waters). For all tissues, retinyl butyrate was used to quantify R and its esters at 325 nm. 3,4-Didehydroretinyl acetate was used to quantify DR and its esters at 350 nm.
Adipose tissue, kidney, and lung analyses for DR and R by HPLC
Adipose tissue (50 mg), in triplicate, was homogenized (PT 10/35 Brinkmann Instruments, Westbury, NY) in PBS (500 μl). Ethanol (1 ml) was added to denature proteins and internal standard was added (0.07 nmol retinyl butyrate). Hexanes (1 × 2 ml, 2 × 1 ml) were added, mixed for 30 s, pooled, and dried under argon. The film was redissolved in benzene (40 μl); an aliquot was injected (30 μl). The mobile phase (70:15:15 acetonitrile/methanol/ dichloromethane) was run at 1.8 ml/min.
Kidney and lung were analyzed similarly; tissue (0.5 g) was ground with sodium sulfate (1.5 g). After internal standard was added (retinyl butyrate), the tissue was extracted repeatedly with dichloromethane to 10 ml. Half of this volume was removed and dried under argon. The film was redissolved (80 μl, 50:50 methanol/dichloroethane); an aliquot (32 μl) was injected. The mobile phase (85:15 acetonitrile:dichloroethane with 0.05% triethylamine) was run at 1 ml/min up to 5 min to separate DR and R, increased to 2 ml/min by 7 min and held until 35 min by which time retinyl stearate had eluted.
Adipose, kidney, and lung were analyzed on the same column (Sunfire® C18 column, 4.6 × 250 mm, 5 μm, Waters Corp, Milford, MA) and system (600 Pump and Controller, Waters; SPD-10A VP UV-VIS Detector set to 325 and 350 nm, C-R7A plus Chromatopac both Shimadzu, Kyoto, Japan). The detection limit for R and DR, respectively, was 0.8 and 1.2 pmol.
Liver percent fat by Folch extraction
Liver dichloromethane extract (as described above; 10 ml) was evaporated under argon. The remaining film was placed in a desiccator, evacuated for 2 h, and left overnight. The mass of fat was normalized per liver (36).
Serum C-reactive protein
Serum CRP was measured using a commercial high-sensitivity ELISA kit specific for rat CRP (Alpha Diagnostic, San Antonio, TX) and microplate reader (Molecular Devices, Sunnyvale, CA). All samples and standards were analyzed in triplicate. The detection limit was 6.0 ng/ml and the CV was 7.5%.
Kinetic analysis
Initially, fraction of DR dose [estimating plasma volume as 0.038 ml/g BW (37) using body weights on the day serum was drawn] was modeled by a “front door approach” (38) in a steady-state model. The fractional transfer coefficients (FTCs) resulting from this analysis were not physiological and were an indication that the DR dose represented a perturbation (39) to the vitamin A metabolism of these rats. Thus, model-based compartmental analysis with time invariant FTCs and a steady-state solution was applied to the plasma DR data. The absorption function was fixed to reflect the liver maximum fraction of dose measured by HPLC. Initial conditions were set to 700 nmol DR. Plasma DR (nmol) data were fit from a published tritiated R model in rats with similar liver stores (40, 41). FTCs [L(I,J)s or the fraction of compartment J transferred to compartment I per unit time] were then determined. The Windows version of the Simulation, Analysis and Modeling (WinSAAM) software (17, 38) was used for this analysis.
Statistical analysis
Descriptive data are presented as group means ± SD. Tissue data were compared using the “proc mixed covtest” command of SAS. Significance at P < 0.05 was reported for the main effects of fat and vitamin A as well as their interaction. Geometric mean serum DR (nmol) data from each treatment group (6 vs. 21% dietary fat on the 17 nmol/day retinyl acetate dose) was modeled in order to approach central tendency. A superrat model (42) was built because the same rats could not be used for all time points. In the superrat approach, groups of animals are killed at different times, and tissue data are pooled into one data set and used for modeling. Because compartmental modeling was done using group mean data at each time point, it was not possible to compare kinetic parameters statistically between the two groups. For weighting purposes, model development based on observed data was set at a fractional standard deviation (SD/mean) of 5%. Fit between the predicted model and the observed data was evaluated by comparing the weighted sum of squares, representing the sum of the squared differences between the observed and model-predicted data points.
RESULTS
Effects of dietary fat on body and tissue weights
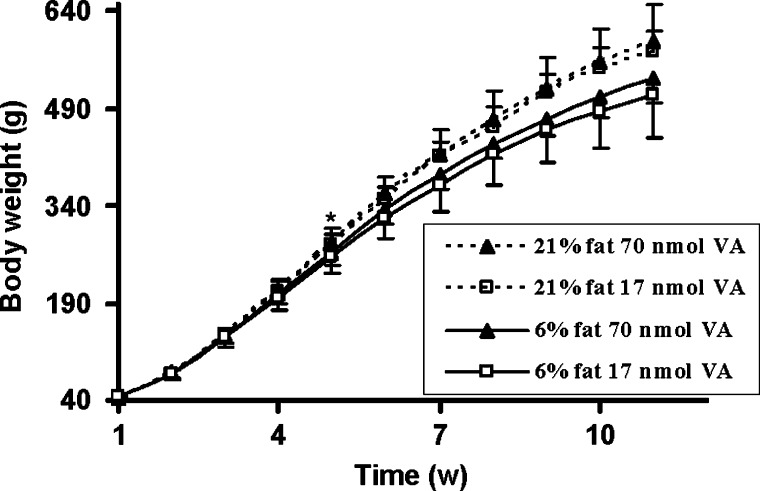
Final body weights of rats on the 21% fat diet were higher than those on 6% dietary fat (Table 1). From weeks 5 to 11, rats fed the 21% fat diet weighed significantly more than rats fed the 6% fat diet (Fig. 2). There was a significant overall main effect of week (P < 0.0001) as well as fat (P = 0.0029). While rats dosed with 17 nmol vitamin A/day consistently weighed less each week than rats fed the higher dose of vitamin A, this was not statistically significant (vitamin A main effect, P = 0.33), except for the last week (ANOVA, P = 0.026).
TABLE 1.
Effects of fat and vitamin A on body weights, tissue weights, and serum vitamin A in rats fed two levels of fat and vitamin Aa
| Fat ± Vitamin Ab |
Factors (P)c |
||||||
|---|---|---|---|---|---|---|---|
| Variable | NF− | NF+ | HF− | HF+ | F | VA | F × VA |
| Final body weight (g) | 509.8 ± 64.4 | 536.1 ± 36.9 | 577.1 ± 33.1 | 593.7 ± 55.2 | <0.0001 | 0.14 | 0.82 |
| Liver weight (g) | 19.3 ± 4.2 | 20.5 ± 2.8 | 22.5 ± 3.0 | 21.2 ± 2.9 | 0.047 | 0.97 | 0.19 |
| Perirenal adipose (g) | 14.3 ± 5.2 | 16.8 ± 3.4 | 21.8 ± 6.1 | 23.7 ± 6.9 | <0.0001 | 0.17 | 0.83 |
| Epididymal adipose (g) | 9.3 ± 2.1 | 11.9 ± 1.9 | 15.8 ± 3.0 | 15.7 ± 4.1 | <0.0001 | 0.15 | 0.12 |
| Serum R (μmol/L) | 0.50 ± 0.12 | 1.41 ± 0.31 | 0.47 ± 0.12 | 1.47 ± 0.34 | 0.86 | <0.0001 | 0.58 |
| Serum CRP (mg/L)d | 150.3 ± 24.8 | 114.5 ± 20.6 | 143.3 ± 57.2 | 126.8 ± 31.6 | 0.86 | 0.094 | 0.52 |
| Liver fat (mg/g) | 33.3 ± 14.6 | 34.1 ± 11.0 | 44.6 ± 9.5 | 44.7 ± 10.5 | 0.002 | 0.88 | 0.93 |
| MRDR ratioe | 1.23 ± 0.37 | 0.16 ± 0.05 | 1.58 ± 0.37 | 0.16 ± 0.08 | 0.21 | <0.0001 | 0.21 |
Values (n = 12 per treatment group) represent least squares means ± SD, with significant differences (P < 0.05) by variable.
