Abstract
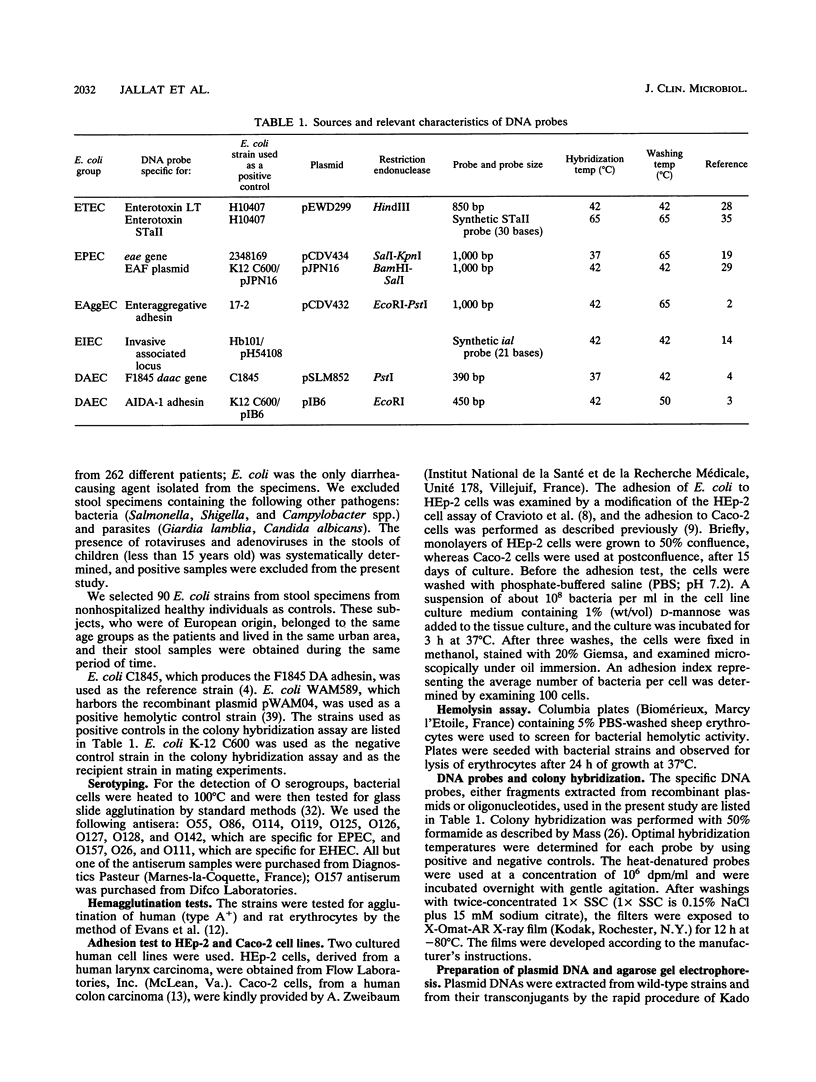
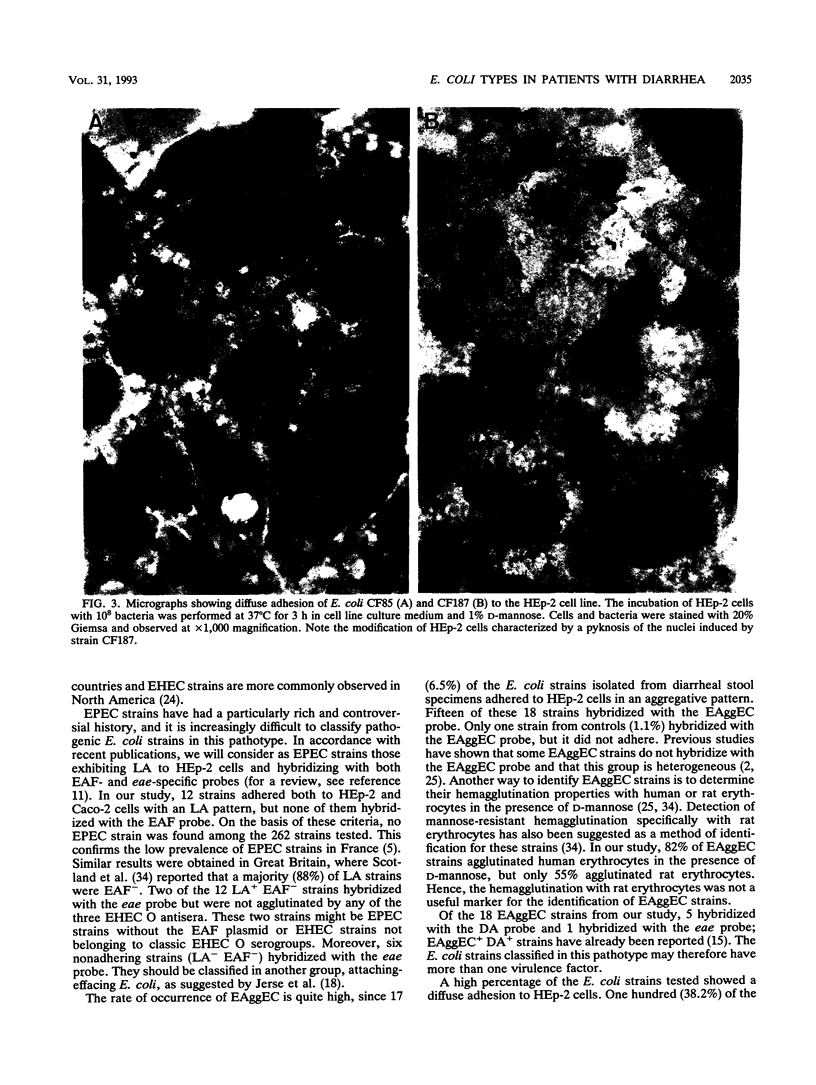
Two hundred sixty-two strains of Escherichia coli isolated from diarrheal stool specimens from infants, children, and adults hospitalized in Clermont-Ferrand, France, were studied to classify them in the previously described pathogenic groups of E. coli involved in diarrheal diseases. A total of 1.5% of them belonged to the enterotoxigenic E. coli pathotype, but none belonged to the enteroinvasive E. coli, enterohemorrhagic E. coli, or enteropathogenic E. coli pathotypes. Seventeen strains (6.5%) exhibited an aggregative pattern of adhesion to HEp-2 cells (EAggEC pathotype), but of these, three (17.6%) did not hybridize with the EAggEC DNA probe. Most of the strains involved in diarrhea belonged to the diffusely adhering E. coli group; 100 strains (38.2%) exhibited a diffuse adhesion (DA) to HEp-2 cells. Only eight strains (8.9%) from controls diffusely adhered to HEp-2 cells. The highly significant difference (P < 0.0001) between DA strains from patients and from controls suggests that the diffusely adhering E. coli strains should be considered pathogens. Only 33 of them (33%) hybridized with the previously described DA DNA probe, and only 2 (2%) hybridized with the AIDA DNA probe. Four different major proteins were observed in the bacterial surface extracts of the 33 strains positive with the DA DNA probe. In addition, 16 strains that diffusely adhered to HEp-2 cells induced a cytotoxic effect on HEp-2 cells that was characterized by pyknosis and lysis of the cytoplasmic membrane. This cytotoxic effect was correlated with the synthesis of a hemolysin. The genes involved in diffuse adhesion to HEp-2 cells were located on conjugative R plasmids in strains that did not hybridize with the DA or AIDA DNA probes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Nataro J. P., Kaper J. B. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986 Apr;52(1):334–336. doi: 10.1128/iai.52.1.334-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry B., Savarino S. J., Vial P., Kaper J. B., Levine M. M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990 Jun;161(6):1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- Benz I., Schmidt M. A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989 May;57(5):1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert M. G., d'Hauteville H. M., Sansonetti P. J. Detection of enteric pathotypes of Escherichia coli by hybridization using six DNA probes. Ann Inst Pasteur Microbiol. 1988 Mar-Apr;139(2):189–202. [PubMed] [Google Scholar]
- Colonna B., Nicoletti M., Visca P., Casalino M., Valenti P., Maimone F. Composite IS1 elements encoding hydroxamate-mediated iron uptake in FIme plasmids from epidemic Salmonella spp. J Bacteriol. 1985 Apr;162(1):307–316. doi: 10.1128/jb.162.1.307-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna B., Ranucci L., Fradiani P. A., Casalino M., Calconi A., Nicoletti M. Organization of aerobactin, hemolysin, and antibacterial resistance genes in lactose-negative Escherichia coli strains of serotype O4 isolated from children with diarrhea. Infect Immun. 1992 Dec;60(12):5224–5231. doi: 10.1128/iai.60.12.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Frankel G., Riley L., Giron J. A., Valmassoi J., Friedmann A., Strockbine N., Falkow S., Schoolnik G. K. Detection of Shigella in feces using DNA amplification. J Infect Dis. 1990 Jun;161(6):1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- Girón J. A., Jones T., Millán-Velasco F., Castro-Muñoz E., Zárate L., Fry J., Frankel G., Moseley S. L., Baudry B., Kaper J. B. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991 Mar;163(3):507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- Gomes T. A., Blake P. A., Trabulsi L. R. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J Clin Microbiol. 1989 Feb;27(2):266–269. doi: 10.1128/jcm.27.2.266-269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Gicquelais K. G., Kaper J. B. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect Immun. 