Abstract
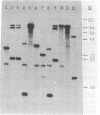
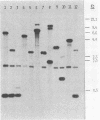
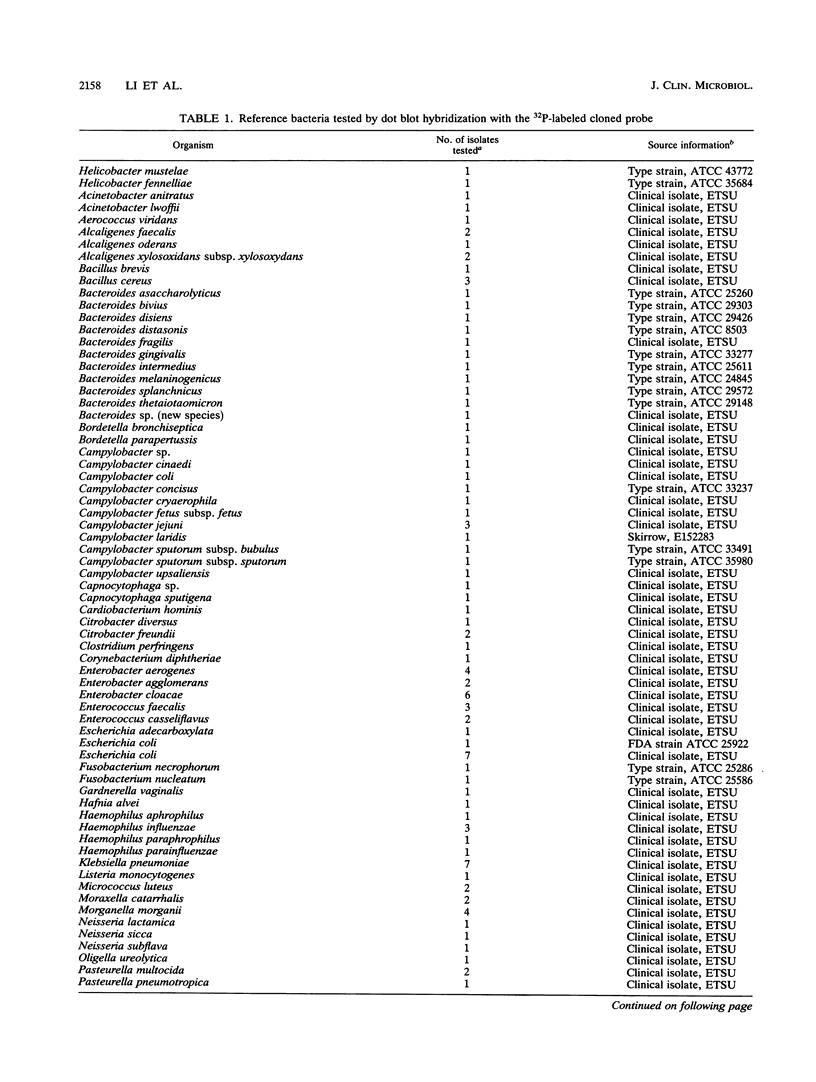
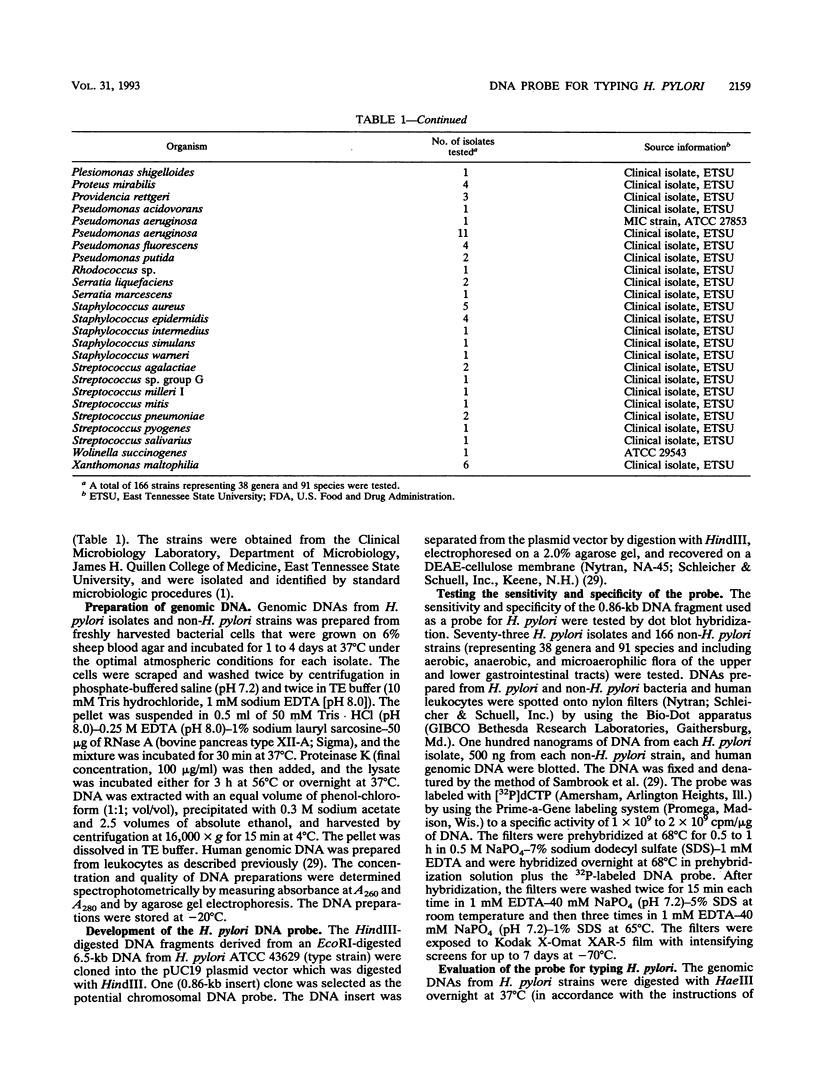
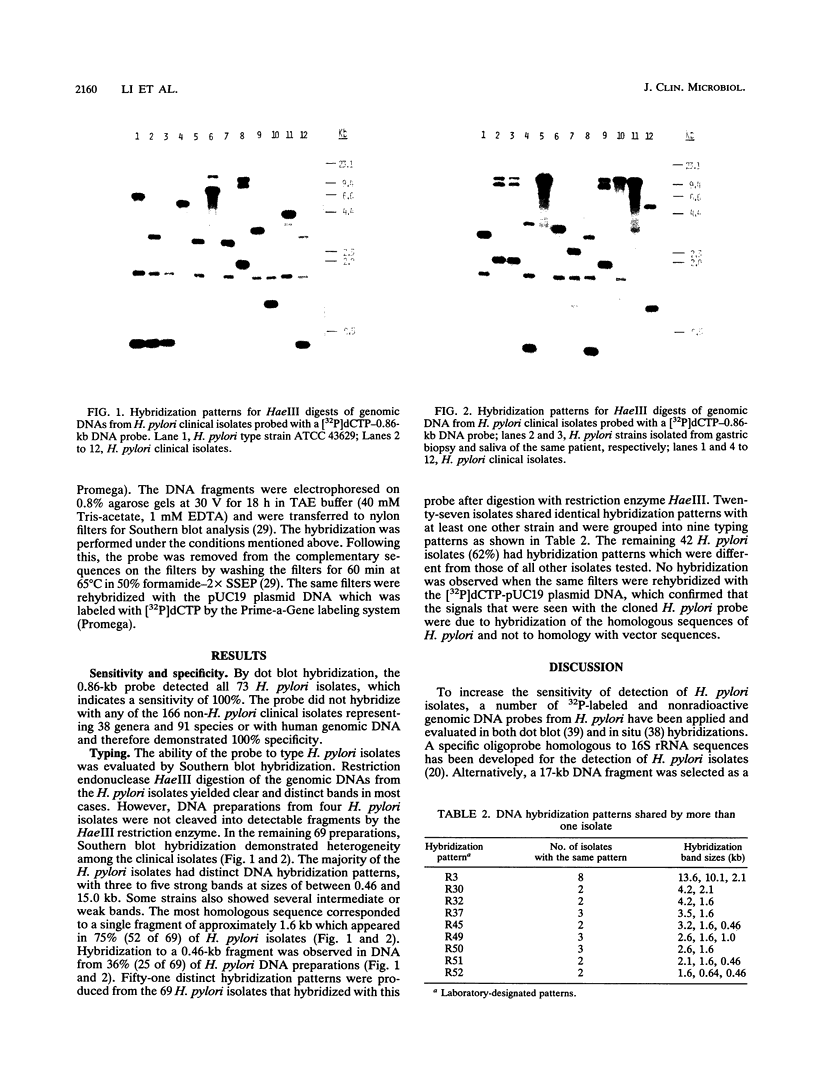
HindIII-digested DNA fragments derived from an EcoRI-digested 6.5-kb fragment of chromosomal DNA prepared from Helicobacter pylori ATCC 43629 (type strain) were cloned into the pUC19 vector. A 0.86-kb insert was identified as a potential chromosomal DNA probe. The specificity of the probe was evaluated by testing 166 non-H. pylori bacterial strains representing 38 genera and 91 species which included aerobic, anaerobic, and microaerophilic flora of the upper and lower gastrointestinal tracts. None of the 166 non-H. pylori strains hybridized with this probe (100% specificity), and the sensitivity of this probe was also 100% when H. pylori isolates from 72 patients with gastritis and with the homologous ATCC type strain were tested by dot blot hybridization. The capability of this probe for differentiating between strains of H. pylori was evaluated by Southern blot hybridization of HaeIII-digested chromosomal DNA from 68 clinical isolates and the homologous ATCC type strain of H. pylori. Fifty-one unique hybridization patterns were seen among the 69 strains tested, demonstrating considerable genotypic variation among H. pylori clinical isolates. We propose that this probe would be of significant value for conducting epidemiologic studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton C. L., Kleanthous H., Coates P. J., Morgan D. D., Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. L., Wren B. W., Mullany P., Topping A., Tabaqchali S. Molecular cloning and expression of Campylobacter pylori species-specific antigens in Escherichia coli K-12. Infect Immun. 