Abstract
Oxidative stress has been implicated as a key event in the degenerative process of dopaminergic neurons; however, the cellular mechanisms underlying chronic oxidative stress-induced neurodegeneration remain to be established. In this study, N27 cells, a dopaminergic neuronal cell line derived from rat mesencephalon, exposed to low doses of H2O2 (0–30 μM for 12–24 hr) exhibited dose- and time-dependent increases in cytotoxicity and ROS generation. In addition, the H2O2-induced neurotoxicity was accompanied by increased caspase-3 activity and PKCδ cleavage. Notably, treatment with antioxidants Trolox and MnTBAP or PKCδ cleavage inhibitor zDIPDfmk significantly protected against oxidative stress-induced apoptotic cell death. These results demonstrate that the N27 cell line is a useful model for the study of the chronic low dose oxidative stress-induced apoptotic cell death cascade and that caspase-3-dependent PKCδ proteolytic activation may be important in the apoptotic process in dopaminergic neurons undergoing chronic oxidative insult.
INTRODUCTION
The cellular mechanisms underlying nigral dopaminergic degeneration associated with various pathological conditions such as Parkinson’s disease (PD) and methamphetamine abuse are yet to be clarified. In recent years, studies have implicated oxidative stress as a key contributor to dopaminergic cell degeneration, including PD-associated degeneration.1, 2 Oxidative damage triggered by reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) can activate a series of signaling events ultimately leading to programmed cell death, or apoptosis.3–5 Caspase-3 represents a convergence point for both mitochondria-dependent and -independent pathways in cells undergoing apoptotic cell death in response to oxidative stress6–8 or to various dopaminergic neurotoxins.9–11 In fact, caspase-3 activity is significantly increased in the brains of PD patients.12–14 Consistent with these reports, we have demonstrated that oxidative stress induced by exposure to neurotoxins such as methylcyclopentadienyl manganese tricarbonyl (MMT), 1-methyl–4-phenylpyridinium (MPP+), or dieldrin causes cell death in dopaminergic neurons via caspase-3 activation and the subsequent proteolytic cleavage of the proapoptotic kinase protein kinase C-δ (PKCδ).15–18
Hydrogen peroxide has been widely used to induce oxidative stress-mediated apoptosis in various cellular models.15–18 Recently, our lab demonstrated that high doses of H2O2 (100–300 μM) induce dose-dependent increases in cytotoxicity, caspase-3 activity, and PKCδ cleavage over short exposure periods (4 hr) in a mesencephalic dopaminergic neuronal cell line (N27).19 However, the mechanisms underlying chronic low dose oxidative stress-induced apoptosis in dopaminergic cells have not been well established. Herein, we sought to investigate the susceptibility of the N27 dopaminergic cell line to chronic low doses of H2O2 (3–30 μM), and to delineate oxidative stress-dependent cell death signaling events. Our results demonstrate increases in cytotoxicity, caspase-3 activity, and PKCδ cleavage in N27 cells following longer term exposure (12–24 hr) to approximately 10-fold lower doses of H2O2 (3–30 μM) than those used in the previous study.19 We also show that a competitive peptide inhibitor, Z-Asp(OMe)-Ile-Pro-Asp(OMe)-FMK (z-DIPD-fmk), which mimics the caspase-3 cleavage site of PKCδ, can effectively reduce the cleavage and activation of PKCδ, caspase-3 activity, and DNA fragmentation during H2O2 exposure in this model. Furthermore, treatment with antioxidants such as Trolox, MnTBAP, or the PKCδ cleavage inhibitor zDIPDfmk significantly attenuated apoptotic cell death. These results indicate that the N27 cell line is a useful model for the study of chronic low dose oxidative stress-induced apoptosis in dopaminergic degeneration. Importantly, our results highlight the significance of PKCδ proteolytic signaling in the demise of dopaminergic cells during chronic oxidative insult.
