Abstract
In eukaryotic cells, control mechanisms have developed that restrain cell-cycle transitions in response to stress. These regulatory pathways are termed cell-cycle checkpoints. The G2/M checkpoint prevents cells from entering mitosis when DNA is damaged in order to afford these cells an opportunity to repair the damaged DNA before propagating genetic defects to the daughter cells. If the damage is irreparable, checkpoint signaling might activate pathways that lead to apoptosis. Since alteration of cell-cycle control is a hallmark of tumorigenesis, cell-cycle regulators represent potential targets for therapy. The centrosome has recently come into focus as a critical cellular organelle that integrates G2/M checkpoint control and repairs signals in response to DNA damage. A growing number of G2/M checkpoint regulators have been found in the centrosome, suggesting that centrosome has an important role in G2/M checkpoint function. In this review, we discuss centrosome-associated regulators of the G2/M checkpoint, the dysregulation of this checkpoint in cancer, and potential candidate targets for cancer therapy.
Introduction
With the aging of the world's population, the westernization of diet, and the increasing environmental pollution associated with the global economy, cancer has emerged as the top threat to human life worldwide [1,2]. To advance our progress against this disease, the two most important goals for cancer researchers are to fully understand the molecular basis of cancer and to develop effective therapies for it. One of the hallmarks of carcinogenesis is dysregulation of the cell cycle [3]. Cell cycle is controlled at a number of checkpoints. When cells suffer extracellular or intracellular stress or both, the cell-cycle checkpoints, especially G1/S and G2/M checkpoints which are controlled by a number of complexes that are composed of cyclin-dependent kinases (Cdks), cyclins, and their negative regulators including the Cip/Kip family members and the INK4a/ARF family members [4-6], are activated. The G1/S checkpoint is the first surveillance system to stop DNA synthesis when cells suffer from extracellular stresses and it is an effective step to control cell proliferation and apoptosis. The mechanism of G1/S checkpoint is extensively studied [5-8]. The G2/M checkpoint prevents DNA-damaged cells from entering mitosis and allows for the repair of DNA that was damaged in late S or G2 phases prior to mitosis. The G2/M checkpoint is controlled by Cdc2/cyclinB, and their negative regulators including p21Cip1 and p27 [9]. Weakened G2/M checkpoint under therapeutic setting may trigger cell death via mitotic catastrophe for cells with unrepairable DNA lesions and mitosis machinery. This may represent a novel strategy to kill cancer cells, especially those with the p53 mutant phenotype which could result in inactivation or lost of the G1/S checkpoint in cancer [10,11]. Thus, the G2/M checkpoint is a potential target for cancer therapy.
As the primary microtubule-organizing center (MTOC), the centrosome plays an important role in maintaining chromosome stability by establishing bipolar mitotic spindles. Accumulating evidence suggests that centrosome integrates cell-cycle arrest and repair signals in response to genotoxic stress [12]. A growing number of important cell cycle regulators such as Cdks, checkpoint kinases (Chks), polo-like kinases (Plks), Aurora kinases, NIMA-related kinases (Neks), p53, BRCA1, and cyclin B1 have been shown to localize to the centrosome (Table 1). All of those proteins have been implicated in participating in G2/M checkpoint control and in the regulation of centrosome separation [13-20]. Abnormal expression (either under or over) of these proteins has been observed in most cancers [21] and they have been found to directly influence the efficacy of antitumor agents [22]. Thus, manipulating these G2/M checkpoint proteins could enhance cancer's sensitivity to radiotherapy and chemotherapy. In this review we focus on centrosome-associated regulators of G2/M checkpoint and potential targets for cancer chemotherapeutic therapy.
Table 1.
Centrosome-associated G2/M checkpoint proteins
| Centrosome proteins | Substrates | Functions | Effects of expression manipulation |
| cyclin B/Cdk1 [33] | Drp1/Dnml1, HuR, hnRNP-k, TPX2 | mitosis entry, bipolar spindle assembly | inhibition: induce cell cycle arrest and apoptosis |
| Aurora A[34,35,76] | centrosomin, γ-TuRC, Eg5, Ran-TPX2, CENP-A, PP1, p53, Cdh1, NM23-H1, CPEB, Cdc25B, TPX2 | mitotic entry and exit, centrosome mutation and separation, spindle formation | inhibition: monopolar spindle overexpression: centrosome amplification and loss of mitotic checkpoint |
| Aurora B[34,35] | INCEP, Survivin, BubR1, Mad2 | chromatid separation, spindle assembly checkpoint | inhibition: multinucleate cells |
| Plk1[21,34,36] | Cdc25, cyclinB/Cdk1, p53, Nlp1, ATM/ATR, BRCA1, Chk1, Emi1, Wee1 | mitotic entry and exit, APC/C regulation, bipolar spindle formation, centrosome maturation, | inhibition: smaller centrosomes |
| Nek2A[18,34,101] | PP1, C-Nap1 | centrosome separation and maturation, mitotic entry | overexpression: split centrosomes |
| Survivin[90,91,102] | Caspases 3, 7, 9, Aurora B, INCENP | anti-apoptosis | inhibition: loss of mitotic kinases and checkpoint, supernumerary centrosome |
| p53[47,48] | p21, 14-3-3, GADD45 | centrosome duplication | inhibition: centrosome amplication |
| BRCA1[51,52] | γ-Tubulin, Chk1/2, p53, Cdc25, Wee1, Aurora A | centrosome duplication | inhibition: centrosome re-duplication and hyperactive MT nucleation |
| APC/C[99] | Cyclin B/Cdk1, securin, Aurora A, Plk1, Cdk2 | sister chromatid separation, mitotic exit, proteasomal degradation | NA |
| ATM/ATR[55] | p53, Chk1/2, BRCA1, Mdm2 | initiation of genotoxic stress response | NA |
| Chk1/2 [56-59] | Cdc25, BRCA1, E2F, p73α | centrosome separation, mitotic entry | inhibition: centrosome amplification and mitotic arrest |
Cell cycle and centrosomal cycle
The cell cycle entails a recurring sequence of events that include the duplication of cellular contents and subsequent cell division. Traditionally, the cell cycle in the eukaryotic cell is divided into four phases: Gap phase 1 (G1); DNA synthesis phase (S); Gap phase 2 (G2), during which the cell prepares itself for division; and mitosis phase (M), during which the chromosomes separate and the cell divides. The M phase includes prophase, metaphase, anaphase, and telophase [23].
Centrosome, the nonmembranous organelles that occupy a tiny volume near the center of the cell, are usually proximal to the nucleus. In most vertebrate cells, the centrosome is classically depicted as having two orthogonally positioned cylindrical centrioles surrounded by a matrix of fibrous and globular proteins that constitute the pericentriolar material (PCM)[24]. The cell cycle involves an intricate process of DNA replication and cell division that concludes with the formation of two genetically equivalent daughter cells. In this progression, the centrosome is duplicated only once to produce the bipolar spindle and ensure proper chromosome segregation. Centrosome maturation and separation are tightly regulated during the cell cycle. Centrosome duplication consists of the five morphological steps during cell cycle progression [25]. 1) In early G1/S phase, the mother and daughter centrioles separate slightly and lose their orthogonal orientation; 2) in S phase, synthesis of a daughter centriole occurs in the vicinity of each preexisting centriole; 3) in G2 phase, the procentrioles elongate to complete the duplication process. The duplicated centrosome disjoins into two functionally separate centrosome, each containing a mother-daughter pair of centrioles; 4) in late G2 phase, the centrosome increases in size and separate to allow the formation of a bipolar spindle; 5) in M phase, the original mother and daughter centrioles detach from each other in an event termed centrosome disjunction. Since centrosome duplicates only once during the normal cell cycle, duplication of centrosome must proceed in coordination with DNA synthesis to synchronize with cell division [26]. Centrosome appears to be a critical organelle for G2/M checkpoint. Centrosome separation is initiated at the G2 phase and completed in the M phase. Several key proteins involved in controlling the G2/M checkpoint have been shown to physically associate with centrosome.
