Abstract
The nuclear receptor constitutive active/androstane receptor (CAR) up-regulated expression of the apoptotic growth arrest and DNA-damage-inducible 45 β (GADD45B) gene in HepG2 cells. Overexpression of GADD45B augmented CAR-mediated induction of the human CYP2B gene by the CAR activator 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) and coactivated CAR-dependent transcription of the NR1-luciferase reporter gene. Small interfering RNA knockdown of GADD45B resulted in repression of both the induction and the coactivation. Induction of the mouse Cyp2b10 gene by TCPOBOP was profoundly attenuated in the primary hepatocytes prepared from GADD45B-knockout mice compared with those from wild-type mice. Because CAR is a key transcription factor that activates the genes that encode for xenobiotic metabolizing enzymes and transporters, GADD45B, acting as a CAR coactivator and coregulating CAR target genes, may be involved in hepatic drug metabolism and excretion of xenobiotics.
The nuclear receptor CAR is a member of the nuclear steroid/thyroid hormone receptor superfamily (Timsit and Negishi, 2007). CAR is characterized as a xenobiotic-sensing nuclear receptor that can be activated by a large number of xenobiotics, including therapeutic drugs such as phenobarbital, phenytoin, and various statins (Honkakoski et al., 1998; Jackson et al., 2004; Kobayashi et al., 2005). The activated CAR translocates from the cytoplasm to the nucleus of hepatocytes (Kawamoto et al., 1999). Once in the nucleus, the CAR forms a heterodimer with RXR and binds to the promoters of its target genes and activates their transcription. These genes include the large set of hepatic genes that encode enzymes and transporters that are involved in xenobiotic metabolism and excretion (Sueyoshi et al., 1999; Sueyoshi and Negishi, 2001). Human CYP2B6 is the classic CAR-regulated enzyme that metabolizes an ever-increasing number of therapeutic drugs (e.g., bupropion, efavirenz, ifosfamide, methadone, and meperidine) and activates the anticancer prodrug cyclophosphamide (Schwartz et al., 2003; Rodriguez-Antona and Ingelman-Sundberg, 2006; Zanger et al., 2007). Through these processes, CAR constitutes a key part of the cellular defense mechanism against xenobiotic toxicity and carcinogenicity by increasing hepatic capability to detoxify and excrete xenobiotics. Similar to the other nuclear receptors, the CAR-RXR heterodimer requires coregulators in this activation (Muangmoonchai et al., 2001; Mäkinen et al., 2003; Ueda et al., 2005). A number of coregulators have been demonstrated to be CAR coactivators or corepressors; for example, GRIP1, SRC1, PGC-1α, PBP, FoxO1, SMRT, and N-CoR (Min et al., 2002, 2005; Ueda et al., 2002; Kodama et al., 2004; Shiraki et al., 2003; Jia et al., 2005). We have now characterized GADD45B as being a novel CAR coactivator.
GADD45B, an antiapoptotic factor, is known to directly bind to MKK7 and inhibit MKK7-dependent phosphorylation of JNK, thereby repressing JNK-mediated apoptosis (Papa et al., 2004). Also, the Gadd45b gene is one of the CAR-regulated genes that is induced by drugs such as PB and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (Columbano et al., 2005; manuscript submitted for publication). In addition, we have shown that CAR directly binds to the GADD45B protein, increasing the ability of GADD45B to inhibit MKK7 activity (manuscript submitted for publication). Consistent with the CAR-dependent inhibition of MKK7 activity, treatment with TCPOBOP attenuates JNK-mediated apoptosis in wild-type mouse primary hepatocytes but not in GADD45B-KO primary hepatocytes. Thus, protein-protein interactions of CAR with GADD45B confer upon CAR the ability to determine GADD45B activity. Given their interactions, we examine here whether or not GADD45B can regulate the trans-activation activity of CAR. To this end, we performed real-time PCR and cell-based transient transfection assays to examine coactivation activity of GADD45B, employed siRNA to knockdown GADD45B, and used primary hepatocytes of GADD45B-KO mice to determine the role of GADD45B in CAR-mediated gene induction. The experimental observations obtained are consistent with the conclusion that GADD45B coactivates CAR-mediated transcription and is critically involved in drug induction of both human and mouse CYP2B genes.