MATERIALS AND METHODS
Chemicals
Hydrogen peroxide (H2O2, 30% w/v) was obtained from Sigma-Aldrich (St. Louis, MO). RPMI 1640, fetal bovine serum (FBS), L-glutamine, penicillin, and streptomycin were purchased from InVitrogen (Gaithersburg, MD). SYTOX® Green and dihydroethidine (DhEt) dyes were acquired from InVitrogen (Gaithersburg, MD); Ac-DEVD-AFC (caspase-3 substrate) and zDIPDfmk (PKCδ cleavage inhibitor) were obtained from MP Biomedicals (Irvine, CA); Cell Death Detection ELISA Plus Assay Kit was purchased from Roche Biochemicals (Indianapolis, IN); PKCδ antibody was acquired from Santa Cruz Laboratories (Santa Cruz, CA).
Cell culture and treatment paradigm
N27 cells were maintained in RPMI 1640 with 10% FBS and supplementary penicillin/streptomycin and L-glutamine in T-175 culture flasks or 6- or 24-well sterile culture plates at 37°C in humidified CO2. Cells were treated with 3–30 μM H2O2 with or without inhibitors 24 hours after plating and were harvested post-treatment for analysis.
ROS generation assay
Reactive oxygen species (ROS) generation was detected as previously described.15 Cells grown in T-175 flasks were harvested with trypsin-EDTA and resuspended in serum-free Hanks’ balanced salt solution (HBSS). Dihydroethidine (DhEt), a sodium borohydride-reduced derivative of ethidium bromide, was used to detect the ROS O2·−. A final concentration of 1 μM DhEt was added to a 1-ml cell suspension and measured for oxidative changes upon addition of 0, 10, or 30 μM H2O2 with or without inhibitors using a Becton Dickenson FACScan™ flow cytometer (Becton Dickenson, San Francisco, CA). Data were analyzed with Cellquest™ data analysis software (Becton Dickenson, San Francisco, CA).
Cytotoxicity measurement
N27 cells were grown in 24-well plates and exposed to 0, 3, 10, or 30 μM H2O2. The cells were treated with 1 μM SYTOX® Green, a cell-impermeable dye that enters dead cells and intercalates with DNA to produce green fluorescence.20 Dead cells were then quantified by measuring fluorescence excitation at 485 nm and emission at 538 nm using a multi-well plate reader (SpectraMax Gemini XS Model, Molecular Devices, Sunnyvale, CA).
Enzymatic detection of caspase-3 activation
Caspase-3 activity was measured as previously described, using 50 μM Ac-DEVD-AMC as the fluorometric caspase-3 substrate for the reaction. 15 Caspase activity was measured at 405 nm with a SpectraMax microplate reader.
Western blot analysis
Following exposure to 3, 10, or 30 μM H2O2, cells were resuspended in 500 μl homogenization buffer (20 mM Tris HCl (pH 8.0), 2 mM EDTA, 10 mM EGTA, 2 mM DTT, 1 mM PMSF, 25 μg/ml aprotinin, and 10 μg/ml leupeptin), sonicated, and centrifuged at 10,000 × g for 1 hour at 4°C as described previously.15 Cells were also cotreated with or without 50 μM zDIPDfmk (PKCδ cleavage inhibitor). Proteins were separated on a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane, which was blotted with PKCδ polyclonal antibody (1:2000). Western blot was also performed using IR dye-800 conjugated anti-rabbit dye and Alexa Flour 680 conjugated anti-mouse IgG as secondary antibodies. To confirm equal protein loading, blots were reprobed with a β-actin antibody (1:5000 dilution). Western blot images were captured and analyzed with either a Kodak 2000 MM imaging system or Odyssey IR Imaging system (LICOR).