Centrosome-associated regulators of G2/M checkpoint
An increasingly number of cancer related proteins have been shown to reside in or traffic in and out of centrosomes. These regulators include: 1) A number of cell cycle-regulated proteins, including cyclin B1, Cdks, Chks, Plks, aurora kinases, and Neks [27-36]; 2) Oncogenes, such as Survivin, Ras, Rad6, and HER2/neu [37-40]; 3) Tumor suppressors including p53, Rb, p21, XRCC2/3, APC, NM23-R1/H1, Gadd45 and BRCA l/2 [19,20,41-48]; and 4) Ubiquitination and degradation related proteins, including anaphase-promoting complex/cyclosome (APC/C), BRCA1, Cdc20, and Cdh1 [49-52]; 5) DNA damage checkpoint proteins including ATM, ATR, p53, BRCA1, Chk1, and Chk2 [29,53-59]. More detailed information about these regulators is listed in Table 1. The roles of these centrosome-associated regulators have been extensively investigated and some of the current understanding of their roles in G2/M checkpoint and in response to DNA damage is summarized in Fig 1. In this section, we will review the regulatory roles of the key centrosome-related kinases and some cancer related genes involved in G2/M transition.
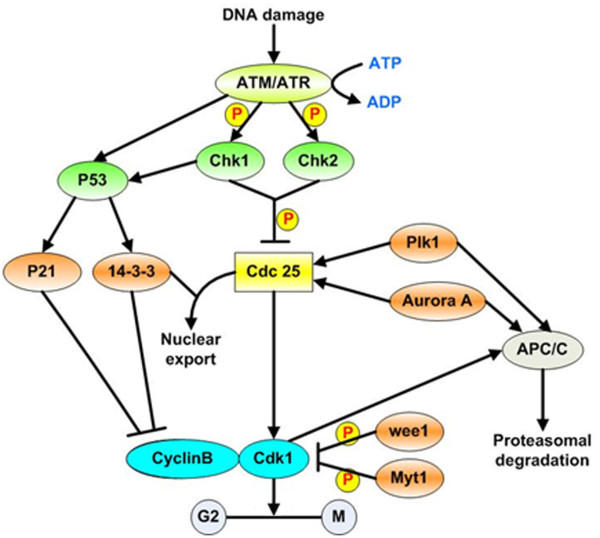
Figure 1.
Activation of the G2/M checkpoint after DNA damage. In response to DNA damage, the ATM, ATR signaling pathway is activated, which leads to the phosphorylation and activation of Chk1 and Chk2 and to the subsequent phosphorylation of Cdc25. Phosphorylated Cdc25 is sequestered in the cytoplasm by 14-3-3 proteins, which prevents activation of cyclinB/Cdk1 by Cdc25 and results in G2 arrest. Activated ATM/ATR also activates p53-dependent signaling. This contributes to the maintenance of G2 arrest by upregulating 14-3-3, which sequesters Cdk1 in the cytoplasm. In addition, p53 induces the transactivation of p21, a Cdk inhibitor that binds to and inhibits cyclinB/Cdk1 complexes. P: phosphorylation.
Cdc2 (also termed Cdk1) and its regulator cyclin B drive cells into mitosis from G2 phase. In early G2 phase, Cdk1 is inactivated by phosphorylation of T14 and Y15 residues by Wee1 and Myt1 kinases [60]. The initial activation of cyclin B/Cdk1 occurs at the centrosome in prophase. This involves Cdk1 dephosphorylation at T14 and Y15 by Cdc25 phosphatase family (Cdc25A, B, and C) and cyclin B phosphorylation at Ser126/128 by MPF and Ser133 by Plk1 [61-65].
Chk1 and Chk2 are transducers of ATR- and ATM-dependent signaling in response to DNA damage. Chk1 has been detected at the interphase centrosome, and inhibition of Chk1 resulted in premature centrosome separation [29]. Chk2 was also reported to localize to the centrosome and could be phosphorylated at Thr 68/26 and Ser 28 by Plk1, which co-localized with Chk2 at the centrosome in early mitosis [66]. Chk1 is activated by ATR in cells treated with ultraviolet radiation [67], whereas Chk2 is activated by ATM in cells exposed to ionizing radiation [68]. Activation of ATM/ATR initiates the subsequent protein kinase cascade through both p53 dependent and independent pathways. In p53 dependent pathways, p53 is phosphorylated on Ser 15 and Ser 20 [69-71] and then activates downstream targets genes, such as p21 and 14-3-3, which play an important role in G2/M checkpoint through inhibition of Cdk1/cyclin B [72,73]. In the p53 independent pathway, Chk1 and Chk2 phosphorylate Cdc25 at Ser 216, which down-regulate Cdc25 activity by promoting 14-3-3 protein and nuclear export [53,61]. Chk1/2 also phosphorylates Wee 1 and increases Wee 1 activity. It is known that both Cdc25C and Wee 1 phosphorylation cooperatively reduce Cdk1/cyclin B1 activity leading to G2/M arrest [61,74,75].
In mammalian cells, three members of the Aurora family have been identified: Aurora A, B, and C. Among them, Aurora A is associated with the centrosome and microtubules. Aurora A is essential for controlling multiple steps in the cell cycle from late S phase through M phase, including centrosome maturation and separation, mitotic spindle formation, and mitotic entry and exit. Aurora A mediates its multiple functions by interacting with other centrosome proteins including p53, centrosomin, centromere protein A, Eg5, and BRCA1[76].
Plk1, which is the best studied member of the Plk family in mammalian cells, is involved in various events in mitotic progression [77]. Plk1 increases during S and G2/M [78]. Plk1 phosphorylates and activates Cdc25, which leads to activation of Cdk1/cyclin B1 and G2/M checkpoint [79,80]. Plk1 also plays a role in mitosis exit by regulating the anaphase-promoting complex [81,82]. In response to DNA damage, Plk1 activity is inhibited in an ATM/ATR dependent manner [83], preventing mitosis entry.
Nek2, which is a member of the Nek kinase family, has a role in regulation of the G2/M checkpoint and is localized to the centrosome. Nek2 has two splice variants: Nek2A and Nek2B. Nek2A is required for centrosome separation at the G2/M transition and forms a complex with the catalytic subunit of protein phosphatase 1 (PP1) and a large coiled-coil protein called C-Nap1 [84,85]. Nek2 can phosphorylate its substrates, C-Nap1 and Nlp, contributing to their displacement from the centrosome, which is an essential step for subsequent splitting of the centrosome [85-87].
Survivin is a member of the inhibitor of apoptosis protein (IAP) family that plays an essential role in the control of cell division and the inhibition of apoptosis [88]. Survivin is expressed in a cell cycle-dependent manner and regulates G2/M phase by localizing to multiple sites on the mitotic apparatus including the centrosome, microtubules, and the mitotic spindle [37]. Also, Survivin performs its mitotic roles by cooperating with inner centromere protein (INCENP) and Aurora B [89]. A basic event for Survivin regulation is phosphorylation of the Thr34 by the p34(Cdc2) kinase [90,91]. Survivin induces apoptosis by inhibiting, directly or indirectly, the activity of Caspases 3, 7, and 9.
Accumulating evidence indicates that BRCA1 is located in the centrosome and binds to γ-tubulin [92]. BRCA1 has an important role in regulating centrosome duplication. This tumor suppressor is involved in all phases of the cell cycle and regulates orderly events during cell-cycle progression through its transcriptional activity and ubiquitination ligase E3 function [92-95]. BRCA1 interacts with many proteins that play important roles in multiple biological pathways. These proteins include ATM, ATR, Chk1/2, Wee1, p53, Aurora A, and Cdc25C [93,94,96,97], all of which have important roles in G2/M cell cycle regulation.
The ubiquitin-proteasome pathway (UPP) is essential for degrading intracellular proteins, which plays a key role in maintaining cellular homeostasis. Polymers of ubiquitin are covalently attached to protein targets by three key enzymes: ubiquitin-activating enzyme E1, ubiquitin-conjugating enzymes E2, and ubiquitin ligases E3. The resulting ubiquitinated proteins are then recognized and degraded by the 26S proteasome [98]. Cyclin B/Cdk1 is a master regulator during G2/M transition, and cyclin B/Cdk1 activity is strictly governed by the anaphase-promoting complex/cyclosome (APC/C), a ring-finger-type E3 that plays an important role in sister chromatid separation and exit from mitosis by degrading mitotic substrates. The APC/C is activated by its adaptor and regulators, such as Cdc20 and Cdh1, to target Securin and mitotic cyclins. Activation of APC/C is required for anaphase onset and mitotic exit [99].