1991 Nov;59(11):3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988 Mar;56(3):640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesage D. D., Gerbaud G. R., Chabbert Y. A. Carte génétique et strucutre chez Escherichia coli K12 d'un plasmide de résistance isolé de Salmonella ordonez. Ann Microbiol (Paris) 1975 May-Jun;126A(4):435–448. [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Prado V., Robins-Browne R., Lior H., Kaper J. B., Moseley S. L., Gicquelais K., Nataro J. P., Vial P., Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988 Jul;158(1):224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Oberhelman R. A., Dupont H. L., Javier de la Cabada F., Garibay E. V. Enteroadherent Escherichia coli as a cause of diarrhea among children in Mexico. J Clin Microbiol. 1987 Oct;25(10):1917–1919. doi: 10.1128/jcm.25.10.1917-1919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Labigne A., Moseley S., Hull R., Hull S., Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990 Jan;58(1):279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Said B., Willshaw G. A., Cheasty T., Rowe B. Identification of enteropathogenic Escherichia coli isolated in Britain as enteroaggregative or as members of a subclass of attaching-and-effacing E. coli not hybridising with the EPEC adherence-factor probe. J Med Microbiol. 1991 Nov;35(5):278–283. doi: 10.1099/00222615-35-5-278. [DOI] [PubMed] [Google Scholar]
- Sommerfelt H., Svennerholm A. M., Kalland K. H., Haukanes B. I., Bjorvatn B. Comparative study of colony hybridization with synthetic oligonucleotide probes and enzyme-linked immunosorbent assay for identification of enterotoxigenic Escherichia coli. J Clin Microbiol. 1988 Mar;26(3):530–534. doi: 10.1128/jcm.26.3.530-534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson T. N., Bilge S. S., Nowicki B., Moseley S. L. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect Immun. 1991 Jan;59(1):261–268. doi: 10.1128/iai.59.1.261-268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Gonzalez-Carrero M. I., Sekizaki T., Rojo F. Biological activities specified by antibiotic resistance plasmids. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):1–12. doi: 10.1093/jac/18.supplement_c.1. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