Treatment groups follow: NF- 6% dietary fat, 17 nmol retinyl acetate daily; NF+ 6% dietary fat, 70 nmol retinyl acetate daily; HF- 21% dietary fat, 17 nmol retinyl acetate daily; HF+ 21% dietary fat, 70 nmol retinyl acetate daily.
Factors include the main effects of dietary fat (F) and vitamin A (VA) and the interaction between fat and vitamin A (F × VA).
Serum CRP is n = 6 per treatment group.
The modified relative dose response (MRDR) ratio is the molar concentration of 3,4-didehydroretinol to retinol (R) in the serum 4 h after dosing with 3,4-didehydroretinyl acetate (n = 4 per treatment group).
Fig. 2.
Effects of dietary fat on body weights over time. Rats fed the 21% fat diet (n = 24) are represented by a dashed line, whereas rats fed the 6% fat diet (n = 24) are represented by a solid line. Rats dosed with 70 nmol daily vitamin A (VA) are represented by a solid triangle, whereas rats dosed daily with 17 nmol VA are represented by an open square. Values are means ± SD of 12 rats per treatment per week. * P < 0.05 comparing 6 versus 21% dietary fat; indicates the first week that a significant difference was observed; differences were maintained throughout the study duration (Fat × week P < 0.05 week 5, P < 0.05 weeks 6 to 11).
Liver, perirenal fat pads, and epididymal fat pads weighed significantly more in the 21% fat versus 6% fat group (Table 1). For all tissues, there was no effect of vitamin A. When these organ weights were normalized to body weight, only the fat main effect for liver mass was no longer significant (fat main effect, P = 0.74). Nonetheless, we investigated whether differences in liver weight could be explained by dietary fat treatment. Rats maintained on the 21% fat diet compared with those on the 6% fat diet had more fat per g liver. Serum CRP was higher in the 17 nmol compared with 70 nmol vitamin A group, but the difference did not reach significance.
Effects of dietary fat on serum and tissue retinoids
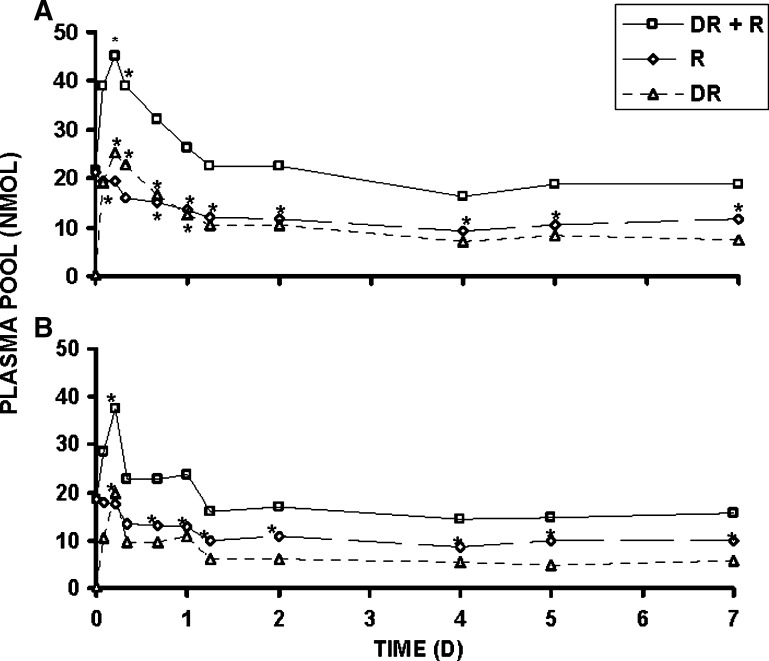
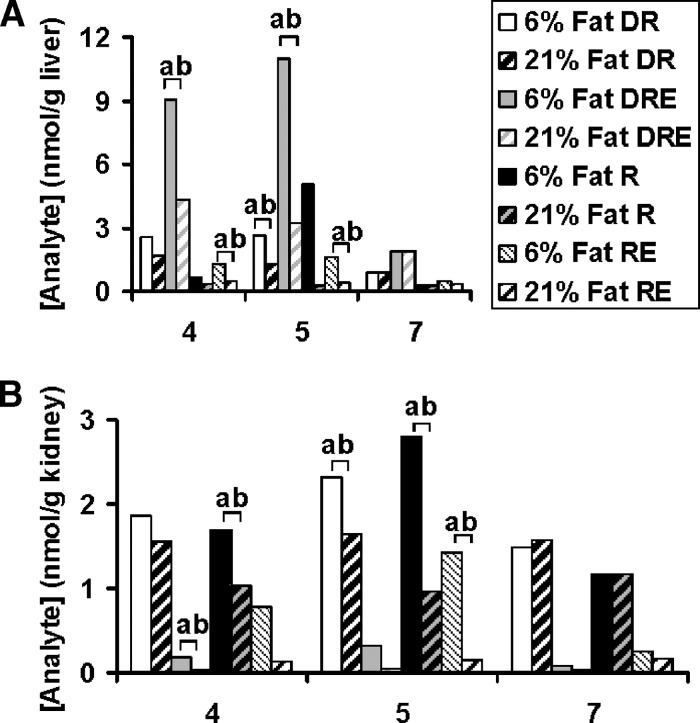
Serum R concentration, at the time of kill, was significantly greater in rats receiving 70 nmol compared with 17 nmol retinyl acetate/day (Table 1). Total serum R decreased after the DR dose illustrating the dose's perturbing effect. By 16 h, significantly lower total serum R was observed compared with baseline for both dietary fat groups dosed daily with 17 nmol retinyl acetate (Fig. 3). Dietary fat treatment had no effect on the modified relative dose-response test, the plasma ratio of DR to R at 4 h, but as expected was significantly different by vitamin A treatment. A modified relative dose-response ratio ⩾ 0.060 corresponds to deficient liver reserves defined as < 70 nmol/g (20). The liver total R concentration data reflect values below this cutoff (Table 2). Rats dosed with 17 nmol daily were in slight negative R and severe negative DR balance (17) between day 4 and 7 post DR dose, although more pronounced in 6% (respectively, 6.7 and 50.4 nmol/day) than 21% fat fed rats (respectively, 2.2 and 27.3 nmol/day). Only hepatic total DR (nmol/g liver tissue) was sensitive to fat treatment; elevated levels of DR and 3,4-didehydroretinyl esters (DRE) were observed in 6% compared with 21% fat fed rats. Liver total R concentration, as expected, was only affected by vitamin A treatment. For other tissues, only kidney (Table 2) not lung (data not shown), perirenal (Table 2), nor epididymal adipose tissue (data not shown) showed a main effect of dietary fat treatment. Rats on the 6% compared with 21% fat diet had increased renal R, retinyl esters (RE), and DRE. While epididymal (data not shown) and perirenal fat pads contained RE, DRE were not detected. The effect of dietary fat on DRE concentrations in liver (Fig. 4A) and kidney (Fig. 4B) was even more pronounced when the data were further examined on kill day 4, 5, and 7 for the rats dosed daily with 17 nmol vitamin A. The effect of dietary fat on DRE concentrations in liver and kidney was not observed in either of these tissues (see supplementary Fig. I) for rats dosed daily with 70 nmol retinyl acetate.
