Abstract
Mycobacterium tuberculosis has evolved a number of strategies to survive within the hostile environment of host phagocytes. Reactive nitrogen and oxygen intermediates (RNI and ROI) are among the most effective antimycobacterial molecules generated by the host during infection. Lsr2 is a M. tuberculosis protein with histone-like features, including the ability to regulate a variety of transcriptional responses in mycobacteria. Here we demonstrate that Lsr2 protects mycobacteria against ROI in vitro and during macrophage infection. Furthermore, using macrophages derived from NOS−/− and Phox−/− mice, we demonstrate that Lsr2 is important in protecting against ROI but not RNI. The protection provided by Lsr2 protein is not the result of its ability to either bind iron or scavenge hydroxyl radicals. Instead, electron microscopy and DNA-binding studies suggest that Lsr2 shields DNA from reactive intermediates by binding bacterial DNA and physically protecting it. Thus, Lsr2 appears to be a unique protein with both histone-like properties and protective features that may be central to M. tuberculosis pathogenesis. In addition, evidence indicates that lsr2 is an essential gene in M. tuberculosis. Because of its essentiality, Lsr2 may represent an excellent candidate as a drug target.
Keywords: Mycobacterium tuberculosis, ROI, DPS, DNA
Mycobacterium tuberculosis is a facultative intracellular bacterium that has evolved sophisticated mechanisms to survive and replicate inside host mononuclear phagocytes (1). Phagocytes exert much of their antimycobacterial activity by generating reactive nitrogen and oxygen intermediates (RNI and ROI, respectively), which kill bacteria by damaging macromolecules such as bacterial DNA. Nitric oxide synthase-2 (NOS2) knockout mice, which cannot generate RNI, and phagocyte oxidase (PHOX) knockout mice, which cannot generate ROI, exhibit increased sensitivity to M. tuberculosis infection (2, 3, 4). Conversely, M. tuberculosis strains deficient in catalase-peroxidase (KatG), or other antioxidative mechanisms, are less virulent in mouse models (5, 6). Moreover, the observation that children with defective oxidative burst mechanisms because of chronic granulomatous disease develop numerous complications from bacillus Calmette–Guérin vaccination and have a high incidence of tuberculosis, highlights the importance of ROI in protection against M. tuberculosis in humans (7).
Dps (DNA-binding protein from starved cells) is an important bacterial virulence factor that protects DNA from ROI and other toxic molecules. Dps appears to protect DNA through the dual mechanisms of iron sequestration, which prevents Fenton-mediated ROI generation and DNA binding, which compacts DNA and creates a protective physical barrier (8, 9). Dps has been identified in a diverse group of bacteria, including Mycobacterium smegmatis (10); however, structural homologs have not been detected in the M. tuberculosis genome. This is relatively surprising given the importance of ROI in antituberculosis immunity. It is reasonable to hypothesize that M. tuberculosis expresses proteins with Dps-like characteristics and functions despite the lack of an identifiable homolog. In fact, the M. tuberculosis histone-like protein, Lsr2, shares a number of physical properties with Dps, including small size, high isoelectric point, and an ability to bind DNA with little specificity (11). These observations suggested that Lsr2 might have a functional role in M. tuberculosis that is similar to the role of Dps in other bacteria.
Results
Protecting DNA Against Hydroxyl Radical Damage.
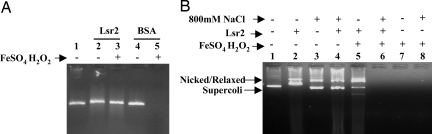
As a first step in determining whether Lsr2 had a Dps-like functionality, we studied the ability of M. tuberculosis Lsr2 to protect DNA against damage by hydroxyl radicals. A linear 700-bp fragment of DNA was incubated in vitro with H2O2 and Fe(II) in the presence or absence of recombinant M. tuberculosis Lsr2 or a BSA (BSA) control protein. The DNA was completely destroyed by Fe(II)/H2O2 treatment (Fig. 1A). Notably, DNA was substantially protected from degradation by pretreatment with Lsr2 (Fig. 1A, lane 3). In contrast, pretreatment with an equivalent concentration of BSA suspended in the imidazole buffer did not result in any protection (Fig. 1A, lane 5).
Fig. 1.
DNA protection studies. (A) Linear DNA (≈0.2 μM) was used alone or after incubation with either 2 μM of purified Lsr2 or BSA. Both proteins were suspended in identical imidazole buffers. Samples were treated for 5 min with either FeSO4 (20 mM) plus H2O2 (5 mM) or left untreated as indicated. The samples were analyzed on an ethidium bromide stained 1% agarose gel. Lane 1, linear DNA; lane 2, linear DNA + Lsr2; lane 3, linear DNA + Lsr2 + FeSO4/H2O2; lane 4, linear DNA + BSA; lane 5, linear DNA + BSA + FeSO4/H2O2. (B) Supercoiled φhX174 plasmid (≈0.1 μM) was used alone or incubated with Lsr2 in imidazole buffer. The Lsr2 was used without or with prior extensive dialysis in buffer containing 800 mM NaCl. Samples were treated for 5 min with either FeSO4 (20 mM) plus H2O2 (5 mM) or left untreated as indicated and then analyzed on an ethidium bromide stained 1% agarose gel. Lane 1, φhX174 plasmid; lane 2, φhX174 plasmid + Lsr2; lane 3, φhX174 plasmid in buffer containing 800 mM NaCl; lane 4, φhX174 plasmid in buffer containing 800 mM NaCl + dialyzed Lsr2; lane 5, φhX174 plasmid + Lsr2 + FeSO4/H2O2; lane 6, φhX174 plasmid in buffer containing 800 mM NaCl + dialyzed Lsr2 + FeSO4/H2O2; lane 7, φhX174 plasmid + FeSO4/H2O2; lane 8, φhX174 plasmid in buffer containing 800 mM NaCl + FeSo4/H2O2.