Dysregulation of the centrosome-associated regulators of G2/M checkpoint in cancer
Mounting evidence indicates that cell-cycle dysregulation is a common feature of cancer. The G2/M checkpoint in particular is an area of focus for cancer research. Abnormalities of several of above mentioned centrosome-associated regulators of the G2/M checkpoint have been detected in human tumors, as detailed below [21,100-102]:
The Aurora A gene is located on chromosome 20q13.2, a region that is commonly amplified in many epithelial cancers. Both mRNA and protein levels of Aurora A are overexpressed in a variety of tumor tissues and tumor cell lines, suggesting its potential role in tumorigenesis. Aurora A mRNA upregulation has been significantly associated with advanced tumor stage, the presence of positive regional lymph nodes, as well as distant metastasis in head and neck squamous cell carcinoma [100]. Aurora A also promotes cell migration and reduces the radiosensitivity of laryngeal squamous cell carcinoma [103]. In ovarian cancer, overexpression of Aurora A is associated with centrosome amplification and poor survival [104]. Overexpression of Aurora A was significantly associated with aggressive clinical behavior including high histologic grade, invasion, metastasis and overall survival of patients with bladder cancer. Aurora A gene copy number has been reported to be a promising biomarker for detection of bladder cancer [105,106].
Plk1 expression has been showed to be elevated in non-small-cell lung, head and neck, esophageal, gastric, breast, ovarian, endometrial, colorectal, and thyroid carcinomas; melanomas, and gliomas [21]. Overexpression of Plk1 correlates positively with tumor stage, nodal status, and diffuse growth pattern in human gastric cancer [107]. In a study of 158 colon cancer patients, Weichert et al. found that overexpression of Plk1 correlated positively with Dukes stage and nodal status [108].
Overexpression of active Nek2A kinase leads to premature splitting of the mother and daughter centrioles [18,109], whereas expression of inactive Nek2A kinase causes the formation of centrosomal abnormalities, monopolar spindles, and aneuploidy [110], all of which are involved in regulating genetic stability and tumorigenesis. Elevated protein expression of Nek2 leads to centrosome abnormality and, consequently, tumorigenesis. Nek2 expression is elevated in breast, ovary, cervical, prostate cancers, and leukemia [101].
Abnormal expression of Survivin in mammalian cells could result in aberrant mitotic progression characterized by cell division defects that include supernumerary centrosomes, mislocalization of mitotic kinases, and loss of mitotic checkpoint. Survivin is overexpressed in a wide spectrum of human cancer, including lung, breast, colon, gastric, liver, bladder, uterine, and ovary cancer [102].
Heat-shock protein 90 (hsp90), a molecular chaperone, plays a role in G2/M checkpoint regulation by associating with its client proteins including Chk1, Cdk1, Wee1, Myt1, Plk1, and cyclinB through regulation of their stability. Hsp90 inhibitors could result in targeting of these client proteins to the proteasome to be degraded which may explain the substantial G2/M peak in cell cycle [111-114].
The APC/C, a multisubunit ubiquitin ligase E3, is a gatekeeper for mitosis by balancing the amount of checkpoint regulators. Two key activators for APC/C function are Cdh1 and Cdc20. Dysfunction of APC/CCdh1 might result in abnormal accumulation of both mitotic Cdk activity and non-Cdk kinases activity(such as Aurora A, Plk, and Nek2), leading to the development of cancer [115]. APC/CCdc20 recognizes and marks the key substrate securin and cyclin B1 for degradation and promotes chromosome separation and anaphase onset in a time- and spatial-dependent manner. Deregulation of Cdc20-dependent proteolysis can result in aneuploidy, ultimately resulting in cancer. Securin has been reported to be overexpressed in human breast and colorectal cancers [116,117]. In addition, Hagting et al. found that blocked proteolysis of securin by APC/CCdc20 led to genomic instability in cultured cells [50]. Thus, dysfunction of the APC/C might lead to uncontrolled proliferation, genomic instability, and cancer.
Modulation of G2/M checkpoint proteins and cancer therapy
Although there are defects in G2/M checkpoint proteins in cancer, the nature of these alterations is quite different from that of alterations of the G1/S checkpoint. The presence of p53 mutation in 50% of all cancers renders the G1/S checkpoint less efficient, allowing synthesis of unrepaired DNA [11,118]. For G2/M checkpoint proteins, mutations of key players are not common. Even for BRCA1, mutation is infrequent in sporadic cancers and more concentrated in the familial breast cancers [94]. The impact of p53 as a checkpoint protein is complex because p53 is also a major regulator of apoptosis [119]. Because cell cycle checkpoints also repair DNA damages caused by therapeutics, the role of cell cycle checkpoints are often the cause for resistance. On one hand, increased proliferation is a common feature for aggressive cancers, thus inhibition of cell proliferation is a logical approach. On the other hand, most cancer drugs target cycling cells, so the fast growing tumor cells are more sensitive to these treatments. It is well-known that slow-growing and more differentiated cancers are generally resistant to chemotherapy. As a matter of fact, the G2/M checkpoint is invariably activated in cancer cells in response to DNA damage partially causing resistance to therapy [120,121]. Specifically, the G2/M checkpoint based anti-cancer strategies have been focused on targeting and inactivating the G2/M checkpoint, thus forcing the cancer cells into mitosis with increased DNA damage and finally into mitotic catastrophe and cell death. Following is a brief review on some of the checkpoint related cancer therapies under development [122-127] (Table 2).
Table 2.
Small molecule inhibitors targeting centrosome associated G2/M checkpoint regulators
| Specific targets | Functions | Clinical development | Combination with DNA-damaging agents | |
| Flavopiridol[128] | Cdk1, p21, Survivin | Bind to Cdc2, inhibit apoptosis | Phase I/II | paclitaxel, irinotecan, gemcitabine, IR |
| UCN-01[153-155] | Cdk1, Chk1/2 | Inhibit Chk1 and PKC activity, promote apoptosis | Phase I/II | fluorouracil, topotecan, cisplatin, IR, temozolomide |
| Daidzein[139] | Cdk1, p21Cip1, p57Kip2 | Inhibit Cdk1 and cell proliferation | Preclinical | NA |
| Caffeine [123-125] | ATM/ATR, PI3K | Inhibit ATM/ATR, cause G2 checkpoint abrogation, induce apoptosis | Phase I | Taxol, cisplatin |
| KU-55933[122,126] | ATM | Inhibit ATM | Phase I | NA |
| Berberine[140,141] | Wee1, 14-3-3, Cdk2, cyclinB, Cdc25c | Induce G2/M phase arrest and apoptosis | Preclinical | NA |
| 17AAG [145-147] | Chk1, Hsp90 | Downregulate Chk1, inhibit colony formation, induce apoptosis, abrogate G2/M checkpoint | Phase I/II | gemcitabine, cisplatin, topoisomerase I poisons, taxol, IR |
| XL844[148] | Chk1/2 | Enhance gemcitabine antitumor activity | Phase I | Gemcitabine |
| CHIR-124[149] | Chk1 | abrogate G2/M checkpoint, induce apoptosis | Preclinical | Topoisomerase I poisons |
| PF-00477736[150] | Chk1, cyclin B, Securin, Aurora | Inhibit Chk1, abrogates cell cycle arrest | Preclinical | Gemcitabine, carboplatin |
| PD0166285[127] | Wee1, Aurora A | Inhibit Wee1, abrogate G2/M checkpoint | Preclinical | NA |
| CEP-3891[143,151] | Chk1 | Abrogate G2/M checkpoint | Preclinical | IR |
| N-aryl-N'-pyrazinylurea [142,152] | Chk1 | Inhibit Chk1, inhibit cell proliferation | Preclinical | Doxorubicin, camptothecin |
| VX-680[166,170] | Aurora A, B | Block cell proliferation, disrupt bipolar spindle formation, accumulate of cell with 4N or greater DNA | Phase II | Vorinostat, docetaxel |
| Hesperadin[171] | Aurora kinase | Inhibit Aurora kinase activity | Phase I/II | NA |
| ZM447439[173] | Aurora A, B | Inhibit Aurora A, induce apoptosis | Phase I | NA |
| PHA-739358[164] | Aurora A, B, C | Inhibit proliferation | Phase I | NA |
| PHA-680632 [167,163] | Aurora A | Inhibit Aurora A and proliferation | Phase I | IR |
| ON01910[179] | Plk1, Cdk1 | Inhibit Plk1, induce mitosis arrest | Preclinical | NA |
Abbreviation: NA, not available; IR, ionizing radiation
Cdc2 inhibitors
To date, the majority of the published data suggests that inhibition of cyclin/Cdk complexes may prevent or delay tumor progression in cancer patients. Among a number of Cdk inhibitors under development, flavopiridol and UCN-01 are being tested in clinical trials [3,128]. We will review flavopiridol as an example.
