Abstract
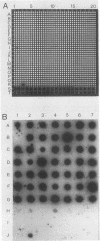
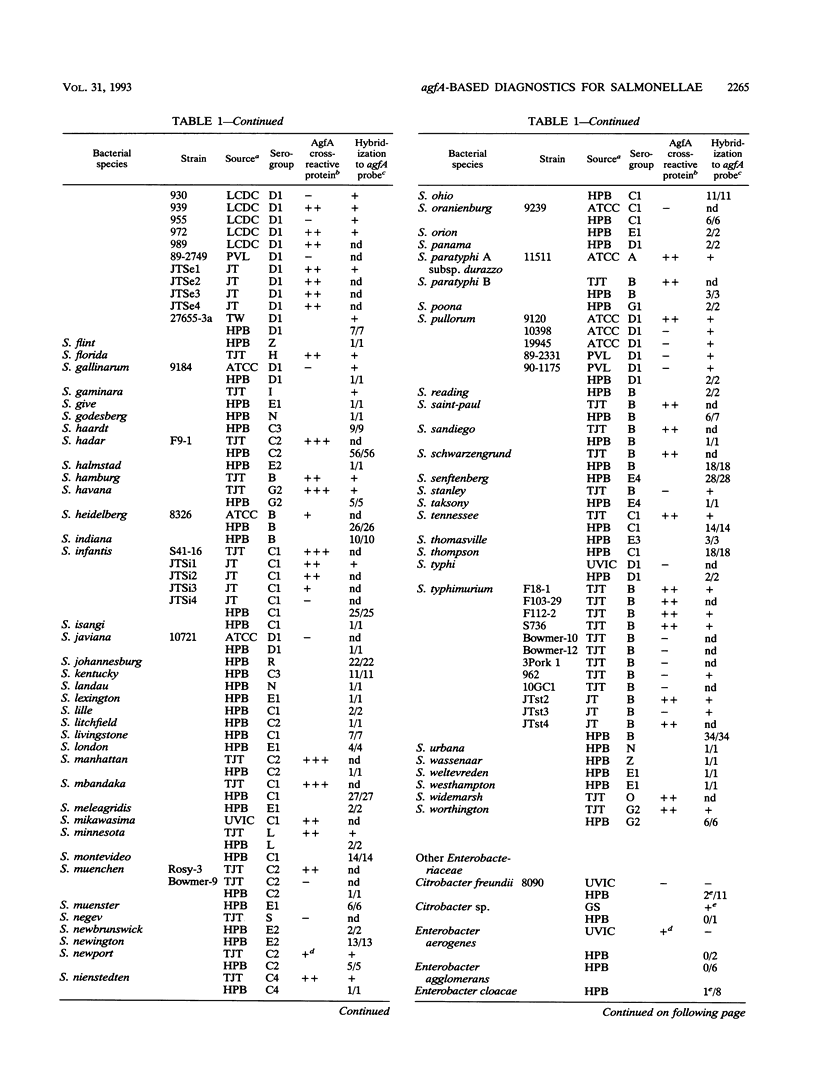
Salmonella enteritidis 27655-3b and a few diarrheagenic Escherichia coli strains produce morphologically and antigenically related, thin, aggregative fimbriae, collectively named GVVPQ fimbriae (S. K. Collinson, L. Emödy, T. J. Trust, and W. W. Kay, J. Bacteriol. 174:4490-4495, 1992). To determine whether GVVPQ fimbriae are common to Salmonella spp. and other enteropathogenic members of the family Enterobacteriaceae, 113 isolates were phenotypically screened for Congo red binding and aggregative colony morphology. Presumptive positive and representative negative strains were examined by Western blotting (immunoblotting) by using antiserum to SEF 17, the native GVVPQ fimbria of S. enteritidis. Only four S. enteritidis strains and six E. coli isolates possessed substantial amounts of GVVPQ fimbriae after 24 h of incubation on T medium. Following 5 days of incubation, 56 of 93 Salmonella isolates (60%) and 1 of 7 additional E. coli clinical isolates possessed detectable levels of GVVPQ fimbriae. Since variable expression of GVVPQ fimbriae was observed among Salmonella isolates and some E. coli strains produced scant amounts, as revealed by immunoelectron microscopy, the ability to produce these fimbriae was evaluated by genotypic screening. The structural gene for the SEF 17 fimbrin, agfA, was amplified by the polymerase chain reaction, cloned, and sequenced to provide a characterized DNA probe. An agfA DNA fragment hybridized strongly to 603 of 604 (99.8%) Salmonella isolates but very weakly to 31 of 266 other members of the family Enterobacteriaceae including 26 of 137 E. coli strains, 3 of 14 Citrobacter spp., and single isolates of Shigella sonnei and Enterobacter cloacae. The agfA DNA probe proved to be a valuable diagnostic tool for Salmonella isolates arrayed on hydrophobic grid membrane filters. Unique agfA sequences were targeted in the development of a polymerase chain reaction assay specific for Salmonella spp.