Fig. 3.
Plasma pool (nmol) of 3,4-didehydroretinol (DR) + R, R, and DR calculated by multiplying the serum concentration of each compound as measured by HPLC with an estimate of plasma volume (0.038 ml/g BW) (37). A: 21% fat group; B: 6% fat group. DR + R (solid line with square), R (long-dashed line with diamond), and DR (dashed line with triangle). Repeated measures using Tukey adjustment for multiple comparisons significantly different from t = 0 for each compound indicated by *; adjusted P value < 0.05. n = 4 per time; except n = 7 at 5 h for 6% fat fed rats, n = 3 and 9, respectively, at 2 and 5 h for 21% fat fed rats. Values are means ± SD.
TABLE 2.
R concentrations in rat liver, kidney, epididymal, and perirenal fat padsa
| Fat ± Vitamin A |
Factors (P)b |
||||||
|---|---|---|---|---|---|---|---|
| Retinoid (nmol/g) | NF- | NF+ | HF− | HF+ | F | VA | F × VA |
| Liver | |||||||
| R | 2.0 ± 5.1 | 4.1 ± 1.9 | 0.3 ± 0.1 | 3.5 ± 1.5 | 0.16 | 0.002 | 0.51 |
| RE | 1.1 ± 0.7 | 37.0 ± 14.0 | 0.4 ± 0.2 | 29.8 ± 13.2 | 0.16 | <0.0001 | 0.25 |
| DR | 2.0 ± 1.1 | 1.8 ± 0.6 | 1.3 ± 0.4 | 1.5 ± 0.5 | 0.017 | 0.900 | 0.26 |
| DRE | 7.3 ± 4.5 | 14.6 ± 4.0 | 3.1 ± 2.1 | 11.8 ± 5.2 | 0.005 | <0.0001 | 0.58 |
| Total R | 3.1 ± 5.2 | 41.1 ± 15.3 | 0.7 ± 0.2 | 33.3 ± 14.5 | 0.110 | <0.0001 | 0.39 |
| Total DR | 9.3 ± 5.4 | 16.4 ± 4.2 | 4.4 ± 2.4 | 13.3 ± 5.6 | 0.004 | <0.0001 | 0.51 |
| Kidney | |||||||
| R | 1.9 ± 1.0 | 5.7 ± 2.0 | 1.1 ± 0.4 | 4.6 ± 0.7 | 0.009 | <0.0001 | 0.73 |
| RE | 0.8 ± 0.7 | 4.8 ± 4.2 | 0.1 ± 0.1 | 1.9 ± 1.6 | 0.011 | 0.0001 | 0.11 |
| DR | 1.9 ± 0.5 | 0.5 ± 0.2 | 1.6 ± 0.5 | 0.4 ± 0.1 | 0.069 | <0.0001 | 0.42 |
| DRE | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0003 | 0.42 | 0.61 |
| Total R | 2.7 ± 1.6 | 10.5 ± 6.0 | 1.2 ± 0.5 | 6.5 ± 2.1 | 0.007 | <0.0001 | 0.21 |
| Total DR | 2.1 ± 0.6 | 0.7 ± 0.3 | 1.6 ± 0.5 | 0.4 ± 0.1 | 0.013 | <0.0001 | 0.42 |
| Perirenal fat pad | |||||||
| R | 0.06 ± 0.03 | 0.28 ± 0.09 | 0.06 ± 0.03 | 0.30 ± 0.05 | 0.68 | <0.0001 | 0.55 |
| RE | 0.11 ± 0.07 | 0.48 ± 0.15 | 0.06 ± 0.05 | 0.43 ± 0.07 | 0.073 | <0.0001 | 0.90 |
| DR | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.11 ± 0.05 | 0.07 ± 0.02 | 0.67 | 0.013 | 0.17 |
| DRE | ND | ND | ND | ND | NA | NA | NA |
| Total R | 0.17 ± 0.10 | 0.76 ± 0.23 | 0.12 ± 0.07 | 0.73 ± 0.12 | 0.30 | <0.0001 | 0.87 |
ND, not detectable; NA, not available.
Rats were fed 6% (NF) versus 21% (HF) fat by weight for 11 weeks and 17 (−) or 70 (+) nmol vitamin A daily for 10 weeks. Rats were administered a one time oral 700 nmol 3,4-didehydroretinyl acetate dose at the beginning of week 10. Values (n = 12 per treatment group) represent least squares means ± SD, with significant differences (P < 0.05) by factor. Retinol (R), retinyl esters (RE), 3,4-didehydroretinol (DR), and 3,4-didehydroretinyl esters (DRE), sum of R and RE (Total R), sum of DR and DRE (Total DR).
Factors include the main effects of dietary fat (F) and vitamin A (VA) and the interaction between fat and vitamin A (F × VA).
Fig. 4.
Concentrations (nmol/g) of 3,4-didehydroretinol (DR), 3,4-didehydroretinyl esters (DRE), retinol (R), and RE in liver (A) and kidney (B) in rats fed 6% (solid; except for retinyl ester = narrow downward diagonal) versus 21% fat (wide upward diagonal) 4, 5, and 7 days following a one time oral 700 nmol 3,4-didehydroretinyl acetate dose at the beginning of week 10. These rats were dosed daily with 17 nmol retinyl acetate. Different lower-case letters indicate a significant difference between 6 and 21% dietary fat group by compound by LSD (P < 0.05). n = 4 per day per dietary fat group. Values are means.