Flavopiridol binds and directly inhibits Cdc2 as well as inhibiting antiapoptotic molecules including p21, Bcl2, and Survivin [129-131]. Flavopiridol has been tested as a novel chemotherapeutic agent for rhabdoid tumors, osteosarcoma, Ewing's family tumor cells, and leukemia [131-133]. The combinations of flavopiridol with paclitaxel, irinotecan, or gemcitabine have shown promising effects in cell line studies and in clinical trials. It was reported that paclitaxel or docetaxel followed by flavopiridol is associated with an increased induction of apoptosis through accelerating exit of cells from mitosis, but the reverse treatment schedule did not show added effect than paclitaxel or docetaxel alone [134-136]. Recently, it was reported that paclitaxel treatment followed by carboplatin for 1 hour and flavopiridol over 24 hours every 3 weeks for 3 cycles was effective and safe in NSCLC patients [137]. A greater antitumor effect was observed with the combination of gemcitabine or irinotecan followed by flavopiridol in several epithelial gastrointestinal cell lines [129,138]. Thus, flavopiridol in combination with chemotherapy may overcome cell cycle mediated drug resistance.
Other regulators of cyclin/Cdk complexes and Cdk inhibitors have been reported. Treatment with the isoflavone daidzein decreased the expression of Cdc2 and increased the expression of the Cdk inhibitors p21Cip1 and p57Kip2 in MCF-7 and MDA-MB-453 cells. Thus, daidzein exerts its anticancer effects in human breast cancer cells via cell-cycle arrest [139]. Berberine has been reported to induce G2/M arrest in leukemia and gastric cancer cells via the inhibition of cyclin B1 and the promotion of Wee1 [140,141].
Chk1 inhibitors
There are a large reservoir of identified Chk1 inhibitors including UCN-01, 17AAG, XL844, CHIR-124, PF-00477736, CEP-3891, and N-aryl-N'-pyrazinylurea. UCN-01, 17AAG, and XL844 are being tested in clinical trials, while the others are still in preclinical studies [142-152]. UCN-01 has been reported to promote apoptosis through G2/M checkpoint abrogation in various human cell lines. Thus, UCN-01 exerts more marked antitumor effects through combination with radio- or chemotherapy [153-155]. Results of three Phase I studies of combination therapy with UCN-01 in patients with solid tumors have been published, in which UCN-01 was combined with fluorouracil [156], topotecan [157], and cisplatin [158], respectively. UCN-01 plus topotecan or carboplatin were found to be generally well tolerated; however, combination of UCN-01 and fluorouracil did not show significant antitumor activity against advanced ovarian cancer [159,160]. Further research to develop these combinations is warranted, especially focusing on reducing side effects.
Aurora Kinase Inhibitors
The evidence linking Aurora kinase overexpression and malignancy has stimulated interest in identifying and developing Aurora kinase inhibitors for cancer therapy. RNA interference targeting Aurora A has been found to suppress tumor growth and enhance sensitivity to chemotherapy- and radiation-induced apoptosis in human cells [161,162]. Several Aurora kinase inhibitors, including VX-680, Hesperadin, ZM447439, AT-9283, MLN-8054, R-763, SU6668, and PHA-739358, have been identified and are undergoing phase I/II clinical trials [163-167].
One of these inhibitors, VX-680, the first Aurora kinase inhibitor to enter clinical trials, not only inhibits cell proliferation but also induces apoptosis in a wide spectrum of tumor types. VX-680 was shown to greatly inhibit tumor growth in vivo in three xenograft models of leukemia, colon, and pancreatic tumors. It was reported that VX-680 has no effect on non-cycling normal cells which makes it a promising anticancer agent [168]. VX-680 also was found to be effective in reducing cell growth in different anaplastic thyroid cancer-derived cell lines [169]. In ovarian cancer, combination of VX-680 (also known as MK-0457) with docetaxel could significantly reduce cell proliferation and increase tumor cell apoptosis than VX-680 or docetaxel alone in vivo [170]. Further investigation of this inhibitor is warranted to exploit its potential value in the treatment of cancer.
In tobacco BY-2 cells, another Aurora kinase inhibitor, Hesperadin, was found to induce delayed transition from metaphase to anaphase and early exit from mitosis after chromosome segregation [171]. It is not clear, however, whether Hesperadin causes tumor cell death. In a colony formation assay, ZM447439, another Aurora kinase inhibitor, was found to be more toxic to proliferating cells than to nondividing cells [172], indicating that it might also be used selectively to kill proliferating tumor cells. ZM447439 is an effective apoptosis-inducing and G2/M phase-arresting agent in acute myeloid leukemia and Hep2 carcinoma cells [173,174].
Inhibitors of Plk1
The G2/M phase regulator Plk1 is frequently overexpressed in cancers and correlates with aggressiveness and poor prognosis. Cogswell et al observed that silencing of Plk1 functions induced apoptosis accompanied by mitotic catastrophe in SAOS-2 and U-2OS tumor cells but not in normal human mammary epithelial cells [175]. Findings from another study suggested that reduction of Plk1 expression via small interfering RNAs (RNAi) could prevent the growth of bladder cancer in vivo [176]. Downregulation of Plk-1 expression by RNAi has been found to cause cell-cycle arrest at the G2/M phase, reduce cellular proliferation, and increase gemcitabine cytotoxicity in pancreatic tumor cells in vitro [177].
Small molecule inhibitors of Plk1 include ATP-competitive and non-ATP-competitive categories. Identifying specific ATP-competitive inhibitors is challenging because of the high degree of structural conservation among ATP-binding domains in various kinases [178]. ON01910, a non-ATP-competitive Plk1 inhibitor, was reported to inhibit cancer cells growth by inducing mitosis arrest and apoptosis in many tumor cell lines. Importantly, ON01910 did not show hematotoxicity, liver damage, or neurotoxicity in vivo [179]. Thus, ON01910 is a promising Plk1 inhibitor that may exhibit beneficial effect in patients.
Summary and future directions
Cell cycle checkpoints provide mechanisms for cells to repair DNA damage. Activated checkpoints slow down cell cycle progression and thus allow normal cells to repair damage to prevent propagation of damaged DNA. The development of anti-cancer therapeutics has capitalized on the fact that activation of checkpoint proteins results in attenuated cell proliferation lead to anti-growth cancer therapeutics. Drugs have been developed to arrest cancer cells and stop cancer cell proliferation. On the other hand, the same mechanism that normally protects cells from DNA damage also repairs DNA following chemotherapy and radiotherapy. Therefore, strategies have been developed to abrogate the checkpoint activation, and drugs that exert this effect are combined with chemo- or radiotherapy to enhance cell kill.
In addition to small molecule inhibitors, gene based therapeutics such as antisense oligonucleotides also show promise. Recently, there is growing interest in a class of small RNA termed microRNAs (miRNAs) [180]. The miRNAs are a class of small (~22 nucleotides) noncoding RNAs that functions as post-transcriptional gene regulators [181]. miRNAs may regulate the expression of many genes, such as tumor-suppressor genes and oncogenes as well as their molecular networks [182-186], which in turn impact cell cycle progression [187,188]. miRNAs regulate a wide range of biological processes, including cell differentiation, proliferation, and apoptosis [189]. Aberrant miRNAs expression is involved in human tumorigenesis [190,191]. Mertens-Talcott et al [184] demonstrated that miR-27a increased the percentage of MDA-MB-231 cells in G2/M by inducing its target gene Myt-1, which inhibits G2/M through enhanced phosphorylation and inactivation of Cdk1. Yang et al [185] showed miR-214 induces cell survival and cisplatin resistance primarily by down-regulation of PTEN protein and activation of the Akt pathway through 3'-untranslated region (UTR) of the PTEN in human ovarian cancer. According to Yang et al [192], let-7i expression was significantly reduced in chemotherapy-resistant epithelial ovarian cancer patients. The in vitro study showed that reduced let-7i expression significantly increased the resistance of ovarian and breast cancer cells to cis-platinum. Thus, it was proposed that let-7i could be targeted in platinum-resistance patients. Taken together, miRNAs emerge as new therapeutic targets as well as tools in cancer treatment.