Materials and Methods
Materials
TCPOBOP was purchased from Sigma-Aldrich (St. Louis, MO), androstenol was purchased from Steraloids (Newport, RI), Superscript First-Strand Synthesis System were from Invitrogen (Carlsbad, CA), fetal bovine serum was from Atlanta Biologicals (Lawrenceville, GA), and the Complete miniprotease inhibitor cocktail tablet was from Roche Applied Science (Indianapolis, IN). All other reagents were purchased from Sigma-Aldrich, unless indicated otherwise.
Plasmids
Using proper sets of primers, the full length of mouse GADD45B was amplified from the mouse liver cDNA library using LA taq DNA polymerase (Takara, Shiga, Japan) and was cloned into pcDNA3.1-TOPO plasmid, designated pcDNA3.1-GADD45B. The sequences of insert were verified by DNA sequencing. pGL3-tk-(NR1)x5-luciferase (Luc) was previously constructed (Kawamoto et al., 1999), and pcDNA3.1 and phRL-TK were purchased from Invitrogen and Promega (Madison, WI), respectively.
Animals
GADD45B-KO and the corresponding C57BL/6 mice, provided by Dr. Binfeng Lu at University of Pittsburgh, were housed in a temperature-controlled environment with 12-h light/dark cycles with access to standard chow and water ad libitum under the protocols and procedures approved by the National Institutes of Health Animal Care and Use Committee in accordance with National Institutes of Health guidelines.
Cell Culture
Ym17 cells, a stable cell line that expresses mCAR tagged with V5-His, were established from HepG2 cells (Swales et al., 2005). HepG2 and Ym17 cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). Cells in a 24-well plate were transfected with a pGL3-TK-NR1-luciferase reporter plasmid (0.2 μg/well) and phRL-TK plasmid (0.05 μg/well) by LipofectAMINE 2000 (Invitrogen) according to the manufacturer's instruction, with an additional cotransfection of 0.2 μg/well pcDNA3.1-GADD45B or pcDNA3.1 empty plasmids. After 16 h, the cells were subjected to treatment with chemicals: DMSO, 250 nM TCPOBOP, or 10 μM androstenol for an additional 24 h. Luciferase activity was measured using the dual-luciferase reporter assay system.
siRNA Transfection
GADD45B SMARTpool siRNAs were obtained from Dharmacon RNAi Technologies (Lafayette, CO). Liposome solutions of 100 pmol GADD45B siRNA or control siRNA (siCONTROL NonTargeting siRNA no. 1) were mixed with a separately prepared liposome solution with the pGL3-TK-NR1-luciferase reporter and phRL-TK plasmids. Subsequently, these liposome mixtures were transfected into Ym17 cells for LUC reporter assays. After 24 h, the cells were treated with chemicals. Reduction of GADD45B expression was confirmed by real-time PCR.
Mouse Primary Hepatocytes
Mouse primary hepatocytes were prepared from male mice by a two-step collagenase perfusion method and cultured as previously described (Honkakoski and Negishi, 1997). Hepatocytes were suspended in Williams' medium E supplemented with 7% fetal bovine serum, 1× liquid media supplement (5 μg/ml insulin, 5 μg/ml transferrin, 5 μg/ml selenite; Sigma-Aldrich), 2 mM l-glutamine, and 30 mM pyruvate and were allowed to adhere to 24-well plates (1 × 105 cells) for 1 h in a CO2 incubator at 37°C. These hepatocytes were treated with DMSO or 250 nM TCPOBOP for 16 h in Williams' medium E supplemented with 5 nM dexamethasone and 1× liquid media supplement.
Real-Time PCR
Total RNA was isolated from HepG2, Ym17, and mouse primary hepatocytes using the TRIzol (Invitrogen). cDNAs were synthesized using the Superscript First-Strand Synthesis System and random hexamer primers. Real-time PCR measurement of individual cDNAs was performed with the ABI prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). For the probes used, assay identification numbers of prede-signed TaqMan gene expression assay (gene, assay identification no., Applied Biosystems) are: human GADD45B, Hs00169587_m1; human GADD45A, Hs00169255_m1; human CYP2B6, Hs00167937_g1; mouse CYP2B10, Mm00456591_m1; mouse CAR, Mm00437986_m1; and mouse GADD45B, Mm00435123_m1. The TaqMan rodent GAPDH and human β-actin controls (Applied Biosystems) were used as internal control.