Dynamics of DR tracer
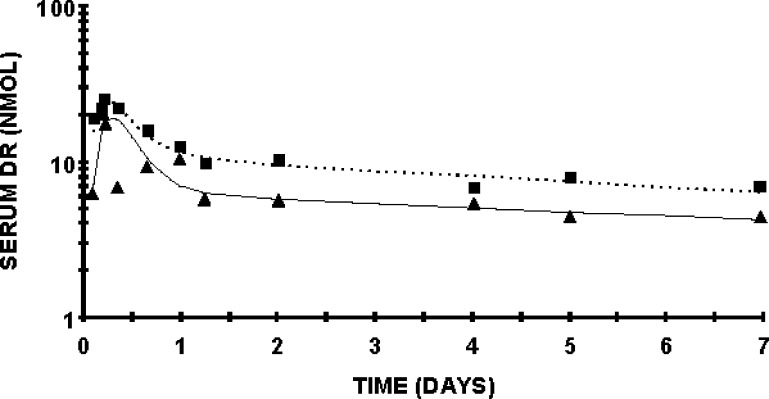
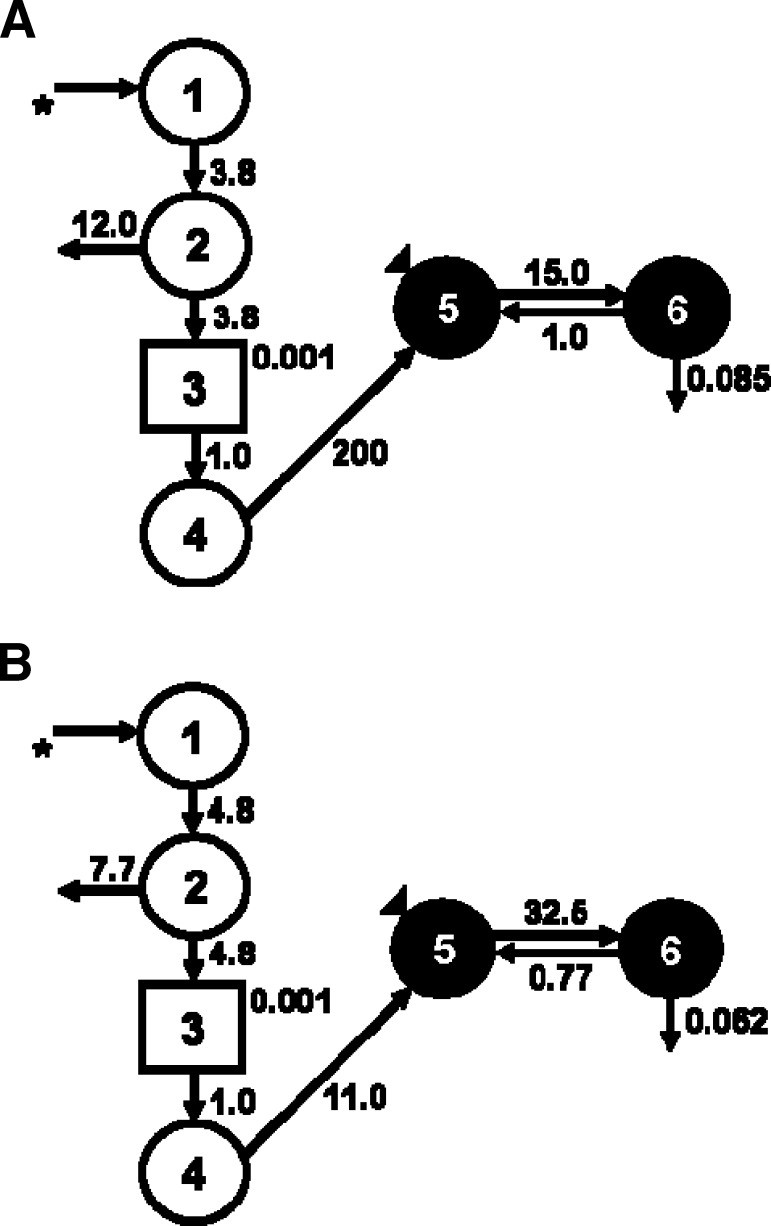
To further investigate the metabolism of recently ingested vitamin A tracer in rats fed a 6% or 21% fat diet for 11 weeks, we applied model-based compartmental analysis to the serum DR data. Because the DR was administered orally, an absorption/processing model was developed. Compartments 1–4 include a delay element representing the digestion and absorption of the dose, chylomicron processing, and hepatic processing of DR onto RBP. After the absorption model was developed, a two-compartment model was fit (Fig. 5), similar to previously published conceptual models (40, 43). The following parameters were adjusted to create a fit: the delay component, transfer from liver to plasma, transfer from plasma to extravascular spaces, and disposal rate. These were represented kinetically, respectively, by DT (3), L(5,4), L(6,5), and L(10,6). Compartment 5 represents plasma DR bound to RBP and compartment 6 is extravascular DR, primarily as store. The 17 nmol retinyl acetate group randomized to the 21% fat diet was used to develop a control model. Next, DR data from the 17 nmol retinyl acetate group randomized to the 6% fat diet were fit to the control model. The plasma-response profile shows that the handling of the DR dose differed by dietary fat treatment (Fig. 5). The FTCs reflect these differences in turnover between rats fed the 6% compared with the 21% fat diet (Fig. 6). L(6,5) is 15 pools/day in the 21% fat group and 32 pools/day in the 6% fat group.
Fig. 5.
Serum DR response profile of rats fed 6 or 21% fat diet dosed daily with 17 nmol retinyl acetate. The dashed line represents the model fit to the 21% fat data. The square represents the actual plasma DR. The solid line represents the model fit to the 6% fat data. The triangles represent the actual plasma DR for that group. For this analysis, the initial conditions were set to 700 nmol DR administered. Total plasma DR in nmol is on the Y axis. n = 4 per time point per dietary fat group, except n = 3 on days 1 and 7 in the 6% dietary fat group. Values are geometric means.
Fig. 6.
Proposed compartmental model for DR turnover in male Sprague Dawley rats dosed with 17 nmol retinyl acetate daily. A: 21% dietary fat group, B: 6% dietary fat group. Circles represent compartments and arrows represent adjustable model parameters (time invariant fractional transfer coefficients [L(I,J)] or the fraction of compartment J transferred to compartment I per day). Compartments 5 and 6 represent, respectively, plasma and extravascular DR pools. Compartments 1–4 include a delay element (rectangle) representing the digestion and absorption of the dose, chylomicron processing, and hepatic processing of DR onto retinol-binding protein (RBP). The asterisk denotes the oral delivery of the 700 nmol 3,4-didehydroretinyl acetate dose. The triangle denotes the sampling site.
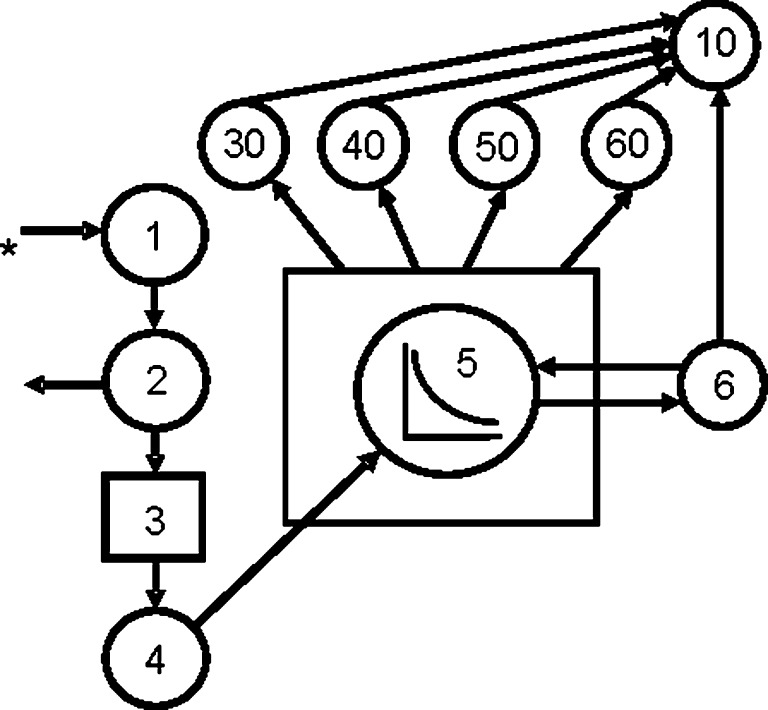
Next, we incorporated plasma DR distribution to extrahepatic tissues (kidney, lung, and epididymal plus perirenal fat pads) into a multitissue model (Fig. 7) by developing a plasma forcing function (44). Extrahepatic DR (nmol) at 4, 5, and 7 days post DR dose was fit (Fig. 8) to the plasma forcing function and plasma distribution FTCs were generated (Table 3). The FTC describing movement of DR from plasma to kidneys [L(30,5)] is close to twice as high in the 6% compared with the 21% dietary fat rats. Similarly, the fraction of the DR pool moving from plasma to either epididymal or perirenal fat pads per day was increased in the 6% relative to the 21% dietary fat group. Because the plasma forcing function was not released as done previously (45), the reported L(10,30) is equal to L(10,30) + L(5,30).