DNA fragmentation assay
DNA fragmentation was measured using a Cell Death Detection ELISA Plus Assay Kit, a fast, highly sensitive, and reliable assay for the detection of early apoptotic death, as described previously.18 After treatment with 0 or 10 μM H2O2 with or without inhibitors, cells were centrifuged at 200 × g for 5 minutes and washed once with phosphate buffered saline (PBS). Cell were then lysed in 450 μl of lysis buffer (included with kit) and centrifuged at 5,000 rpm for 10 minutes. The resulting supernatant was collected and used to determine DNA fragmentation per the manufacturer’s protocol.
RESULTS
Chronic exposure to low dose H2O2 induces a time- and dose-dependent increase in cytotoxic cell death in N27 mesencephalic dopaminergic neuronal cells
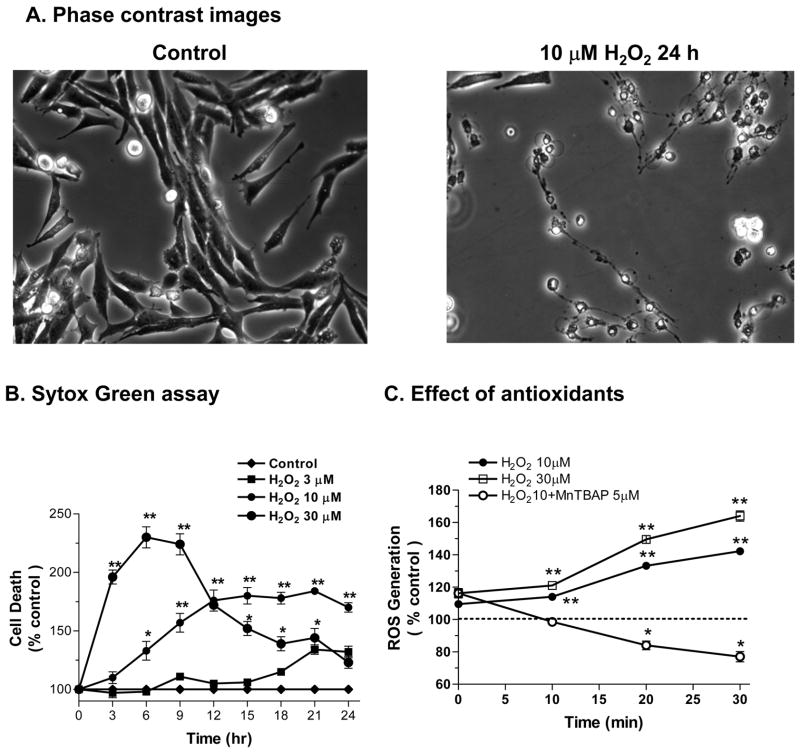
We have previously shown that N27 cells are sensitive to acute exposure to 100 μM H2O2 for 4 hr.19 Therefore, we examined whether a 24 hr exposure to low dose H2O2 (3–30 μM) is cytotoxic to N27 cells using the Sytox® Green assay. An increase in the number of Sytox-positive green cells indicates an increase in cell death, because the Sytox green dye permeates compromised cell membranes to stain nuclear chromatin. N27 cells were exposed to three low doses (3, 10, and 30 μM) of H2O2 for 24 hr. Quantitative analysis of Sytox fluorescence using a fluorescence plate reader revealed that H2O2 induced a dose-dependent increase in cytotoxic cell death (Fig 1B). Exposure to 3 and 10 μM H2O2 increased the cytotoxicity over time, peaking around 24 hr, whereas the 30 μM H2O2-induced increase in cytotoxicity peaked at an earlier time period between 9 and 12 hr, and then gradually decreased to almost baseline levels at 24 hr. A 12 hr exposure resulted in a 5, 72 and 124% increase in the number of Sytox-positive cells in 3, 10, and 30 μM H2O2-treated N27 cells, respectively (compared to untreated cells; Fig 1B). Figure 1A shows the phase-contrast images of N27 cells before and after treatment with 10 μM H2O2 for 24 hr. The cells were significantly reduced in number and size in H2O2-treated N27 cells. Together, these data suggest that N27 dopaminergic cells are sensitive to chronic oxidative damage.