Full text
PDF




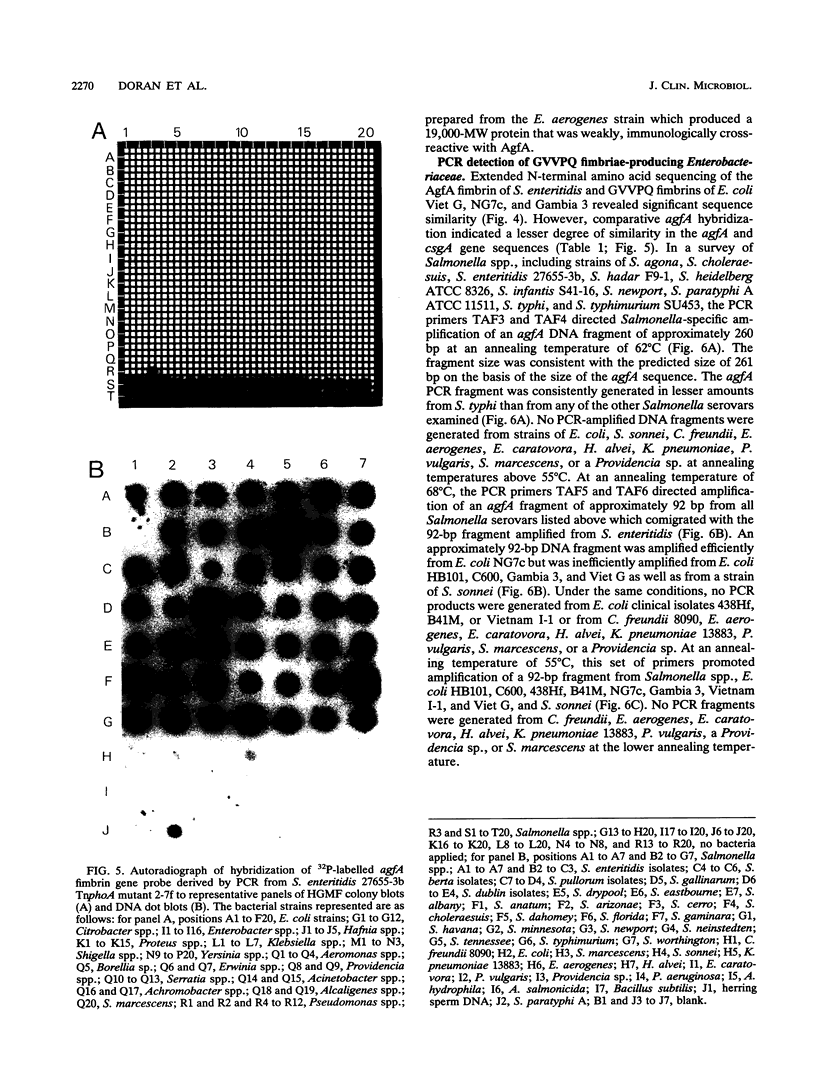



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Guerry P., Trust T. J. Distribution and polymorphism of the flagellin genes from isolates of Campylobacter coli and Campylobacter jejuni. J Bacteriol. 1993 May;175(10):3051–3057. doi: 10.1128/jb.175.10.3051-3057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist A., Olsén A., Pfeifer J., Russell D. G., Normark S. The Crl protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992 Sep;6(17):2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bhaduri S., Turner-Jones C., Lachica R. V. Convenient agarose medium for simultaneous determination of the low-calcium response and Congo red binding by virulent strains of Yersinia enterocolitica. J Clin Microbiol. 1991 Oct;29(10):2341–2344. doi: 10.1128/jcm.29.10.2341-2344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano R. J., Torres M. J., Klem R. E., Palomares J. C., Casadesus J. Detection of salmonellas by DNA hybridization with a fluorescent alkaline phosphatase substrate. J Appl Bacteriol. 1992 May;72(5):393–399. doi: 10.1111/j.1365-2672.1992.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Chan S. W., Wilson S. G., Vera-Garcia M., Whippie K., Ottaviani M., Whilby A., Shah A., Johnson A., Mozola M. A., Halbert D. N. Comparative study of colorimetric DNA hybridization method and conventional culture procedure for detection of Salmonella in foods. J Assoc Off Anal Chem. 1990 May-Jun;73(3):419–424. [PubMed] [Google Scholar]
- Cohen M. L., Tauxe R. V. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science. 1986 Nov 21;234(4779):964–969. doi: 10.1126/science.3535069. [DOI] [PubMed] [Google Scholar]
- Collinson S. K., Doig P. C., Doran J. L., Clouthier S., Trust T. J., Kay W. W. Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J Bacteriol. 1993 Jan;175(1):12–18. doi: 10.1128/jb.175.1.12-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson S. K., Emödy L., Müller K. H., Trust T. J., Kay W. W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson S. K., Emödy L., Trust T. J., Kay W. W. Thin aggregative fimbriae from diarrheagenic Escherichia coli. J Bacteriol. 1992 Jul;174(13):4490–4495. doi: 10.1128/jb.174.13.4490-4495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Fitts R., Diamond M., Hamilton C., Neri M. DNA-DNA hybridization assay for detection of Salmonella spp. in foods. Appl Environ Microbiol. 1983 Nov;46(5):1146–1151. doi: 10.1128/aem.46.5.1146-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Gopo J. M., Melis R., Filipska E., Meneveri R., Filipski J. Development of a Salmonella-specific biotinylated DNA probe for rapid routine identification of Salmonella. Mol Cell Probes. 1988 Dec;2(4):271–279. doi: 10.1016/0890-8508(88)90011-4. [DOI] [PubMed] [Google Scholar]