Fig. 7.
Proposed model for DR metabolism in rats fed 6% or 21% dietary fat and dosed daily with 17 nmol retinyl acetate. Compartments are represented as circles. Interconnectivities between compartments correspond to fractional transfer coefficients or L(I,J)s (Table 3). The asterisk denotes the oral delivery of the 700 nmol 3,4-didehydroretinyl acetate dose. The plasma kinetics forcing function is represented by the box. The circle within the box indicates that the plasma curve is being fit. The curve indicates the shape of the plasma kinetics being used to define the forcing function. The rate constants that depend upon the forcing function originate from the box. In this model, DR flows to extrahepatic tissues, which are listed accordingly: 30 = kidney, 40 = lung, 50 = epididymal fat, and 60 = perirenal fat.
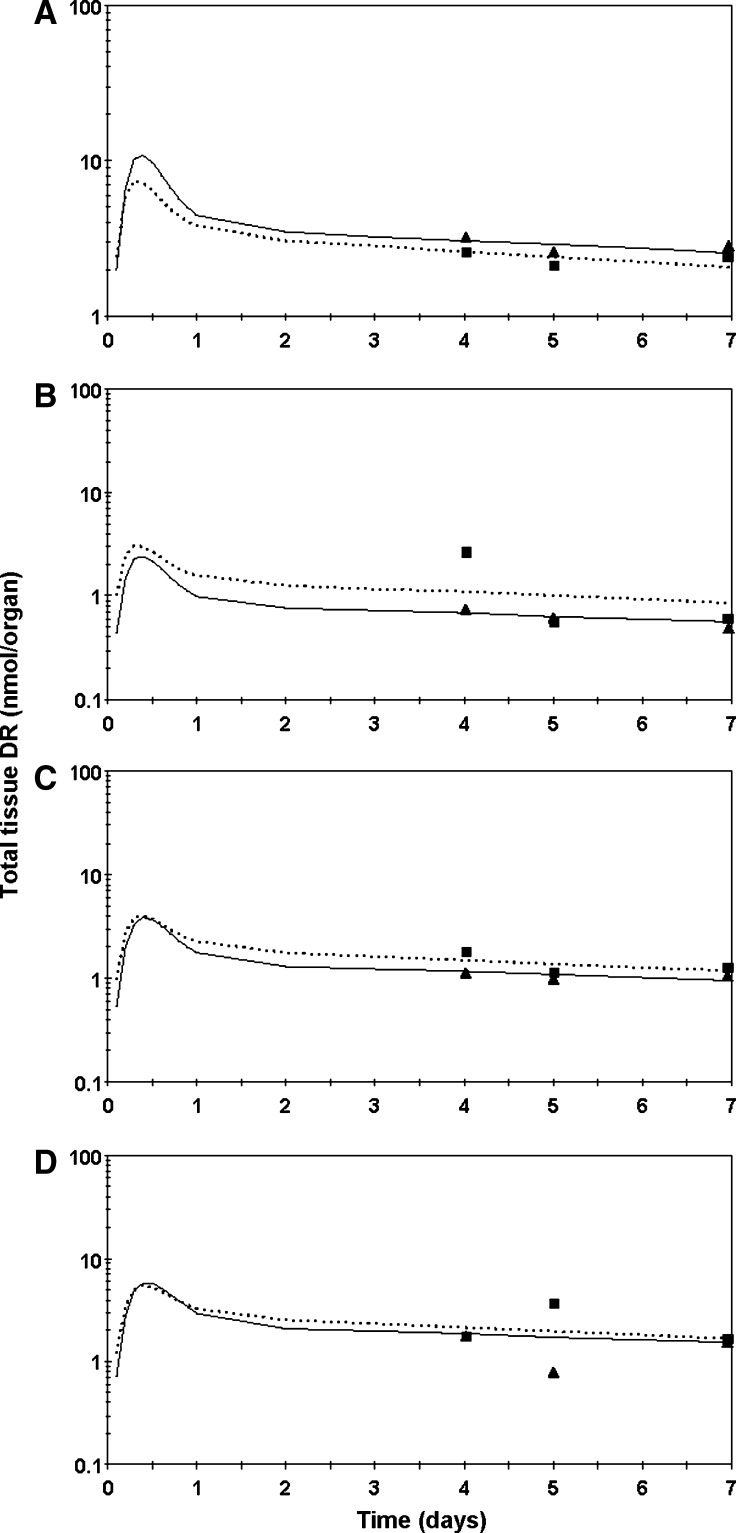
Fig. 8.
Semilog plot of mean observed and model-predicted total tissue DR in rats dosed daily with 17 nmol retinyl acetate kidneys (A), lungs (B), epididymal fat pads (C), and perirenal fat pads (D). Triangles represent observed data for the 6% fat treatment (mean of 4 rats per day); solid line represents the model-predicted curve. Squares represent observed data for the 21% fat treatment (mean of 4 rats per day; except perirenal adipose day 7, n = 3); dashed line represents the model-predicted curve.
TABLE 3.
Kinetic parameters for extrahepatic tissues
| L(I,J) d−1 |
||
|---|---|---|
| (I,J) | 6% | 21% |
| (30,5) | 9.013 | 4.776 |
| (10,30) | 15.000 | 14.990 |
| (40,5) | 2.000 | 2.000 |
| (10,40) | 15.000 | 15.000 |
| (50,5) | 2.100 | 1.703 |
| (10,50) | 9.291 | 9.291 |
| (60,5) | 2.644 | 1.910 |
| (10,60) | 7.333 | 7.333 |
Kinetic parameters for extrahepatic tissues in rats fed 6% versus 21% fat by weight for 77 days and 17 nmol vitamin A daily for 56 days. An oral dose of 3,4-didehydroretinol was administered on day 71. Time invariant fractional transfer coefficients [L(I,J)] or the fraction of compartment J transferred to compartment I per day. Compartments 10, 30, 40, 50, and 60 represent respectively irreversible loss, kidneys, lungs, and epididymal plus perirenal fat pads from n = 12 animals per dietary fat group, except for perirenal adipose tissue in 6% dietary fat fed rats n = 11.