Figure 1. Chronic exposure of low dose H2O2 increases ROS generation and neurotoxicity in N27 dopaminergic cells.
(A) Phase contrast images showing N27 cells before and after treatment with 10 μM H2O2 for 24 hr. (B) Sytox Green cytotoxicity assay: N27 cells were exposed to 3, 10, or 30 μM H2O2 over a 24-hr period. (C) Flow cytometric detection of ROS production: N27 cells were loaded with 10 μM dihydroethidine for 15 min and then treated with 10 and 30 μM H2O2 in the presence or absence of SOD mimetic or ROS inhibitor MnTBAP (5 μM). ROS was detected by flow cytometric measurements over a 30-min period. Data represent results from at least two independent measures performed in triplicate. *p<0.05 and **p<0.01 indicates significant differences compared to untreated control cells.
Chronic exposure to low dose H2O2 induces a time- and dose-dependent increase in ROS production in N27 cells
Next, we examined whether low dose H2O2 treatment generates ROS generation in N27 cells. The results show that low doses of H2O2 induced time- and dose-dependent increases in ROS production over a 30-min exposure (Fig 1C). Exposure to 10 and 30 μM H2O2 for 30 min resulted in 34 and 58% increases in ROS production, respectively, compared to untreated N27 cells. Pretreatment with the cell-permeable SOD mimetic MnTBAP (5 μM) completely blocked the 10 μM H2O2-induced increase in ROS production; interestingly, levels were below the baseline, suggesting that MnTBAP not only blocked the H2O2-induced increase in ROS production, but also decreased baseline ROS levels.
Antioxidants and PKCδ cleavage inhibitor attenuate H2O2-induced increases in caspase-3 enzyme activity
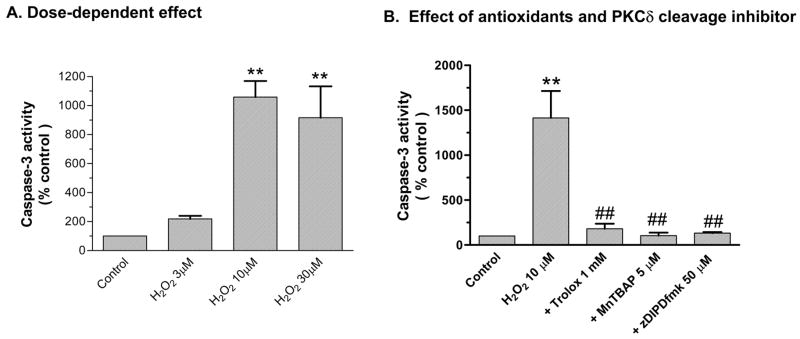
ROS can activate mitochondria- and caspase-dependent apoptotic cell death signaling pathways in both neuronal and non-neuronal cells. Herein, we examined activation of caspase-3, a key effector caspase, in N27 cells chronically exposed to low doses of H2O2. As shown in Fig 2A, exposure to 3, 10, and 30 μM H2O2 for 18 hr resulted in 2-, 10-, and 9-fold increases in caspase-3 enzyme activity. Co-treatment with ROS inhibitors Trolox (1 mM) and MnTBAP (5 μM) almost completely attenuated 10 μM H2O2-induced increases in caspase-3 enzyme activity (Fig 2B). Furthermore, as shown in Fig 2C, co-treatment with 50 μM zDIPDfmk almost completely suppressed 10 μM H2O2-induced increases in caspase-3 enzyme activity, possibly via a positive feedback mechanism reported in our previous studies (Fig 2C).15, 18 Together, these results suggest that antioxidants and the PKCδ cleavage inhibitor can modulate caspase-3 activation in H2O2-treated N27 cells.