Cancer stem cells (CSCs) have become a new focus in cancer research since they may play a role in cancer initiation, metastasis, treatment resistance, and recurrence [193]. CSCs have been found in hematopoietic cancers as well as solid tumors included brain, neck, lung, breast, liver, colon, pancreas, prostate, bone, and melanoma [193-199]. Investigations into characteristics of CSCs improved our understanding of tumor treatment resistance. Conventional chemo- or radiotherapies preferentially kill dividing cells, but CSCs are low-growing, which make them resistant to conventional therapy. It is also likely that conventional therapies actually enrich CSCs and these cells have to potential to repopulate. Therefore, failure to target CSCs predicts for cancer recurrence. Current studies on CSCs zero in on the limitless proliferative capacity, self-renewal pathways, drug efflux pumps, and their niche [200]. Whether and how these features are linked to cell cycle checkpoints are not clear although they will likely be linked. The development of strategies that target CSCs as well as checkpoint will likely crosses paths and has potential in emergence in a new class of highly effective cancer therapeutics.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors have read and approved the final version of the manuscript. YMW drafted the manuscript and generated tables and figure. PJ, JL, RB and FXX contributed to the writing and editing of the manuscript. WZ contributed to the writing of the manuscript and supervised the project.
Acknowledgments
Acknowledgements
We gratefully thank Ms. Diane Hackett at the Department of Scientific Publications of M. D. Anderson Cancer for editing this manuscript.
Contributor Information
Yingmei Wang, Email: yingmeiwang@gmail.com.
Ping Ji, Email: pji@mdanderson.org.
Jinsong Liu, Email: jliu@mdanderosn.org.
Russell R Broaddus, Email: rbroddus@mdanderosn.org.
Fengxia Xue, Email: fengxiaxue1962@163.com.
Wei Zhang, Email: wzhang@mdanderson.org.
References
- McCracken M, Olsen M, Chen MS, Jr, Jemal A, Thun M, Cokkinides V, Deapen D, Ward E. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol. 1998;227:25–41. doi: 10.1007/978-3-642-71941-7_2. [DOI] [PubMed] [Google Scholar]
- Carnero A, Hannon GJ. The INK4 family of CDK inhibitors. Curr Top Microbiol Immunol. 1998;227:43–55. doi: 10.1007/978-3-642-71941-7_3. [DOI] [PubMed] [Google Scholar]
- Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164–1188. doi: 10.2741/1871. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Dash BC, El-Deiry WS. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell Biol. 2005;25:3364–3387. doi: 10.1128/MCB.25.8.3364-3387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Ishii H, Saito T. DNA damage-dependent cell cycle checkpoints and genomic stability. DNA Cell Biol. 2006;25:406–411. doi: 10.1089/dna.2006.25.406. [DOI] [PubMed] [Google Scholar]
- Loffler H, Lukas J, Bartek J, Kramer A. Structure meets function – centrosomes, genome maintenance and the DNA damage response. Exp Cell Res. 2006;312:2633–2640. doi: 10.1016/j.yexcr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol. 2000;12:658–665. doi: 10.1016/S0955-0674(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Walworth NC. DNA damage: Chk1 and Cdc25, more than meets the eye. Curr Opin Genet Dev. 2001;11:78–82. doi: 10.1016/S0959-437X(00)00160-X. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ, Muschel RJ. Inhibition of centrosome separation after DNA damage: a role for Nek2. Radiat Res. 2004;162:128–135. doi: 10.1667/RR3211. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fletcher L, Muschel RJ. The role of Polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. J Biol Chem. 2005;280:42994–42999. doi: 10.1074/jbc.M505450200. [DOI] [PubMed] [Google Scholar]
- Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. Embo J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci USA. 1998;95:12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–717. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. 2003;24:139–145. doi: 10.1016/S0165-6147(03)00026-9. [DOI] [PubMed] [Google Scholar]
- Ivanchuk SM, Rutka JT. The cell cycle: accelerators, brakes, and checkpoints. Neurosurgery. 2004;54:692–699. doi: 10.1227/01.NEU.0000109534.28063.5D. discussion 699–700. [DOI] [PubMed] [Google Scholar]
- Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2:688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- Sankaran S, Parvin JD. Centrosome function in normal and tumor cells. J Cell Biochem. 2006;99:1240–1250. doi: 10.1002/jcb.21003. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Sluder G. "It takes two to tango": understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 2001;15:1167–1181. doi: 10.1101/gad.894001. [DOI] [PubMed] [Google Scholar]
- Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J Cell Sci. 1992;101:529–545. doi: 10.1242/jcs.101.3.529. [DOI] [PubMed] [Google Scholar]
- Pockwinse SM, Krockmalnic G, Doxsey SJ, Nickerson J, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Penman S. Cell cycle independent interaction of CDC2 with the centrosome, which is associated with the nuclear matrix-intermediate filament scaffold. Proc Natl Acad Sci USA. 1997;94:3022–3027. doi: 10.1073/pnas.94.7.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA, Bartek J, Lukas J. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–891. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol. 2002;12:640–647. doi: 10.1016/S0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- Hames RS, Fry AM. Alternative splice variants of the human centrosome kinase Nek2 exhibit distinct patterns of expression in mitosis. Biochem J. 2002;361:77–85. doi: 10.1042/0264-6021:3610077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci USA. 2008;105:1442–1447. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit TL, Ahmad N. Regulation of mitosis via mitotic kinases: new opportunities for cancer management. Mol Cancer Ther. 2007;6:1920–1931. doi: 10.1158/1535-7163.MCT-06-0781. [DOI] [PubMed] [Google Scholar]
- Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R, Nigg EA. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell. 2003;5:113–125. doi: 10.1016/S1534-5807(03)00193-X. [DOI] [PubMed] [Google Scholar]
- Fortugno P, Wall NR, Giodini A, O'Connor DS, Plescia J, Padgett KM, Tognin S, Marchisio PC, Altieri DC. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115:575–585. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Lyakhovich A, Visscher DW, Heng H, Kondrat N. Rad6 overexpression induces multinucleation, centrosome amplification, abnormal mitosis, aneuploidy, and transformation. Cancer Res. 2002;62:2115–2124. [PubMed] [Google Scholar]
- Montagna C, Andrechek ER, Padilla-Nash H, Muller WJ, Ried T. Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene. 2002;21:890–898. doi: 10.1038/sj.onc.1205146. [DOI] [PubMed] [Google Scholar]
- Saavedra HI, Knauf JA, Shirokawa JM, Wang J, Ouyang B, Elisei R, Stambrook PJ, Fagin JA. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. 2000;19:3948–3954. doi: 10.1038/sj.onc.1203723. [DOI] [PubMed] [Google Scholar]
- Iovino F, Lentini L, Amato A, Di Leonardo A. RB acute loss induces centrosome amplification and aneuploidy in murine primary fibroblasts. Mol Cancer. 2006;5:38. doi: 10.1186/1476-4598-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- Mantel C, Braun SE, Reid S, Henegariu O, Liu L, Hangoc G, Broxmeyer HE. p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood. 1999;93:1390–1398. [PubMed] [Google Scholar]
- Griffin CS, Simpson PJ, Wilson CR, Thacker J. Mammalian recombination-repair genes XRCC2 and XRCC3 promote correct chromosome segregation. Nat Cell Biol. 2000;2:757–761. doi: 10.1038/35036399. [DOI] [PubMed] [Google Scholar]