DISCUSSION
All rats were vitamin A deficient despite one of the groups receiving >50 nmol/day, a level estimated to keep rats (∼420 g) in vitamin A balance (46). Given that rats with low liver vitamin A stores (3.5 nmol R/liver) accumulate hepatic RBP (47) and that these rats had very low liver vitamin A, they presumably accumulated a pool of hepatic apo-RBP. Tracer predominantly entered the liver for storage via chylomicron remnants (48). The DR dose resulted in a surge of plasma DR, evidence of an accumulated hepatic apo-RBP pool, that transiently increased total plasma R and DR to levels typically seen in vitamin A-sufficient rats (43). During the time the DR bolus was released on RBP, dietary vitamin A was withheld. Rats dosed with 17 nmol vitamin A/day had low hepatic RE and had already decreased their plasma R pool prior to the DR dose. They could not maintain a normal plasma R concentration because the pool that maintains plasma R was nearly depleted (49). Dietary fat did not affect total plasma R on day 7. The hepatic RE pool (data not shown) was significantly greater at 4 and 5 versus 7 days in 6% fat fed rats dosed with 17 nmol vitamin A daily, whereas in rats fed 21% fat this pool was static. Taken together, these data may indicate that some other mechanism, including a tissue besides liver, maintained the plasma R pool in the rats on the 21% fat diet because the liver RE pool was not significantly depleting between days 4, 5, and 7.
Unlike liver in the vitamin A-deficient rats, kidney R and RE concentrations were elevated in the 6% compared with 21% fat groups (Table 2 and Fig. 4B). These data suggest that kidney storage of vitamin A is more sensitive to dietary fat level and adiposity than hepatic storage of vitamin A. When kidney R and RE were normalized to total kidney mass or 100 g BW, the fat main effect is no longer significant. Kidney masses did not differ between treatment groups. This finding suggests that per g kidney, the 6% fat fed group had more highly concentrated renal R and RE than the 21% fat fed group but the concentration was not elevated enough to achieve significance when normalized to the tissue's weight. Kidney plays a major role in maintaining plasma R homeostasis by maintaining a balance of glomerular filtration and tubular reabsorption of R in its physiological transport complex (50–52). Nagy et al. (19) reported that very few lipid droplet containing stellate cells were found in the interstitial regions of kidney cortex regardless of whether animals were fed a vitamin A-sufficient or excessive diet suggesting that in the vitamin A-deficient rats, little R, RE, and presumably DR and DRE were stored in renal stellate cell lipid droplets. Kato, Kato, and Goodman (53) reported that RBP traveled to the apical portions of the proximal convoluted tubular cells, and this represented RBP that had already undergone glomerular filtration and tubular reabsorption. This report of RBP's renal cellular location suggests that R may colocalize there. RBP staining was increased in kidneys of vitamin A-sufficient compared with deficient rats (53), which can be attributed to the depressed circulating plasma RBP in the deficient rats (54). In the present study, there was no effect of the two fat treatments on the plasma R pool size 4, 5, or 7 days post-DR dose in rats given 17 nmol/day; however, increased R was observed in kidneys of rats fed 6% compared with 21% fat. These data suggest that the rate of R's movement between plasma and the other tissues was affected.
As shown previously for total vitamin A (15, 16), increased vitamin A dosing did increase epididymal (data not shown) and perirenal (Table 2) fat pad R and RE. Perirenal adipose tissue R was elevated in 6% compared with 21% fat fed rats at 4 days, whereas R was elevated in 21% compared with 6% fat fed rats on day 5. This is probably due to the significant decrease in total perirenal adipose R between 4 and 5 days in the 6% fat group (data not shown). The fat effect on renal vitamin A and the shrinking perirenal adipose R pool size suggests that the mechanism setting whole body vitamin A tissue distribution during vitamin A deficiency is intricately involved with kidney and perirenal adipose tissue (17, 50). This supports the previous report that adipose tissue acts as a store during vitamin A restriction (16).
In order to examine the mechanisms responsible for the maintenance of plasma R during vitamin A deficiency, we investigated tissue distribution of recently ingested vitamin A as DR tracer. Based on our tissue vitamin A findings, we hypothesized that kidney vitamin A tracer level alone would be affected by fat treatment. Similar to what was observed for renal R and RE, renal DRE was elevated in the 6% compared with the 21% fat group but not DR. Both hepatic DR and DRE, however, were also elevated in the 6% compared with the 21% fat group. In these same rats dosed daily with 17 nmol vitamin A (6% fat), there was a significant decrease in renal and hepatic DR between days 5 and 7 (Fig. 4). A similar pattern was found for renal R, RE, and hepatic RE. This provides evidence that DR can replace R because they decreased in parallel; thus, the rats had two vitamin A compounds to utilize. Rats on the 6% fat diet drew on liver DR, DRE, and RE to maintain plasma concentrations and to meet extrahepatic vitamin A tissue needs. Consistent with this, rats fed 6% fat maintained perirenal adipose DR pool size (Fig. 8D), whereas in rats fed 21% fat, the perirenal adipose DR pool was significantly larger on day 5 compared with day 4 or 7. DRE were not detected in either adipose depot suggesting that DR cannot be esterified in these tissues. However, in vitamin A-sufficient rats, 75% of the R is unesterified in adipose depots (55). The enzymatic machinery may not be activated to esterify DR in adipose tissue under the vitamin A deficiency observed in these rats. Hepatic and not intestinal lecithin:R acyltransferase, for example, was shown to be inactive in vitamin A-depleted rats (56).
Because plasma vitamin A kinetics are directly related to vitamin A status (43), we compared the kinetic parameters of rats dosed daily with 17 nmol vitamin A fed 6% versus 21% fat. We wanted to determine whether the increased renal DR level in the 6% fat group would be reflected in parameters of DR turnover and distribution. The FTC describing the movement of DR from plasma to extravascular stores [L(6,5)] (Fig. 6) in the 6% fat group was twice that of the 21% fat group, reflecting the more severe vitamin A deficiency in this group. The L(6,5) for rats on 21% fat was comparable to rats with similar liver vitamin A stores dosed with tritiated R and fed 17% dietary fat (40, 57). Thus, the kinetics of DR were similar to those of tritiated R in rats with comparable fat intake and deficient vitamin A status.
Consistent with the difference in L(6,5), the FTC representing DR distribution from plasma to kidney [L(30,5)] in the 6% fat group was twice that of the 21% fat group (Table 3). In support of 6% fat fed rats being in more severe negative vitamin A balance, the FTC representing DR distribution from plasma to perirenal adipose tissue was higher in 6% compared with 21% fat fed rats. Thus, in this 11-week feeding trial in rats, 21% fat promoted DR's distribution to adipose, while 6% fat decreased DR plasma turnover time (58). By day 7 post-DR dose, both dietary groups dosed with 17 nmol retinyl acetate daily had ∼60 nmol total hepatic DR, suggesting that by day 7 both groups had similar liver DR stores to draw on even though the kinetics in these two groups were different. While the differences in tissue R and tracer accumulation by level of dietary fat were presented, the mechanism by which these impacted the extent of negative vitamin A balance remains unclear. Increased renal recycling may explain the kinetic parameters described. The increased body adiposity in the 21% dietary fat group altered the rats' metabolism of the vitamin. Thus, the nearly 50% difference in the mass of the fat pads between the two fat treatments may explain the lower FTC describing DR movement from plasma to kidney for the 21% fat group.
DR is routinely used to assess vitamin A status in human populations around the world (22–30, 32); therefore, it is important to demonstrate its similarity to R in animal models. The kinetic differences by level of dietary fat observed in the present study indicate that vitamin A requirements may be affected in lean versus obese individuals and should be explored in future studies. Specifically, vitamin A utilization rates in lean versus obese individuals should be determined to describe potential differences in human vitamin A requirements. Given increasing evidence that some fat-soluble compounds are distributed differently between adipose and circulation in lean versus obese individuals (1–4), further research is needed to interpret these biomarkers.