- Hacker J., Bender L., Ott M., Wingender J., Lund B., Marre R., Goebel W. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb Pathog. 1990 Mar;8(3):213–225. doi: 10.1016/0882-4010(90)90048-u. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Inagaki Y., Okamura N., Nakaya R., Yamamoto N. Type 1 pili enhance the invasion of Salmonella braenderup and Salmonella typhimurium to HeLa cells. Microbiol Immunol. 1992;36(6):593–602. doi: 10.1111/j.1348-0421.1992.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Johnson K., Charles I., Dougan G., Pickard D., O'Gaora P., Costa G., Ali T., Miller I., Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991 Feb;5(2):401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Westurlund B., Holthöfer H., Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115–127. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- Kukkonen M., Raunio T., Virkola R., Lähteenmäki K., Mäkelä P. H., Klemm P., Clegg S., Korhonen T. K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993 Jan;7(2):229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Lanser J. A., Anargyros P. A. Detection of Escherichia coli adhesins with DNA probes. J Clin Microbiol. 1985 Sep;22(3):425–427. doi: 10.1128/jcm.22.3.425-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Finlay B. B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. H., Collinson S. K., Trust T. J., Kay W. W. Type 1 fimbriae of Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4765–4772. doi: 10.1128/jb.173.15.4765-4772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Qadri F., Hossain S. A., Ciznár I., Haider K., Ljungh A., Wadstrom T., Sack D. A. Congo red binding and salt aggregation as indicators of virulence in Shigella species. J Clin Microbiol. 1988 Jul;26(7):1343–1348. doi: 10.1128/jcm.26.7.1343-1348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue D. C., Tauxe R. V., Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990 Aug;105(1):21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönian G., Sokolowska-Köhler W., Bollmann R., Schubert A., Gräser Y., Presber W. Determination of S fimbriae among Escherichia coli strains from extraintestinal infections by colony hybridization and dot enzyme immunoassay. Zentralbl Bakteriol. 1992 Jan;276(2):273–279. doi: 10.1016/s0934-8840(11)80014-9. [DOI] [PubMed] [Google Scholar]
- Sockett P. N. The economic implications of human Salmonella infection. J Appl Bacteriol. 1991 Oct;71(4):289–295. doi: 10.1111/j.1365-2672.1991.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Campbell M. E., Farmer S. W., Volpel K., Joffe A. M., Paranchych W. Use of a pilin gene probe to study molecular epidemiology of Pseudomonas aeruginosa. J Clin Microbiol. 1989 Nov;27(11):2589–2593. doi: 10.1128/jcm.27.11.2589-2593.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierings G., Elders R., van Lith B., Hofstra H., Tommassen J. Characterization of the Salmonella typhimurium phoE gene and development of Salmonella-specific DNA probes. Gene. 1992 Dec 1;122(1):45–52. doi: 10.1016/0378-1119(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Stugard C. E., Daskaleros P. A., Payne S. M. A 101-kilodalton heme-binding protein associated with congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun. 1989 Nov;57(11):3534–3539. doi: 10.1128/iai.57.11.3534-3539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjojoatmodjo M. N., Fluit A. C., Torensma R., Keller B. H., Verhoef J. Evaluation of the magnetic immuno PCR assay for rapid detection of Salmonella. Eur J Clin Microbiol Infect Dis. 1991 Nov;10(11):935–938. doi: 10.1007/BF02005447. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]