- Roymans D, Vissenberg K, De Jonghe C, Willems R, Engler G, Kimura N, Grobben B, Claes P, Verbelen JP, Van Broeckhoven C, Slegers H. Identification of the tumor metastasis suppressor Nm23-H1/Nm23-R1 as a constituent of the centrosome. Exp Cell Res. 2001;262:145–153. doi: 10.1006/excr.2000.5087. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000;148:505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Bennett RA, Tarapore P, Fukasawa K. Direct evidence for the role of centrosomally localized p53 in the regulation of centrosome duplication. Oncogene. 2007;26:2939–2944. doi: 10.1038/sj.onc.1210085. [DOI] [PubMed] [Google Scholar]
- D'Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. Embo J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125–1137. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, Gygi SP, Parvin JD. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24:8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S, Starita LM, Simons AM, Parvin JD. Identification of domains of BRCA1 critical for the ubiquitin-dependent inhibition of centrosome function. Cancer Res. 2006;66:4100–4107. doi: 10.1158/0008-5472.CAN-05-4430. [DOI] [PubMed] [Google Scholar]
- Loffler H, Bochtler T, Fritz B, Tews B, Ho AD, Lukas J, Bartek J, Kramer A. DNA damage-induced accumulation of centrosomal Chk1 contributes to its checkpoint function. Cell Cycle. 2007;6:2541–2548. doi: 10.4161/cc.6.20.4810. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Yakushijin K, Horne D, Medema R, Kroemer G. The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene. 2004;23:4353–4361. doi: 10.1038/sj.onc.1207573. [DOI] [PubMed] [Google Scholar]
- Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ. ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis. 2003;24:1571–1580. doi: 10.1093/carcin/bgg137. [DOI] [PubMed] [Google Scholar]
- Menoyo A, Alazzouzi H, Espin E, Armengol M, Yamamoto H, Schwartz S., Jr Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727–7730. [PubMed] [Google Scholar]
- Kim MA, Kim HJ, Brown AL, Lee MY, Bae YS, Park JI, Kwak JY, Chung JH, Yun J. Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Exp Mol Med. 2007;39:205–212. doi: 10.1038/emm.2007.23. [DOI] [PubMed] [Google Scholar]
- Bourke E, Dodson H, Merdes A, Cuffe L, Zachos G, Walker M, Gillespie D, Morrison CG. DNA damage induces Chk1-dependent centrosome amplification. EMBO Rep. 2007;8:603–609. doi: 10.1038/sj.embor.7400962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni F, Codegoni AM, Furlan D, Tibiletti MG, Capella C, Broggini M. CHK1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Genes Chromosomes Cancer. 1999;26:176–180. doi: 10.1002/(SICI)1098-2264(199910)26:2<176::AID-GCC11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Leise WF., 3rd Measurement of Wee kinase activity. Methods Mol Biol. 2005;296:299–328. doi: 10.1385/1-59259-857-9:299. [DOI] [PubMed] [Google Scholar]
- Porter LA, Donoghue DJ. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog Cell Cycle Res. 2003;5:335–347. [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 2002;21:8282–8292. doi: 10.1038/sj.onc.1206011. [DOI] [PubMed] [Google Scholar]
- Hagting A, Jackman M, Simpson K, Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr Biol. 1999;9:680–689. doi: 10.1016/S0960-9822(99)80308-X. [DOI] [PubMed] [Google Scholar]
- Tsvetkov L, Xu X, Li J, Stern DF. Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J Biol Chem. 2003;278:8468–8475. doi: 10.1074/jbc.M211202200. [DOI] [PubMed] [Google Scholar]
- Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc Natl Acad Sci USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita TK, Lieberman HB, Lim DS, Dhar S, Zheng W, Taya Y, Kastan MB. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene. 2000;19:1386–1391. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/S1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Dulic V, Stein GH, Far DF, Reed SI. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–420. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/S0955-0674(98)80121-X. [DOI] [PubMed] [Google Scholar]
- Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–411. doi: 10.1016/S0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/S1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Golan A, Yudkovsky Y, Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem. 2002;277:15552–15557. doi: 10.1074/jbc.M111476200. [DOI] [PubMed] [Google Scholar]
- van Vugt MA, Smits VA, Klompmaker R, Medema RH. Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J Biol Chem. 2001;276:41656–41660. doi: 10.1074/jbc.M101831200. [DOI] [PubMed] [Google Scholar]
- Helps NR, Luo X, Barker HM, Cohen PT. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem J. 2000;349:509–518. doi: 10.1042/0264-6021:3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. 1998;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor T, Hacker U, Stierhof YD, Nigg EA. The mechanism regulating the dissociation of the centrosomal protein C-Nap1 from mitotic spindle poles. J Cell Sci. 2002;115:3275–3284. doi: 10.1242/jcs.115.16.3275. [DOI] [PubMed] [Google Scholar]
- Rapley J, Baxter JE, Blot J, Wattam SL, Casenghi M, Meraldi P, Nigg EA, Fry AM. Coordinate regulation of the mother centriole component nlp by nek2 and plk1 protein kinases. Mol Cell Biol. 2005;25:1309–1324. doi: 10.1128/MCB.25.4.1309-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2005;9:360–372. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Reed JC. IAP family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/S1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Doan TP, White RL. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 2001;61:7713–7718. [PubMed] [Google Scholar]
- Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA. 2001;98:9587–9592. doi: 10.1073/pnas.171174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30:285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- Ye Q, Hu YF, Zhong H, Nye AC, Belmont AS, Li R. BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J Cell Biol. 2001;155:911–921. doi: 10.1083/jcb.200108049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Ouchi T. Cell cycle differences in DNA damage-induced BRCA1 phosphorylation affect its subcellular localization. J Biol Chem. 2003;278:2015–2020. doi: 10.1074/jbc.M208685200. [DOI] [PubMed] [Google Scholar]
- Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha BH, Kim EE. Structures of proteases for ubiqutin and ubiquitin-like modifiers. BMB Rep. 2008;41:435–443. doi: 10.5483/bmbrep.2008.41.6.435. [DOI] [PubMed] [Google Scholar]
- Wasch R, Engelbert D. Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene. 2005;24:1–10. doi: 10.1038/sj.onc.1208017. [DOI] [PubMed] [Google Scholar]
- Reiter R, Gais P, Jutting U, Steuer-Vogt MK, Pickhard A, Bink K, Rauser S, Lassmann S, Hofler H, Werner M, Walch A. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. 2004;64:7370–7376. doi: 10.1158/0008-5472.CAN-04-0960. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Guan Z, Wang XR, Zhu XF, Huang XF, Xu J, Wang LH, Wan XB, Long ZJ, Liu JN, Feng GK, et al. Aurora-A, a negative prognostic marker, increases migration and decreases radiosensitivity in cancer cells. Cancer Res. 2007;67:10436–10444. doi: 10.1158/0008-5472.CAN-07-1379. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Lin YG, Immaneni A, Deavers MT, Merritt WM, Spannuth WA, Bodurka DC, Gershenson DM, Brinkley WR, Sood AK. Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin Cancer Res. 2007;13:4098–4104. doi: 10.1158/1078-0432.CCR-07-0431. [DOI] [PubMed] [Google Scholar]