Supplementary Material
Acknowledgments
We thank Peter Crump for statistical analyses and Chris Davis for assistance in caring for the rats.
Abbreviations
DR, 3,4-didehydroretinol
DRE, 3,4-didehydroretinyl esters
FTC, fractional transfer coefficient
R, retinol
RBP, retinol-binding protein
RE, retinyl esters
Published, JLR Papers in Press, November 30, 2008.
Footnotes
Supported by the International Life Sciences Institute Alex Malaspina Future Leader Award (SAT), NIHNIDDK 61973, and USDA NRI 2007-35200-17729. Part of this work was presented at the Experimental Biology Meeting in 2007, Washington, DC, by A.L.E.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
References
- 1.Pereira, S., C. Saboya, G. Chaves, and A. Ramalho. 2008. Class III obesity and its relationship with the nutritional status of vitamin A in pre- and postoperative gastric bypass. Obes. Surg. In press. [DOI] [PubMed]
- 2.Villaca Chaves G., S. E. Pereira, C. J. Saboya, and A. Ramalho. 2008. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin A in individuals with class III obesity. Obes. Surg. 18 378–385. [DOI] [PubMed] [Google Scholar]
- 3.Vilarrasa N., J. Maravall, A. Estepa, R. Sanchez, C. Masdevall, M. A. Navarro, P. Alia, J. Soler, and J. M. Gomez. 2007. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J. Endocrinol. Invest. 30 653–658. [DOI] [PubMed] [Google Scholar]
- 4.Botella-Carretero J. I., F. Alvarez-Blasco, J. J. Villafruela, J. A. Balsa, C. Vazquez, and H. F. Escobar-Morreale. 2007. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin. Nutr. 26 573–580. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q., T. E. Graham, N. Mody, F. Preitner, O. D. Peroni, J. M. Zabolotny, K. Kotani, L. Quadro, and B. B. Kahn. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 436 356–362. [DOI] [PubMed] [Google Scholar]
- 6.Mills J. P., H. C. Furr, and S. A. Tanumihardjo. 2008. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp. Biol. Med. 233 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messerli F. H., H. O. Ventura, E. D. Reisin, G. R. Dreslinski, F. G. Dunn, and E. D. Frohlich. 1982. Obesity and essential hypertension. Contrib. Nephrol. 30 116–123. [DOI] [PubMed] [Google Scholar]
- 8.Dagadu J. M. 1967. Distribution of carotene and vitamin A in liver, pancreas and body fat of Ghanaians. Br. J. Nutr. 21 453–456. [DOI] [PubMed] [Google Scholar]
- 9.Ernst B., M. Thurnheer, S. M. Schmid, and B. Schultes. 2009. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes. Surg. 19 66–73. [DOI] [PubMed] [Google Scholar]
- 10.Roodenburg A. J., R. Leenen, K. H. van het Hof, J. A. Weststrate, and L. B. Tijburg. 2000. Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. Am. J. Clin. Nutr. 71 1187–1193. [DOI] [PubMed] [Google Scholar]
- 11.Pelletier C., J. P. Despres, and A. Tremblay. 2002. Plasma organochlorine concentrations in endurance athletes and obese individuals. Med. Sci. Sports Exerc. 34 1971–1975. [DOI] [PubMed] [Google Scholar]
- 12.Messerli F. H. 1982. Cardiovascular effects of obesity and hypertension. Lancet. 1 1165–1168. [DOI] [PubMed] [Google Scholar]
- 13.Lavie C., and F. Messerli. 1986. Cardiovascular adaptation to obesity and hypertension. Chest. 90 275–279. [DOI] [PubMed] [Google Scholar]
- 14.Goodman D. W., H. S. Huang, and T. Shiratori. 1965. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J. Lipid Res. 6 390–396. [PubMed] [Google Scholar]
- 15.Blaner W. S., J. C. Obunike, S. B. Kurlandsky, M. al-Haideri, R. Piantedosi, R. J. Deckelbaum, and I. J. Goldberg. 1994. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J. Biol. Chem. 269 16559–16565. [PubMed] [Google Scholar]
- 16.O'Byrne S. M., N. Wongsiriroj, J. Libien, S. Vogel, I. J. Goldberg, W. Baehr, K. Palczewski, and W. S. Blaner. 2005. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 280 35647–35657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green M. H., L. Uhl, and J. B. Green. 1985. A multicompartmental model of vitamin A kinetics in rats with marginal liver vitamin A stores. J. Lipid Res. 26 806–818. [PubMed] [Google Scholar]
- 18.Moriwaki H., W. S. Blaner, R. Piantedosi, and D. S. Goodman. 1988. Effects of dietary retinoid and triglyceride on the lipid composition of rat liver stellate cells and stellate cell lipid droplets. J. Lipid Res. 29 1523–1534. [PubMed] [Google Scholar]
- 19.Nagy N. E., K. B. Holven, N. Roos, H. Senoo, N. Kojima, K. R. Norum, and R. Blomhoff. 1997. Storage of vitamin A in extrahepatic stellate cells in normal rats. J. Lipid Res. 38 645–658. [PubMed] [Google Scholar]
- 20.Tanumihardjo S. A., A. B. Barua, and J. A. Olson. 1987. Use of 3,4-didehydroretinol to assess vitamin A status in rats. Int. J. Vitam. Nutr. Res. 57 127–132. [PubMed] [Google Scholar]
- 21.Tanumihardjo S. A., and J. A. Olson. 1988. A modified relative dose-response assay employing 3,4-didehydroretinol (vitamin A2) in rats. J. Nutr. 118 598–603. [DOI] [PubMed] [Google Scholar]
- 22.Tanumihardjo S. A., Muhilal, Y. Yuniar, D. Permaesih, Z. Sulaiman, D. Karyadi, and J. A. Olson. 1990. Vitamin A status in preschool-age Indonesian children as assessed by the modified relative-dose-response assay. Am. J. Clin. Nutr. 52 1068–1072. [DOI] [PubMed] [Google Scholar]
- 23.Tanumihardjo S. A., P. G. Koellner, and J. A. Olson. 1990. The modified relative-dose-response assay as an indicator of vitamin A status in a population of well-nourished American children. Am. J. Clin. Nutr. 52 1064–1067. [DOI] [PubMed] [Google Scholar]
- 24.Tanumihardjo S. A., H. C. Furr, J. W. Erdman, Jr., and J. A. Olson. 1990. Use of the modified relative dose response (MRDR) assay in rats and its application to humans for the measurement of vitamin A status. Eur. J. Clin. Nutr. 44 219–224. [PubMed] [Google Scholar]
- 25.Tanumihardjo S. A., and J. A. Olson. 1991. The reproducibility of the modified relative dose response (MRDR) assay in healthy individuals over time and its comparison with conjunctival impression cytology (CIC). Eur. J. Clin. Nutr. 45 407–411. [PubMed] [Google Scholar]
- 26.Tanumihardjo S. A., Muherdiyantiningsih, D. Permaesih, A. M. Dahro, Muhilal, D. Karyadi, and J. A. Olson. 1994. Assessment of the vitamin A status in lactating and nonlactating, nonpregnant Indonesian women by use of the modified-relative-dose-response (MRDR) test. Am. J. Clin. Nutr. 60 142–147. [DOI] [PubMed] [Google Scholar]