Figure 2. Antioxidants and PKCδ cleavage inhibitor attenuate H2O2-induced increases in caspase-3 enzyme activity.
(A) Caspase-3 activity: N27 cells were exposed to 3, 10, and 30 μM H2O2 for 18 hr and activity was measured using fluorescent substrates. (B) Effect of antioxidants and PKCδ cleavage site blocker. N27 cells were exposed to H2O2 in the presence or absence of 5μM MnTBAP, 1 mM Trolox and 50 μM zDIPDfmk for 18 hr, followed by measurement of caspase-3 enzyme activity. Data represent results from at least two independent measures performed in triplicate. **p<0.01 and **p<0.001 indicates significant differences compared to untreated control cells and ##p<0.01 indicate significant differences between inhibitor-treated and H2O2-treated cells.
Chronic exposure to low dose H2O2 induces caspase-3-dependent proteolytic cleavage of PKCδ
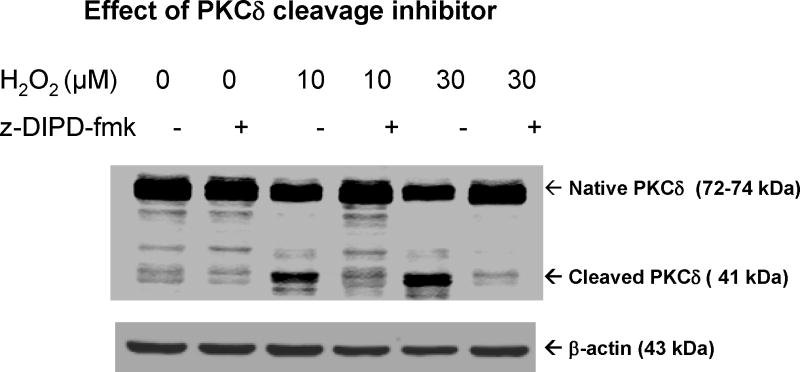
Recently, we and others demonstrated that PKCδ is a prominent endogenous substrate for caspase-3 in many different cell types undergoing apoptotic cell death.15, 17, 18, 21 Caspase-3 cleaves PKCδ to yield a 41 kDa catalytically active fragment and a 38 kDa regulatory subunit. Proteolytic cleavage of PKCδ results in the permanent dissociation of the regulatory domain from the catalytic fragment, resulting in a persistently active kinase. In the present study, we examined the effect of low dose H2O2 on PKCδ cleavage in N27 cells. As shown in Fig 3, a 24-hr exposure to 10 and 30 μM H2O2 dose-dependently induced proteolytic cleavage of PKCδ, compared to untreated N27 cells. Only a very weak cleavage of PKCδ was observed in untreated cells. Co-treatment with the cell-permeable PKCδ cleavage-specific inhibitor Z-DIPD-fmk (50 μM) almost completely attenuated 10 and 30 μM H2O2-induced PKCδ cleavage in N27 cells (Fig 3), suggesting that the cleavage is indeed mediated at the caspase-3 cleavage site. β-Actin western blot confirmed equal protein loading. Overall, these data suggest that proteolytic activation of PKCδ occurs via caspase-3 activation in N27 cells chronically exposed to low doses of H2O2.
Figure 3. Low dose chronic oxidative stress induces PKCδ proteolytic cleavage.
Cell lysates were isolated from N27 cells exposed to 10 or 30 μM H2O2 for 24 hr in the presence or absence of 50 μM z-DIPDfmk as described in methods. The proteins were resolved in SDS-PAGE and immunoblotted with anti-PKCδ antibody. To confirm equal protein loading in each lane, the membranes were reprobed with β-actin antibody. The immunoblots were visualized with Odyssey IR Imaging system (LICOR).