- Park HS, Park WS, Bondaruk J, Tanaka N, Katayama H, Lee S, Spiess PE, Steinberg JR, Wang Z, Katz RL, et al. Quantitation of Aurora kinase A gene copy number in urine sediments and bladder cancer detection. J Natl Cancer Inst. 2008;100:1401–1411. doi: 10.1093/jnci/djn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–1329. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- Weichert W, Ullrich A, Schmidt M, Gekeler V, Noske A, Niesporek S, Buckendahl AC, Dietel M, Denkert C. Expression patterns of polo-like kinase 1 in human gastric cancer. Cancer Sci. 2006;97:271–276. doi: 10.1111/j.1349-7006.2006.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W, Kristiansen G, Schmidt M, Gekeler V, Noske A, Niesporek S, Dietel M, Denkert C. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol. 2005;11:5644–5650. doi: 10.3748/wjg.v11.i36.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Nigg EA. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci. 2001;114:3749–3757. doi: 10.1242/jcs.114.20.3749. [DOI] [PubMed] [Google Scholar]
- Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–61. doi: 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- Basto R, Gergely F, Draviam VM, Ohkura H, Liley K, Raff JW. Hsp90 is required to localise cyclin B and Msps/ch-TOG to the mitotic spindle in Drosophila and humans. J Cell Sci. 2007;120:1278–1287. doi: 10.1242/jcs.000604. [DOI] [PubMed] [Google Scholar]
- Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, Tominaga M, Irie K, Hayashi S, Sueoka E. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. J Cancer Res Clin Oncol. 2006;132:150–158. doi: 10.1007/s00432-005-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac M, Moneo MV, Carnero A, Benitez J, Martinez-Delgado B. Mitotic catastrophe cell death induced by heat shock protein 90 inhibitor in BRCA1-deficient breast cancer cell lines. Mol Cancer Ther. 2008;7:2358–2366. doi: 10.1158/1535-7163.MCT-08-0327. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Hlubek F, Pfeiffer S, Budczies J, Spaderna S, Jung A, Kirchner T, Brabletz T. Securin (hPTTG1) expression is regulated by beta-catenin/TCF in human colorectal carcinoma. Br J Cancer. 2006;94:1672–1677. doi: 10.1038/sj.bjc.6603155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbagabriel S, Fernando M, Waldman FM, Bose S, Heaney AP. Securin is overexpressed in breast cancer. Mod Pathol. 2005;18:985–990. doi: 10.1038/modpathol.3800382. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Crescenzi E, Palumbo G, de Boer J, Brady HJ. Ataxia telangiectasia mutated and p21CIP1 modulate cell survival of drug-induced senescent tumor cells: implications for chemotherapy. Clin Cancer Res. 2008;14:1877–1887. doi: 10.1158/1078-0432.CCR-07-4298. [DOI] [PubMed] [Google Scholar]
- Cortez D. Caffeine inhibits checkpoint responses without inhibiting the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases. J Biol Chem. 2003;278:37139–37145. doi: 10.1074/jbc.M307088200. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Tsuchiya H, Yamamoto N, Hayashi K, Yamauchi K, Kawahara M, Miyamoto K, Tomita K. Caffeine-potentiated chemotherapy for patients with high-grade soft tissue sarcoma: long-term clinical outcome. Anticancer Res. 2007;27:3489–3495. [PubMed] [Google Scholar]
- Qamar L, Davis R, Anwar A, Behbakht K. Protein kinase C inhibitor Go6976 augments caffeine-induced reversal of chemoresistance to cis-diamminedichloroplatinum-II (CDDP) in a human ovarian cancer model. Gynecol Oncol. 2008;110:425–431. doi: 10.1016/j.ygyno.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Shinkawa M, Torimura T, Nakamura T, Selvendiran K, Sakamoto M, Koga H, Ueno T, Sata M. Cell cycle regulation by the Wee1 inhibitor PD0166285, pyrido [2,3-d] pyimidine, in the B16 mouse melanoma cell line. BMC Cancer. 2006;6:292. doi: 10.1186/1471-2407-6-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton M, Chung G, Yakowec P, Wong A, Powers D, Xiong L, Zhang N, Leal J, Bush TL, Santora V, et al. Discovery and evaluation of dual CDK1 and CDK2 inhibitors. Cancer Res. 2006;66:4299–4308. doi: 10.1158/0008-5472.CAN-05-2507. [DOI] [PubMed] [Google Scholar]
- Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, Schwartz GK. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clin Cancer Res. 2001;7:4209–4219. [PubMed] [Google Scholar]
- Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230–235. [PubMed] [Google Scholar]
- Li Y, Tanaka K, Li X, Okada T, Nakamura T, Takasaki M, Yamamoto S, Oda Y, Tsuneyoshi M, Iwamoto Y. Cyclin-dependent kinase inhibitor, flavopiridol, induces apoptosis and inhibits tumor growth in drug-resistant osteosarcoma and Ewing's family tumor cells. Int J Cancer. 2007;121:1212–1218. doi: 10.1002/ijc.22820. [DOI] [PubMed] [Google Scholar]
- Smith ME, Cimica V, Chinni S, Challagulla K, Mani S, Kalpana GV. Rhabdoid tumor growth is inhibited by flavopiridol. Clin Cancer Res. 2008;14:523–532. doi: 10.1158/1078-0432.CCR-07-1347. [DOI] [PubMed] [Google Scholar]
- Jackman KM, Frye CB, Hunger SP. Flavopiridol displays preclinical activity in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50:772–778. doi: 10.1002/pbc.21386. [DOI] [PubMed] [Google Scholar]
- Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK. Flavopiridol enhances the effect of docetaxel in vitro and in vivo in human gastric cancer cells. Mol Cancer Ther. 2003;2:549–555. [PubMed] [Google Scholar]
- Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997;57:3375–3380. [PubMed] [Google Scholar]
- Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999;5:1876–1883. [PubMed] [Google Scholar]
- George S, Kasimis BS, Cogswell J, Schwarzenberger P, Shapiro GI, Fidias P, Bukowski RM. Phase I study of flavopiridol in combination with Paclitaxel and Carboplatin in patients with non-small-cell lung cancer. Clin Lung Cancer. 2008;9:160–165. doi: 10.3816/CLC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- Jung CP, Motwani MV, Schwartz GK. Flavopiridol increases sensitization to gemcitabine in human gastrointestinal cancer cell lines and correlates with down-regulation of ribonucleotide reductase M2 subunit. Clin Cancer Res. 2001;7:2527–2536. [PubMed] [Google Scholar]
- Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–90. doi: 10.1016/j.phymed.2008.04.006. Epub 2008 Jun 9. [DOI] [PubMed] [Google Scholar]
- Lin CC, Lin SY, Chung JG, Lin JP, Chen GW, Kao ST. Down-regulation of cyclin B1 and up-regulation of Wee1 by berberine promotes entry of leukemia cells into the G2/M-phase of the cell cycle. Anticancer Res. 2006;26:1097–1104. [PubMed] [Google Scholar]
- Lin JP, Yang JS, Lee JH, Hsieh WT, Chung JG. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol. 2006;12:21–28. doi: 10.3748/wjg.v12.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GT, Li G, Mantei RA, Chen Z, Kovar P, Gu W, Xiao Z, Zhang H, Sham HL, Sowin T, et al. 1-(5-Chloro-2-alkoxyphenyl)-3-(5-cyanopyrazin-2-yl)ureas [correction of cyanopyrazi] as potent and selective inhibitors of Chk1 kinase: synthesis, preliminary SAR, and biological activities. J Med Chem. 2005;48:3118–3121. doi: 10.1021/jm048989d. [DOI] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–1960. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- Tse AN, Sheikh TN, Alan H, Chou TC, Schwartz GK. 90-kDa heat shock protein inhibition abrogates the topoisomerase I poison-induced G2/M checkpoint in p53-null tumor cells by depleting Chk1 and Wee1. Mol Pharmacol. 2009;75:124–133. doi: 10.1124/mol.108.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SJ, Dai NT, Heldebrant MP, Vroman BT, Smith BD, Karp JE, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106:318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- Matthews DJ, Yakes FM, Chen J, Tadano M, Bornheim L, Clary DO, Tai A, Wagner JM, Miller N, Kim YD, et al. Pharmacological abrogation of S-phase checkpoint enhances the anti-tumor activity of gemcitabine in vivo. Cell Cycle. 2007;6:104–110. doi: 10.4161/cc.6.1.3699. [DOI] [PubMed] [Google Scholar]
- Tse AN, Rendahl KG, Sheikh T, Cheema H, Aardalen K, Embry M, Ma S, Moler EJ, Ni ZJ, Lopes de Menezes DE, et al. CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin Cancer Res. 2007;13:591–602. doi: 10.1158/1078-0432.CCR-06-1424. [DOI] [PubMed] [Google Scholar]