Results
CAR Regulates the GADD45B Gene
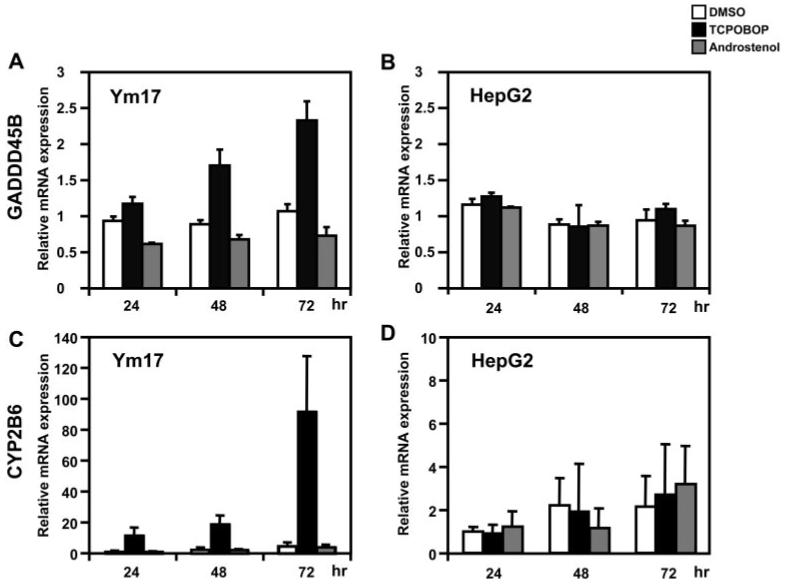
We previously characterized the GADD45B gene as being CAR-regulated in liver and primary hepatocytes using CAR-KO mice (manuscript submitted for publication). For us to use HepG2 cells as an experimental system, we first tested whether CAR can regulate the GADD45B gene in HepG2 cells. Ym17 cells are derived from HepG2 cells and stably express mouse CAR tagged with V5-His and the mCAR-V5, spontaneously present in the nucleus, can be activated by the CAR ligand TCPOBOP (Swales et al., 2005). Treatment with TCOPBOP increased the levels of GADD45B mRNA in a time-dependent manner, approximately 2.5-fold at 72 h in Ym17 cells (Fig. 1A). On the other hand, treatment with the CAR antagonist androstenol slightly decreased the mRNA levels (Fig. 1A). These increases and decreases of GADD45B mRNA were not observed in HepG2 cells (Fig. 1B), indicating the direct involvement of CAR in the activation of the GADD45B gene. The CYP2B6 gene was induced by TCPOBOP in Ym17 but not in HepG2 cells (Fig. 1, C and D).
Fig. 1.
CAR regulates expression of the Gadd45b gene. Ym17 and HepG2 cells were treated with DMSO, TCPOBOP, and androstenol. At various time points after treatments, total RNAs were prepared from these cells and were subjected to real-time PCR to measure the levels of GADD45B and CYP2B6 mRNAs. The levels of mRNA were normalized to β-actin mRNA levels and were shown relative to mRNA levels in cells treated with DMSO set at one. Error bars, SD values from at least three experiments.