Antioxidants and PKCδ inhibitor rescue N27 cells from H2O2-induced apoptotic cell death
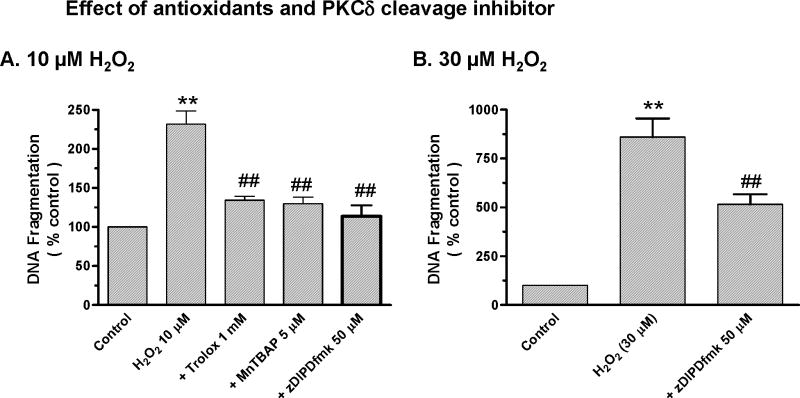
To understand the functional consequence of ROS production and caspase-3 and PKCδ activation, we performed a quantitative DNA fragmentation ELISA assay to determine whether low doses of H2O2 induced apoptosis. As shown in Fig 4, exposure to 10 μM H2O2 for 24 hr resulted in a significant increase in DNA fragmentation compared to untreated cells. Co-treatment with ROS inhibitors Trolox (1mM) or MnTBAP (5μM) or with PKCδ cleavage-specific inhibitor zDIPDfmk (50 μM) significantly suppressed 10 μM H2O2-induced increases in DNA-fragmentation (Fig 4A). Additionally, co-treatment with the PKCδ cleavage-specific inhibitor zDIPDfmk (50 μM) also significantly suppressed an even higher dose (30 μM) of H2O2-induced increases in DNA fragmentation (Fig 4B). Together, these data suggest that ROS, caspase-3, and PKCδ indeed mediate apoptotic cell death in H2O2-treated N27 cells, and that antioxidants and PKCδ inhibitor can rescue cells from apoptotic cell death during chronic oxidative insult.
Figure 4. Antioxidants and PKCδ cleavage site inhibitor attenuate apoptotic cell death during chronic oxidative insult.
N27 cells were exposed to 10 μM H2O2 for 24 hr and DNA fragmentation was measured using ELISA assay. N27 cells were exposed to H2O2 in the presence or absence of 5μM MnTBAP, 1 mM Trolox or 50 μM zDIPDfmk for 24 hr, followed by measurement of DNA fragmentation. Data represent results from at least two independent measures performed in triplicate. **p<0.01 indicates significant differences compared to untreated control cells and ##p<0.01 indicates significant differences between inhibitor-treated cells and H2O2-treated cells.
DISCUSSION
In the present study, N27 cells were exposed to low doses of H2O2 (3–30 μM) over an extended time period (6–24 hr) to ascertain the involvement of caspase-3 activation and PKCδ cleavage in apoptotic cell death. We demonstrate that chronic exposure to low dose H2O2 resulted in a sequential activation of ROS production, activation of caspase-3, proteolytic activation of PKCδ, and nuclear DNA breakdown. Furthermore, blockade of ROS production and caspase-3 activity with Trolox or MnTBAP can significantly rescue N27 cells from H2O2-induced apoptotic cell death. Additionally, suppression of caspase-3 activity and proteolytic activation of PKCδ with the cleavage-specific inhibitor zDIPDfmk rescues N27 cells from H2O2-induced apoptotic cell death. Previously, we showed that oxidative stress induced by neurotoxins mediates apoptosis in dopaminergic neurons via caspase-3 activation and proteolytic activation of PKCδ and that cleaved, active PKCδ regulates the caspase cascade by positive feedback amplification.7, 15–19, 21 Recently, we demonstrated caspase-3-dependent PKCδ cleavage mediates apoptotic cell death in an N27 cell model of PD exposed to high-dose oxidative stress (100–300 μM H2O2) over short exposure periods (4 hr).19 Together, our data strongly suggest that caspase-3 and proteolytic activation of PKCδ mediate oxidative stress-induced apoptotic cell death in N27 cells following low dose chronic H2O2 insult.