1989 Feb;57(2):623–629. doi: 10.1128/iai.57.2.623-629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas M., Owen R. J., Bickley J., Morgan D. R. Molecular techniques for studying the epidemiology of infection by Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;181:20–32. doi: 10.3109/00365529109093204. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D., Sitas F., Newell D. G., Stacey A. R., Boreham J., Peto R., Campbell T. C., Li J., Chen J. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990 Oct 15;46(4):608–611. doi: 10.1002/ijc.2910460410. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Paster B. J., Dewhirst F. E., Taylor N. S., Yan L. L., Macuch P. J., Chmura L. M. Helicobacter mustelae isolation from feces of ferrets: evidence to support fecal-oral transmission of a gastric Helicobacter. Infect Immun. 1992 Feb;60(2):606–611. doi: 10.1128/iai.60.2.606-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall P. A., Hu L. T., Mobley H. L. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. J Clin Microbiol. 1992 Mar;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Hammar M., Tyszkiewicz T., Wadström T., O'Toole P. W. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):54–58. doi: 10.1128/jcm.30.1.54-58.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. A., Hoyle J. A., Lewis F. A., Secker A. D., Cross D., Mapstone N. P., Dixon M. F., Wyatt J. I., Tompkins D. S., Taylor G. R. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol. 1991 Nov;29(11):2543–2549. doi: 10.1128/jcm.29.11.2543-2549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks A. C. Helicobacter pylori (formerly Campylobacter pyloridis/pylori) 1986-1989: a review. J Clin Pathol. 1990 May;43(5):353–356. doi: 10.1136/jcp.43.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Morgan D. D., Owen R. J. Use of DNA restriction endonuclease digest and ribosomal RNA gene probe patterns to fingerprint Helicobacter pylori and Helicobacter mustelae isolated from human and animal hosts. Mol Cell Probes. 1990 Aug;4(4):321–334. doi: 10.1016/0890-8508(90)90023-s. [DOI] [PubMed] [Google Scholar]