GADD45B Coactivates CAR-Mediated Transcription
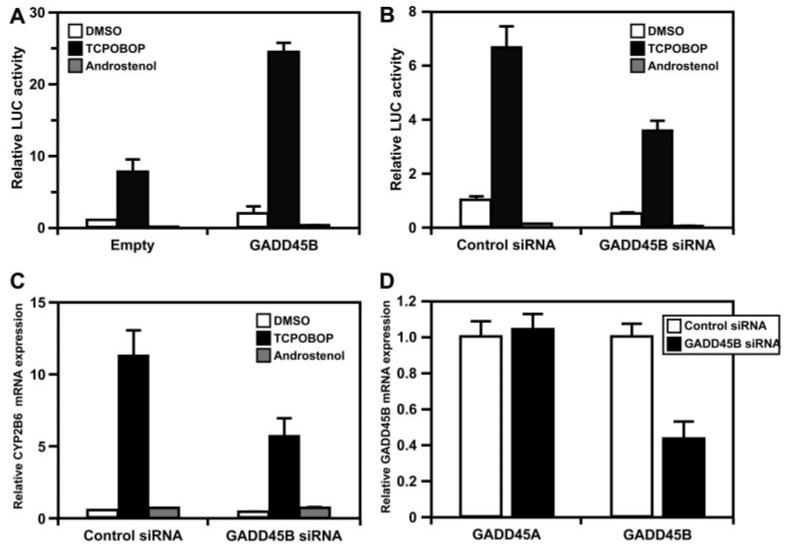
Given our previous observation that GADD45B and CAR interact (manuscript submitted for publication), we examined whether or not this affects CAR and how CAR activates enhancers. CAR activated a Luc reporter gene bearing the CAR binding site NR1 in Ym17 cells after treatment with TCPOBOP, whereas androstenol treatment repressed transcription (Fig. 2A). Subsequently, coexpression of GADD45B was found to up-regulate TCPOBOP-activated reporter activity 3- to 4-fold. To provide further evidence supporting this coactivation by GADD45B, we employed siRNA to knockdown endogenous GADD45B in Ym17 cells. In cells transfected with siRNA GADD45B, the CAR-mediated activation of NR1-Luc reporter gene and the expression of the endogenous CYP2B6 gene were attenuated approximately 50% compared with those in cells treated with control siRNA (Fig. 2, B and C). Under the conditions used, siRNA GADD45B down-regulated GADD45B mRNA approximately 60% and appeared to be specific to GADD45B because no decrease of the GADD45A mRNA occurred (Fig. 2D). These results are consistent with the conclusion that GADD45B is the coactivator of CAR.
Fig. 2.
GADD45B coactivates CAR-mediated transcription. A, Ym17 cells were cotransfected with pcDNA3.1-GADD45B and NR1-luciferase reporter plasmid and were subsequently treated with DMSO, TCPOBOP, and androstenol. These cells were homogenized to prepare cell extracts for measuring luciferase activity. The levels of Luc activity were normalized by with renilla luciferase activities. -Fold activation was calculated relative to those in cells transfected with pcDNA3.1 and treated DMSO as one. B, prior to cotransfection with pcDNA3.1-GADD45B and NR1-luciferase reporter plasmid, Ym17 cells were treated with GADD45B siRNA. Luciferase activities were measured and calculated by taking those in control siRNA- and DMSO-treated cells as one. C, total RNAs were prepared from the siRNA-treated cells and subjected to real-time PCR to measure CYP2B6 mRNA. The levels of mRNA were normalized to β-actin mRNA levels and were shown in relative to mRNA levels in cells transfected with control siRNA and treated with DMSO as one. D, using total RNAs prepared from Ym17 cells treated with control and GADD45B siRNAs, the levels of GADD45A and GADD45B mRNAs were measured by real-time PCR and were shown relative to mRNA levels in cells transfected with control siRNA and treated with DMSO set at one. Error bars, SD values from at least three experiments
GADD45B-KO Attenuates CYP2B Expression
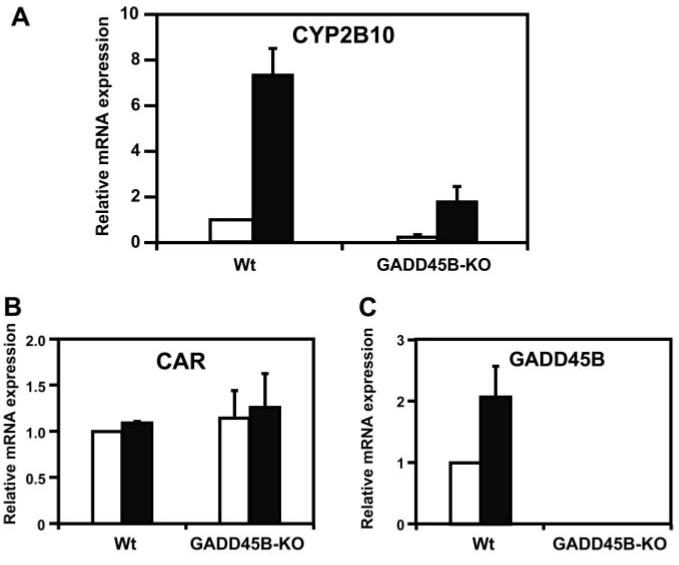
If, in fact, GADD45B regulates CAR-mediated trans-activation in hepatocytes, the expression of the CAR target gene by CAR activators such as TCPOBOP should be attenuated in hepatocytes that lack GADD45B. To this end, primary hepatocytes were prepared from wild-type and GADD45B-KO mice and were treated with TCPOBOP. The Cyp2b10 gene is the CAR-regulated CYP2B in mouse liver. TCPOBOP treatment increased the levels of CYP2B10 mRNA in wild-type primary hepatocytes approximately 7-fold. In GADD45B-KO primary hepatocytes, both basal and induced levels of CYP2B10 mRNA were sharply repressed to approximately 20% of those as observed in the wild-type primary hepatocytes (Fig. 3A). Despite the repression in GADD45B-KO primary hepatocytes, the -fold induction of CYP2B10 mRNA by TCPOBOP remained approximately 9-fold, which is similar to that observed in wild-type primary hepatocytes. These results suggest that GADD45B regulates, possibly acting as a coactivator, the maximal induction of the Cyp2b10 gene. GADD45B did not regulate the expression of the CAR gene in the primary hepatocytes; as expected (Fig. 3B). GADD45B mRNA was increased by TCPOBOP in wild-type primary hepatocytes and was not detected in the GADD45B-KO primary hepatocytes (Fig. 3C). Thus, the effective repression of the CYP2B10 mRNA in the GADD45B-KO primary hepatocytes confirmed that GADD45B performs the critical role in the CAR-mediated induction by TCPOBOP of the Cyp2b10 gene.