Environmental exposure to neurotoxic agents such as pesticides and metals is considered a dominant risk factor of PD.22–27 Additionally, methamphetamine abuse is known to cause damage to the nigrostriatal dopaminergic system via oxidative stress.28, 29 Due to the chronic nature of neurotoxin exposure in humans, chronic experimental models are needed to elucidate disease progression. While caspase-3-mediated apoptosis has been demonstrated in dopaminergic neurons exposed to low doses of the pesticide rotenone over a period of 11 hr10, the relevance of caspase-3-mediated proteolytic cleavage of the proapoptotic kinase PKCδ in the chronic cell culture PD model has not been established. So the recently established N27 cell culture model of dopaminergic degeneration was used for developing chronic exposure models in which the oxidative insult is generated by very low doses of H2O2 over a 24-hr exposure period. In this study, exposure to low dose (3–30 μM) H2O2 induced cytotoxic cell death and elevated intracellular ROS levels in a time- and dose-dependent manner. ROS was generated as early as 10 min and continued to increase over 30 min; these increases were completely blocked by the cell-permeable SOD mimetic MnTBAP. ROS has been shown to activate mitochondrial transition pore opening, which induces cytochrome c release and subsequent activation of initiator and effector caspases. In this study, low dose H2O2 treatment of N27 cells activated caspase-3 over 18 hr; this activation was effectively blocked by treatment with the antioxidant Trolox or SOD mimetic MnTBAP. These data are consistent with the effects of the other dopaminergic toxins dieldrin, MMT, manganese, MPP+, 6-OHDA, and methamphetamine in our cell culture models.
Recently, we and others established PKCδ as an emerging putative substrate for caspase-3.15, 18, 30 Chronic exposure to low dose H2O2 induces both caspase-3 activation and PKCδ cleavage in N27 cells, which can be effectively blocked by a cleavage-specific inhibitor, indicating that the cleavage is mediated by caspase-3. Proteolytic cleavage of PKCδ by caspase-3 results in the persistent activation of PKCδ, which then mediates apoptotic cell death in N27 cells.7, 15–18, 21, 30 DNA fragmentation and chromatin condensation are terminal events in the apoptotic process and are considered biochemical hallmarks of apoptosis.7, 15–19, 21, 30–32 Quantitative ELISA assays revealed that 24-hr exposure to low dose H2O2 induced chromatin condensation and DNA fragmentation in N27 cells. Furthermore, pretreatment with Trolox, MnTBAP, and zDIPDfmk significantly suppressed H2O2-induced DNA fragmentation, indicating that ROS, caspase-3, and PKCδ mediate the cell death event.
In conclusion, the present study demonstrates that low dose H2O2 induces a sequential activation of cell signaling events beginning with ROS production, and followed by caspase-3 activation and proteolytic activation of PKCδ and DNA fragmentation, which eventually results in apoptotic cell death. Although a number of cell lines are available for the study of oxidative damage, a well-characterized dopaminergic cell model is needed to investigate the mechanisms underlying nigrostriatal dopaminergic neurons. We show that a dose as low as 10 μM for 12 hr in the N27 cell model can induce some key oxidative signaling events including caspase-3 activation, PKCδ cleavage, and DNA fragmentation. Our data also suggest that caspase-3 activation and PKCδ cleavage may be important events in the apoptotic process in dopaminergic neurons undergoing chronic oxidative insult. Furthermore, our results demonstrate that antioxidants and a PKCδ cleavage site inhibitor can effectively intervene in PKCδ-dependent proapoptotic signaling in dopaminergic cells during low dose oxidative insult, further supporting the usefulness of the chronic low dose N27 cell culture model in testing the efficacy of novel neuroprotective agents.