- Morotomi M., Hoshina S., Green P., Neu H. C., LoGerfo P., Watanabe I., Mutai M., Weinstein I. B. Oligonucleotide probe for detection and identification of Campylobacter pylori. J Clin Microbiol. 1989 Dec;27(12):2652–2655. doi: 10.1128/jcm.27.12.2652-2655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudbier J. H., Langenberg W., Rauws E. A., Bruin-Mosch C. Genotypical variation of Campylobacter pylori from gastric mucosa. J Clin Microbiol. 1990 Mar;28(3):559–565. doi: 10.1128/jcm.28.3.559-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Bickley J., Costas M., Morgan D. R. Genomic variation in Helicobacter pylori: application to identification of strains. Scand J Gastroenterol Suppl. 1991;181:43–50. doi: 10.3109/00365529109093207. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Fraser J., Costas M., Morgan D., Morgan D. R. Signature patterns of DNA restriction fragments of Helicobacter pylori before and after treatment. J Clin Pathol. 1990 Aug;43(8):646–649. doi: 10.1136/jcp.43.8.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Hunton C., Bickley J., Moreno M., Linton D. Ribosomal RNA gene restriction patterns of Helicobacter pylori: analysis and appraisal of Hae III digests as a molecular typing system. Epidemiol Infect. 1992 Aug;109(1):35–47. [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Vandersteen D., Goates J., Sibley R. K., Pritikin J., Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991 May 1;83(9):640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- Queiroz D. M., Mendes E. N., Rocha G. A. Indicator medium for isolation of Campylobacter pylori. J Clin Microbiol. 