Fig. 3.
GADD45B-KO attenuates expression of the Cyp2b10 gene. Primary hepatocytes were prepared from wild-type and GADD45B-KO mice and were treated with DMSO (open bars) and TCPOBOP (closed bars). Total RNAs were prepared from these hepatocytes and were subjected to real-time PCR. The levels of CYP2B10, CAR, and GADD45B mRNAs are shown relative to those of corresponding mRNAs in DMSO-treated wild-type primary hepatocytes set at one. Error bars, SE values from at least three experiments.
Discussion
The antiapoptotic factor GADD45B has now been shown to regulate CAR-mediated transcription of the CYP2B6 gene in HepG2 cells and the Cyp2b10 in mouse primary hepatocytes. Together with our previous finding of direct CAR interaction with GADD45B (manuscript submitted for publication), present cell-based transfection assays have demonstrated that GADD45B is a new transcriptional coactivator of CAR. Because GADD45B is a factor that may transfer signals into various cellular pathways, an indirect effect of GADD45B to coactivate CAR cannot totally be eliminated as the possible regulatory mechanism at this present time. Nevertheless, the fact that expression levels of CYP2B10 mRNA by TCPOBOP in GADD45B-KO primary hepatocytes was reduced to 20% to the corresponding levels observed in wild-type primary hepatocytes indicates that coactivation by GADD45B appears to be critical for maximal CAR activation of transcription of the Cyp2b10 gene.
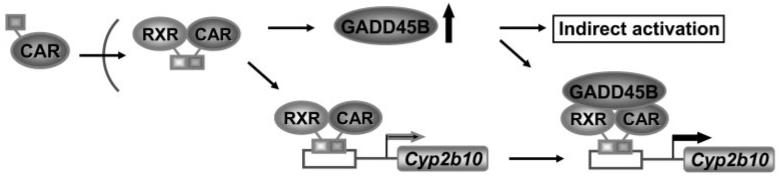
Responding to various endogenous stimuli, the Gadd45b gene is activated, and GADD45B protein is involved in transducing various cell signals (Abdollahi et al., 1991). It is now found that CAR enables the Gadd45b gene to respond to xenobiotics. The biological consequences of xenobiotic activation of the Gadd45b gene remain an attractive subject for future research. The CAR activators PB and TCPOBOP are nongenotoxic carcinogens, and chronic CAR activation by these activators promotes development of hepatocellular carcinoma (HCC) in rodents (Yamamoto et al., 2004; Huang et al., 2005). We have demonstrated that treatment with TCPOBOP attenuates tumor necrosis factor α-induced cell death of mouse primary hepatocytes only in the presence of both CAR and GADD45B, suggesting that CAR may promote HCC by down-regulating the apoptotic JNK pathway (manuscript submitted for publication). PB induction of xenobiotic metabolizing enzymes such as CYP2B has also been suggested to be a factor responsible for PB promotion of HCC (Diwan et al., 2001), although this suggestion has not yet been experimentally demonstrated. Our present finding that GADD45B is a CAR coactivator raises an intriguing question as to whether the role of CYP enzymes in HCC development should be revisited. When rodents are treated with CAR activators, liver GADD45B increases and perhaps puts the animals into a vicious cycle of continuous induction of the CYP2B enzyme (Fig. 4). Thus, in response to CAR activation, GADD45B coordinates attenuation of apoptosis and induction of the CYP2B enzyme and/or other CYP enzyme that may synthesize chemicals assisting HCC development.