Acknowledgments
This study was supported by grants from the National Institute of Health NS 38644, ES10586 and RO3 NS54016. W. Eugene and Linda Lloyd Endowed Professorship and Chair to AGK is acknowledged. The authors also acknowledge Ms. Keri Henderson for her assistance in the preparation of this manuscript.
References
- 1.Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal. 2005;7:685–93. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- 2.Casetta I, Govoni V, Granieri E. Oxidative stress, antioxidants and neurodegenerative diseases. Curr Pharm Des. 2005;11:2033–52. doi: 10.2174/1381612054065729. [DOI] [PubMed] [Google Scholar]
- 3.Tada-Oikawa S, et al. Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 2003;73:3277–88. doi: 10.1016/j.lfs.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Konat GW. Higher order chromatin degradation: implications for neurodegeneration. Neurochem Res. 2002;27:1447–51. doi: 10.1023/a:1021688119574. [DOI] [PubMed] [Google Scholar]
- 5.Chun HS, et al. Dopaminergic cell death induced by MPP(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem. 2001;76:1010–21. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 6.Ueda S, et al. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–14. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 7.Kanthasamy AG, et al. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–20. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- 8.Annunziato L, et al. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–33. doi: 10.1016/s0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 9.Akao Y, et al. Apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol, is mediated by activation of caspase 3. Neurosci Lett. 1999;267:153–6. doi: 10.1016/s0304-3940(99)00361-4. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi FA, et al. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem. 2003;87:914–21. doi: 10.1046/j.1471-4159.2003.02068.x. [DOI] [PubMed] [Google Scholar]
- 11.Turmel H, et al. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord. 2001;16:185–9. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann A, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:2875–80. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandini F, et al. Peripheral markers of apoptosis in Parkinson’s disease: the effect of dopaminergic drugs. Ann N Y Acad Sci. 2003;1010:675–8. doi: 10.1196/annals.1299.123. [DOI] [PubMed] [Google Scholar]
- 14.Blandini F, et al. Modifications of apoptosis-related protein levels in lymphocytes of patients with Parkinson’s disease. The effect of dopaminergic treatment. J Neural Transm. 2004;111:1017–30. doi: 10.1007/s00702-004-0123-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaul S, et al. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa M, et al. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–64. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 18.Anantharam V, et al. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–51. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaul S, et al. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J Biol Chem. 2005;280:28721–30. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- 20.Roth BL, et al. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–31. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, et al. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25:406–21. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Kanthasamy AG, et al. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–19. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Jenner P. Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci. 2001;24:245–7. doi: 10.1016/s0166-2236(00)01789-6. [DOI] [PubMed] [Google Scholar]
- 24.Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 25.Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J Neurol Sci. 2007;262:37–44. doi: 10.1016/j.jns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Li AA, et al. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47:1059–87. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- 27.Sun F, et al. Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacol Ther. 2007;114:327–44. doi: 10.1016/j.pharmthera.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Kanthasamy A, et al. Methamphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin-D in methamphetamine-induced apoptotic cell death. Ann N Y Acad Sci. 2006;1074:234–44. doi: 10.1196/annals.1369.022. [DOI] [PubMed] [Google Scholar]
- 29.Kanthasamy A, et al. Unilateral infusion of a dopamine transporter antisense into the substantia nigra protects against MDMA-induced serotonergic deficits in the ipsilateral striatum. Neuroscience. 2002;114:917–24. doi: 10.1016/s0306-4522(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 30.Reyland ME, et al. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–23. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 31.Nagata S. DNA degradation in development and programmed cell death. Annu Rev Immunol. 2005;23:853–75. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- 32.Antolin I, et al. Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res. 2002;943:163–73. doi: 10.1016/s0006-8993(02)02551-9. [DOI] [PubMed] [Google Scholar]