1987 Dec;25(12):2378–2379. doi: 10.1128/jcm.25.12.2378-2379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames B., Krajden S., Fuksa M., Babida C., Penner J. L. Evidence for the occurrence of the same strain of Campylobacter pylori in the stomach and dental plaque. J Clin Microbiol. 1989 Dec;27(12):2849–2850. doi: 10.1128/jcm.27.12.2849-2850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T. V., Schroth M. N. Bacterial culture preservation in frozen and dry-film methylcellulose. Appl Environ Microbiol. 1981 Nov;42(5):872–877. doi: 10.1128/aem.42.5.872-877.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Eaton M., Chang N., Salama S. M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992 Nov;174(21):6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Blaser M. J. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- Tee W., Lambert J., Smallwood R., Schembri M., Ross B. C., Dwyer B. Ribotyping of Helicobacter pylori from clinical specimens. J Clin Microbiol. 1992 Jun;30(6):1562–1567. doi: 10.1128/jcm.30.6.1562-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. L., Arthur R. R., Mobley H. L., Dick J. D. Detection of Helicobacter pylori by using the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):689–695. doi: 10.1128/jcm.29.4.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg F. M., Zijlmans H., Langenberg W., Rauws E., Schipper M. Detection of Campylobacter pylori in stomach tissue by DNA in situ hybridisation. J Clin Pathol. 1989 Sep;42(9):995–1000. doi: 10.1136/jcp.42.9.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherall B. L., McDonald P. J., Johnson A. M. Detection of Campylobacter pylori DNA by hybridisation with non-radioactive probes in comparison with a 32P-labelled probe. J Med Microbiol. 1988 Aug;26(4):257–263. doi: 10.1099/00222615-26-4-257. [DOI] [PubMed] [Google Scholar]