Fig. 4.
Cross talk regulation of GADD45B to coactivate CAR. Whether CAR directly activates the Gadd45b gene remains a subject for future investigations.
The liver is endowed with the metabolic capability to detoxify xenobiotics, including therapeutic drugs to counter toxicity and carcinogenicity caused by xenobiotics. Microsomal CYP enzymes provide this metabolic capability with the flexibility and adaptability for the liver to deal with an unlimited number of xenobiotics and their structural diversity. This flexibility and adaptability comes from various unique characteristics of the CYP enzymes and CYP genes, one of which is the capability of CYP genes to exhibit altered transcriptional activity in response to xenobiotic exposure, thereby regulating the levels of CYP enzymes. Human CYP2B6 is one such CYP enzyme. CYP2B6 exhibits extreme individual variations in its basal and induced levels in human livers, which may affect how patients respond to drug therapy and human susceptibility to xenobiotic exposure (Zanger et al., 2007). We previously found that insulin represses the mouse Cyp2b gene by down-regulating CAR-mediated transcription indirectly through the forkhead transcription factor, FoxO1, but not by directly regulating CAR expression (Kodama et al., 2004). Here, we have now identified GADD45B as being an example of indirect up-regulation of CAR-mediated transcription of the CYP2B genes. Thus, factors such as GADD45B and FoxO1 provide CAR with a molecular mechanism by which endogenous hormones and stimuli become the critical determinants of hepatic metabolic capability of xenobiotics.
Inflammatory stimulation by lipopolysaccharide (LPS) treatment increases GADD45B through a nuclear factor-κB pathway in mouse liver (Zhang et al., 2005). LPS treatment is known to strongly repress expression of the Cyp2b gene and its induction by PB in mouse liver and primary hepatocytes (Morgan, 1997; Li-Masters and Morgan, 2001). These responses to LPS treatment appear to contradict our conclusion that GADD45B coactivates CAR-mediated transcription. However, a more recent report demonstrated that LPS treatment greatly reduces the levels of CAR protein in mouse liver, resulting in the liver's inability to activate the Cyp2b10 gene (Beigneux et al., 2002). Although its molecular mechanism is not known, this LPS-dependent reduction seems to be a nonspecific event because LPS reduces not only the levels of CAR but also those of PXR and RXRs as well. Once the nonspecific reduction of CAR is eliminated, increases of GADD45B can be expected to be involved in coregulation of CAR-mediated induction of the CYP2B enzyme. The extent to which the inflammation signal via GADD45B modulates the hepatic levels of CYP2B genes and also the other CAR-target genes remains an intriguing question at the present time.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
ABBREVIATIONS
- CAR
constitutive active/androstane receptor
- RXR
retinoid X receptor
- GADD45B
growth arrest and DNA-damage-inducible 45 β
- MKK7
mitogen-activated protein kinase kinase 7
- JNK
c-Jun NH2-terminal kinase
- PB
phenobarbital
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- KO
knockout
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- Luc
luciferase
- DMSO
dimethyl sulfoxide
- HCC
hepatocellular carcinoma
- LPS
lipopolysaccharide
References
- Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1991;6:165–167. [PubMed] [Google Scholar]
- Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;26:145–149. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, Tian J, Locker J. Gadd45b is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology. 2005;42:1118–1126. doi: 10.1002/hep.20883. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Henneman JR, Nims RW. Enhancement of N-nitrosodimethylamine-initiated hepatocarcinogenesis by phenytoin in male F344/NCr rats at a dose causing maximal induction of CYP2B. Int J Toxicol. 2001;20:81–87. doi: 10.1080/10915810151115191. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Negishi M. Characterization of phenobarbital-responsive enhancer module in mouse P450 Cyp2b10 gene. J Biol Chem. 1997;272:14943–14949. doi: 10.1074/jbc.272.23.14943. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear receptor CAR-retinoid X receptor heterodimer activates the Phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. Xenobiotic stress induces hepatomegary and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- Jackson JP, Ferguson SS, Moore R, Negishi M, Goldstein JA. The constitutive active/androstane receptor regulates phenytoin induction of CYP2C29. Mol Pharmacol. 2004;65:1397–1404. doi: 10.1124/mol.65.6.1397. [DOI] [PubMed] [Google Scholar]