- Blasina A, Hallin J, Chen E, Arango ME, Kraynov E, Register J, Grant S, Ninkovic S, Chen P, Nichols T, et al. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase. Mol Cancer Ther. 2008;7:2394–2404. doi: 10.1158/1535-7163.MCT-07-2391. [DOI] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Nylandsted J, Lukas C, Lukas J, Bartek J. Inhibition of Chk1 by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing Radiation. Cancer Res. 2004;64:9035–9040. doi: 10.1158/0008-5472.CAN-04-2434. [DOI] [PubMed] [Google Scholar]
- Li G, Hasvold LA, Tao ZF, Wang GT, Gwaltney SL, 2nd, Patel J, Kovar P, Credo RB, Chen Z, Zhang H, et al. Synthesis and biological evaluation of 1-(2,4,5-trisubstituted phenyl)-3-(5-cyanopyrazin-2-yl)ureas as potent Chk1 kinase inhibitors. Bioorg Med Chem Lett. 2006;16:2293–2298. doi: 10.1016/j.bmcl.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Yu Q, La Rose J, Zhang H, Takemura H, Kohn KW, Pommier Y. UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1. Cancer Res. 2002;62:5743–5748. [PubMed] [Google Scholar]
- Didier C, Cavelier C, Quaranta M, Galcera MO, Demur C, Laurent G, Manenti S, Ducommun B. G2/M checkpoint stringency is a key parameter in the sensitivity of AML cells to genotoxic stress. Oncogene. 2008;27:3811–3820. doi: 10.1038/sj.onc.1211041. [DOI] [PubMed] [Google Scholar]
- Playle LC, Hicks DJ, Qualtrough D, Paraskeva C. Abrogation of the radiation-induced G2 checkpoint by the staurosporine derivative UCN-01 is associated with radiosensitisation in a subset of colorectal tumour cell lines. Br J Cancer. 2002;87:352–358. doi: 10.1038/sj.bjc.6600492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortmansky J, Shah MA, Kaubisch A, Weyerbacher A, Yi S, Tong W, Sowers R, Gonen M, O'Reilly E, Kemeny N, et al. Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol. 2005;23:1875–1884. doi: 10.1200/JCO.2005.03.116. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Oza A, Winquist EW, Moore M, Chen EX, Brown S, Pond GR, Dancey JE, Hirte HW. Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II Consortium study. Ann Oncol. 2006;17:334–340. doi: 10.1093/annonc/mdj076. [DOI] [PubMed] [Google Scholar]
- Lara PN, Jr, Mack PC, Synold T, Frankel P, Longmate J, Gumerlock PH, Doroshow JH, Gandara DR. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res. 2005;11:4444–4450. doi: 10.1158/1078-0432.CCR-04-2602. [DOI] [PubMed] [Google Scholar]
- Welch S, Hirte HW, Carey MS, Hotte SJ, Tsao MS, Brown S, Pond GR, Dancey JE, Oza AM. UCN-01 in combination with topotecan in patients with advanced recurrent ovarian cancer: a study of the Princess Margaret Hospital Phase II consortium. Gynecol Oncol. 2007;106:305–310. doi: 10.1016/j.ygyno.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Edelman MJ, Bauer KS, Jr, Wu S, Smith R, Bisacia S, Dancey J. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clin Cancer Res. 2007;13:2667–2674. doi: 10.1158/1078-0432.CCR-06-1832. [DOI] [PubMed] [Google Scholar]
- Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, Marumoto T, Saya H, Horii A. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res. 2005;65:2899–2905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006;16:356–366. doi: 10.1038/sj.cr.7310046. [DOI] [PubMed] [Google Scholar]
- Soncini C, Carpinelli P, Gianellini L, Fancelli D, Vianello P, Rusconi L, Storici P, Zugnoni P, Pesenti E, Croci V, et al. PHA-680632, a novel Aurora kinase inhibitor with potent antitumoral activity. Clin Cancer Res. 2006;12:4080–4089. doi: 10.1158/1078-0432.CCR-05-1964. [DOI] [PubMed] [Google Scholar]
- Gontarewicz A, Balabanov S, Keller G, Colombo R, Graziano A, Pesenti E, Benten D, Bokemeyer C, Fiedler W, Moll J, Brummendorf TH. Simultaneous targeting of Aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008;111:4355–4364. doi: 10.1182/blood-2007-09-113175. [DOI] [PubMed] [Google Scholar]
- Agnese V, Bazan V, Fiorentino FP, Fanale D, Badalamenti G, Colucci G, Adamo V, Santini D, Russo A. The role of Aurora-A inhibitors in cancer therapy. Ann Oncol. 2007;18:vi47–52. doi: 10.1093/annonc/mdm224. [DOI] [PubMed] [Google Scholar]
- Fiskus W, Wang Y, Joshi R, Rao R, Yang Y, Chen J, Kolhe R, Balusu R, Eaton K, Lee P, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14:6106–6115. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Zhang P, Frascogna V, Lecluse Y, Auperin A, Bourhis J, Deutsch E. Enhancement of radiation response by inhibition of Aurora-A kinase using siRNA or a selective Aurora kinase inhibitor PHA680632 in p53-deficient cancer cells. Br J Cancer. 2007;97:1664–1672. doi: 10.1038/sj.bjc.6604083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- Arlot-Bonnemains Y, Baldini E, Martin B, Delcros JG, Toller M, Curcio F, Ambesi-Impiombato FS, D'Armiento M, Ulisse S. Effects of the Aurora kinase inhibitor VX-680 on anaplastic thyroid cancer-derived cell lines. Endocr Relat Cancer. 2008;15:559–568. doi: 10.1677/ERC-08-0021. [DOI] [PubMed] [Google Scholar]
- Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, Tsang YT, Armaiz-Pena GN, Lu C, Kamat AA, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008;14:5437–5446. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara D, Matsunaga S, Kawabe A, Fujimoto S, Noda M, Uchiyama S, Fukui K. Aurora kinase is required for chromosome segregation in tobacco BY-2 cells. Plant J. 2006;48:572–580. doi: 10.1111/j.1365-313X.2006.02893.x. [DOI] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby E, Walsh V, Pepper C, Burnett A, Mills K. Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica. 2008;93:662–669. doi: 10.3324/haematol.12148. [DOI] [PubMed] [Google Scholar]
- Long ZJ, Xu J, Yan M, Zhang JG, Guan Z, Xu DZ, Wang XR, Yao J, Zheng FM, Chu GL, et al. ZM 447439 inhibition of aurora kinase induces Hep2 cancer cell apoptosis in three-dimensional culture. Cell Cycle. 2008;7:1473–1479. doi: 10.4161/cc.7.10.5949. [DOI] [PubMed] [Google Scholar]
- Cogswell JP, Brown CE, Bisi JE, Neill SD. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ. 2000;11:615–623. [PubMed] [Google Scholar]
- Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhang X, Sun G, Guo X, Li H, You Y, Gardner K, Yuan D, Xu Z, Du Q, et al. RNA Interference-Mediated Silencing of the Polo-like Kinase 1 Gene Enhances Chemosensitivity to Gemcitabine in Pancreatic Adenocarcinoma Cells. J Cell Mol Med. 2008;12:2334–49. doi: 10.1111/j.1582-4934.2008.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N, Jiang J, Holland J, Reddy EP. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–286. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Coukos G. MicroRNAs: a new insight into cancer genome. Cell Cycle. 2006;5:2216–2219. doi: 10.4161/cc.5.19.3319. [DOI] [PubMed] [Google Scholar]
- Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle. 2007;6:2127–2132. doi: 10.4161/cc.6.17.4641. [DOI] [PubMed] [Google Scholar]
- Rezende RA, Bisol T, Hammersmith K, Rapuano CJ, Lima AL, Webster GF, Freitas JF, Laibson PR, Cohen EJ. Efficacy of oral antiviral prophylaxis in preventing ocular herpes simplex virus recurrences in patients with and without self-reported atopy. Am J Ophthalmol. 2006;142:563–567. doi: 10.1016/j.ajo.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, et al. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Iwatsuki M, Ieta K, Ohta D, Haraguchi N, Mimori K, Mori M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008;99:1871–1877. doi: 10.1111/j.1349-7006.2008.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- Wu A, Oh S, Wiesner SM, Ericson K, Chen L, Hall WA, Champoux PE, Low WC, Ohlfest JR. Persistence of CD133+ cells in human and mouse glioma cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem Cells Dev. 2008;17:173–184. doi: 10.1089/scd.2007.0133. [DOI] [PubMed] [Google Scholar]
- Chumsri S, Burger AM. Cancer stem cell targeted agents: therapeutic approaches and consequences. Curr Opin Mol Ther. 2008;10:323–333. [PubMed] [Google Scholar]