- 27.Tanumihardjo S. A., D. Permaesih, A. M. Dahro, E. Rustan, Muhilal, D. Karyadi, and J. A. Olson. 1994. Comparison of vitamin A status assessment techniques in children from two Indonesian villages. Am. J. Clin. Nutr. 60 136–141. [DOI] [PubMed] [Google Scholar]
- 28.Tanumihardjo S. A., D. Suharno, D. Permaesih, Muherdiyantiningsih, A. M. Dahro, Muhilal, D. Karyadi, and J. A. Olson. 1995. Application of the modified relative dose response test to pregnant Indonesian women for assessing vitamin A status. Eur. J. Clin. Nutr. 49 897–903. [PubMed] [Google Scholar]
- 29.Tanumihardjo S. A., J. C. Cheng, D. Permaesih, Muherdiyantiningsih, E. Rustan, Muhilal, D. Karyadi, and J. A. Olson. 1996. Refinement of the modified-relative-dose-response test as a method for assessing vitamin A status in a field setting: experience with Indonesian children. Am. J. Clin. Nutr. 64 966–971. [DOI] [PubMed] [Google Scholar]
- 30.Tanumihardjo S. A., D. Permaesih, Muherdiyantiningsih, E. Rustan, K. Rusmil, A. C. Fatah, S. Wilbur, Muhilal, D. Karyadi, and J. A. Olson. 1996. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J. Nutr. 126 451–457. [DOI] [PubMed] [Google Scholar]
- 31.Valentine A. R., and S. A. Tanumihardjo. 2004. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J. Nutr. 134 1186–1192. [DOI] [PubMed] [Google Scholar]
- 32.van Jaarsveld P. J., M. Faber, S. A. Tanumihardjo, P. Nestel, C. J. Lombard, and A. J. Benade. 2005. Beta-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am. J. Clin. Nutr. 81 1080–1087. [DOI] [PubMed] [Google Scholar]
- 33.Surles R. L., J. Li, and S. A. Tanumihardjo. 2006. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J. Nutr. 136 939–945. [DOI] [PubMed] [Google Scholar]
- 34.Molldrem K. L., and S. A. Tanumihardjo. 2004. Lutein supplements are not bioavailable in the Mongolian gerbil while consuming a diet with or without cranberries. Int. J. Vitam. Nutr. Res. 74 153–160. [DOI] [PubMed] [Google Scholar]
- 35.Surles R. L., J. P. Mills, A. R. Valentine, and S. A. Tanumihardjo. 2007. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent vitamin A deficiency. Am. J. Clin. Nutr. 86 1045–1053. [DOI] [PubMed] [Google Scholar]
- 36.Folch J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- 37.Wang L. 1959. Plasma volume, cell volume, total blood volume and F cells factor in the normal and splenectomized Sherman rat. Am. J. Physiol. 196 188–192. [DOI] [PubMed] [Google Scholar]
- 38.Green M. H., and J. B. Green. 1990. The application of compartmental analysis to research in nutrition. Annu. Rev. Nutr. 10 41–61. [DOI] [PubMed] [Google Scholar]
- 39.von Reinersdorff D., M. H. Green, and J. B. Green. 1998. Development of a compartmental model describing the dynamics of vitamin A metabolism in men. Adv. Exp. Med. Biol. 445 207–223. [DOI] [PubMed] [Google Scholar]
- 40.Gieng S. H., M. H. Green, J. B. Green, and F. J. Rosales. 2007. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 48 904–913. [DOI] [PubMed] [Google Scholar]
- 41.Green, M. H., S. H. Gieng, F. J. Rosales, and J. B. Green. 2007. Mathematical Modeling in Nutrition and Agriculture. Virginia Tech, Blacksburg, VA.
- 42.Landaw E. M., and J. J. DiStefano 3rd. 1984. Multiexponential, multicompartmental, and noncompartmental modeling. II. Data analysis and statistical considerations. Am. J. Physiol. 246 R665–R677. [DOI] [PubMed] [Google Scholar]
- 43.Green M. H., and J. B. Green. 1994. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J. Nutr. 124 2477–2485. [DOI] [PubMed] [Google Scholar]
- 44.Robertson, J. S. 1983. Compartmental distribution of radiotracers. CRC Press, Inc., Boca Raton, FL.
- 45.Cifelli C. J., J. B. Green, and M. H. Green. 2005. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J. Nutr. 135 746–752. [DOI] [PubMed] [Google Scholar]
- 46.Green M. H., J. B. Green, and K. C. Lewis. 1987. Variation in retinol utilization rate with vitamin A status in the rat. J. Nutr. 117 694–703. [DOI] [PubMed] [Google Scholar]
- 47.Muto Y., J. E. Smith, P. O. Milch, and D. S. Goodman. 1972. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J. Biol. Chem. 247 2542–2550. [PubMed] [Google Scholar]
- 48.Blomhoff R., M. H. Green, J. B. Green, T. Berg, and K. R. Norum. 1991. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol. Rev. 71 951–990. [DOI] [PubMed] [Google Scholar]
- 49.Green M. H., J. B. Green, T. Berg, K. R. Norum, and R. Blomhoff. 1993. Vitamin A metabolism in rat liver: a kinetic model. Am. J. Physiol. 264 G509–G521. [DOI] [PubMed] [Google Scholar]
- 50.Gerlach T. H., and M. H. Zile. 1990. Upregulation of serum retinol in experimental acute renal failure. FASEB J. 4 2511–2517. [DOI] [PubMed] [Google Scholar]
- 51.Smith F. R., and D. S. Goodman. 1971. The effects of diseases of the liver, thyroid, and kidneys on the transport of vitamin A in human plasma. J. Clin. Invest. 50 2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vahlquist A., P. A. Peterson, and L. Wibell. 1973. Metabolism of the vitamin A transporting protein complex. I. Turnover studies in normal persons and in patients with chronic renal failure. Eur. J. Clin. Invest. 3 352–362. [DOI] [PubMed] [Google Scholar]
- 53.Kato M., K. Kato, and D. Goodman. 1984. Immunocytochemical studies on the localization of plasma and of cellular retinol-binding proteins and of transthyretin (prealbumin) in rat liver and kidney. J. Cell Biol. 98 1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith J. E., Y. Muto, and D. S. Goodman. 1975. Tissue distribution and subcellular localization of retinol-binding protein in normal and vitamin A-deficient rats. J. Lipid Res. 16 318–323. [PubMed] [Google Scholar]
- 55.Tsutsumi C., M. Okuno, L. Tannous, R. Piantedosi, M. Allan, D. S. Goodman, and W. S. Blaner. 1992. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 267 1805–1810. [PubMed] [Google Scholar]
- 56.Randolph R. K., and A. C. Ross. 1991. Vitamin A status regulates hepatic lecithin: retinol acyltransferase activity in rats. J. Biol. Chem. 266 16453–16457. [PubMed] [Google Scholar]
- 57.Reeves P. G., F. H. Nielsen, and G. C. Fahey, Jr. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123 1939–1951. [DOI] [PubMed] [Google Scholar]
- 58.Green M. H., and J. B. Green. 1990. Experimental and kinetic methods for studying vitamin A dynamics in vivo. Methods Enzymol. 190 304–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.