- Jia Y, Guo GL, Surapureddi S, Sarkar J, Qi C, Guo D, Xia J, Kashireddi P, Yu S, Cho Y-W, et al. Transcription coactivator peroxisome proliferator-activated receptor-binding protein/mediator 1 deficiency abrogates acetaminophen hepatotoxicity. Proc Natl Acad Sci U S A. 2005;102:12531–12536. doi: 10.1073/pnas.0506000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore M, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka Y, Iwazaki N, Nakajo I, Hosokawa M, Negishi M, Chiba K. Identification of HMG-CoA reductase inhibitors as activators for human, mouse and rat constitutive androstane receptor. Drug Metab Dispos. 2005;33:924–929. doi: 10.1124/dmd.104.002741. [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FoxO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Masters T, Morgan ET. Effects of bacterial lipopolysaccharide on phenobarbital-induced CYP2B expression in mice. Drug Metab Dispos. 2001;29:252–257. [PubMed] [Google Scholar]
- Mäkinen JM, Reinisalo MK, Niemi KP, Viitala PJ, Jyrkkarinne JH, Chung HO, Pelkone O, Honkakoski P. Dual action of oestrogens on the mouse constitutive androstane receptor. Biochem J. 2003;376:465–472. doi: 10.1042/BJ20030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min GJ, Kemper JK, Byron Kemper B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J Biol Chem. 2002;277:26356–26363. doi: 10.1074/jbc.M200051200. [DOI] [PubMed] [Google Scholar]
- Morgan ET. Regulation of cytochromes P450 during inflammation and Infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- Muangmoonchai R, Smirlis D, Wong SC, Edwards M, Philips IR, Shephard EA. Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem J. 2001;355:71–78. doi: 10.1042/0264-6021:3550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, et al. Gadd45b beta mediates the NF-kappa B suppression of JNK signaling by targeting MEK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- Schwartz PS, Chen CS, Waxman DJ. Sustained P450 expression and prodrug activation in bolus cyclophosphamide-treated cultured tumor cells: impact of prodrug schedule on P450 gene-directed enzyme prodrug therapy. Cancer Gene Ther. 2003;10:571–582. doi: 10.1038/sj.cgt.7700601. [DOI] [PubMed] [Google Scholar]
- Shiraki T, Sakai N, Kanaya E, Jingami H. Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor γ coactivator-1α: a possible link between xenobiotic response and nutritional state. J Biol Chem. 2003;278:11344–11350. doi: 10.1074/jbc.M212859200. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Negishi M. Phenobarbital response element of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- Swales S, Kakizaki S, Yamamoto Y, Inoue K, Kobayashi K, Negishi M. Novel CAR-mediated mechanism for synergistic activation of two distinct elements within the human cytochrome P450 2B6 gene in HepG2 cells. J Biol Chem. 2005;280:3458–3466. doi: 10.1074/jbc.M411318200. [DOI] [PubMed] [Google Scholar]
- Timsit T, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Kakizaki S, Negishi M, Sueyoshi T. Residue threonine 350 confers steroid hormone responsiveness to the mouse nuclear orphan receptor CAR. Mol Pharmacol. 2002;61:1284–1288. doi: 10.1124/mol.61.6.1284. [DOI] [PubMed] [Google Scholar]
- Ueda A, Matsui K, Yamamoto Y, Pedersen LC, Sueyoshi T, Negishi M. Thr176 regulates the activity of the mouse nuclear receptor CAR and is conserved in the NR1I subfamily members PXR and VDR. Biochem J. 2005;388:623–630. doi: 10.1042/BJ20041572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The nuclear receptor constitutive/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievericht J, Hofmann M, Schwab M. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- Zhang N, Ahsan MH, Zhu L, Sambucetti LS, Purchio AF, West DB. NF-kB and not MAPK signaling pathway regulates GADD45β expression during acute inflammation. J Biol Chem. 2005;280:21400–21408. doi: 10.1074/jbc.M411952200. [DOI] [PubMed] [Google Scholar]