Lsr2 could protect DNA through a direct Lsr2–DNA interaction, perhaps by acting as a physical barrier against ROI. Alternatively, unbound Lsr2 could protect DNA by trapping free radicals or sequestering Fe(II) (which would prevent the generation of OH radicals) in solution. We have observed that Lsr2 cannot bind to DNA in high salt concentrations. To determine whether Lsr2 must bind to DNA to protect against H2O2, we incubated plasmid DNA with Lsr2 in low or high salt (0.8 M NaCl) buffer and repeated the test for H2O2-mediated degradation (Fig. 1B). Plasmid DNA was used as the target in this experiment because circular DNA binds sufficient Lsr2 (in imidazole/low salt buffer) to produce an observable gel shift, and this gel shift can be used to verify DNA binding. Incubating the plasmid DNA with Lsr2 in 400 mM imidazole buffer produced the expected shift (Fig. 1B, lane 2), confirming that Lsr2 binds to DNA under these conditions. As expected, the plasmid was protected against FeII/H2O2-induced damage in the presence of bound Lsr2, although the formation of nicked and relaxed circular DNA was observed (Fig. 1B, lane 5). The experiment was then repeated using Lsr2 that had been dialyzed against 50 mM PBS (pH 7.4), 0.8 M NaCl. This formulation of Lsr2 did not produce a gel shift upon incubation with the plasmid DNA, confirming that Lsr2 does not bind DNA in high salt buffer (Fig. 1B, lane 4). Notably, plasmid DNA preincubated with Lsr2 was not protected against degradation upon FeII/H2O2 treatment under these high-salt conditions (Fig. 1B, lane 6). DNA controls incubated in 400 mM imidazole (Fig. 1B, lane 7) or 0.8 M NaCl buffers without Lsr2 (Fig. 1B, lane 8) were also not protected from FeII/H2O2 damage. These results indicate that the presence of Lsr2 in solution is not sufficient to protect DNA from ROI. Rather, Lsr2 must be bound to DNA to provide protection.
We studied whether Lsr2 might protect DNA by trapping free radicals or by scavenging iron. In spin-trapping experiments, we noted a lower signal intensity in the presence of BSA than in the presence of Lsr2, indicating more radical scavenging by BSA under these conditions (Fig. S1). These results suggest that Lsr2 does not efficiently remove hydroxyl radicals. Most proteins of the Dps family have been shown to specifically bind iron (12, 13, 14). Iron sequestration interferes with the Fenton reaction, reduces ROI generation, and thereby reduces potential DNA damage. We performed fluorescence titration experiments using Lsr2 and either Fe2+or Fe3+. Fluorescence was measure using an excitation wavelength of 284 nm and an emission detection wavelength of 330 nm. The changes in fluorescence were small and linear over the range of Fe concentrations used (data not shown), providing no evidence for specific iron binding by Lsr2. Together, these results suggest that Lsr2 derives its principal protective effects by acting as a physical barrier to DNA degradation.
Electron Microscopy of Lsr2 Bound to DNA.
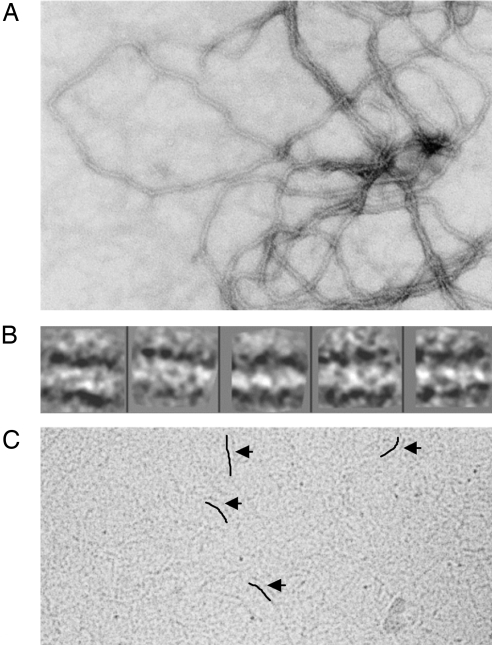
Examination of purified preparations of Lsr2 allowed for a better understanding of how Lsr2 interacts with DNA. The protein sample embedded in uranyl acetate (negative stain) revealed regular fibrils of ≈90 Å diameter (Fig. 2A). The protocol for purifying overexpressed recombinant Lsr2 involves DNase treatment of the lysed Escherichia coli cells. However, absorbance at 260 nm and 280 nm along with bands on both SDS/PAGE gels and agarose DNA gels showed that Lsr2 was copurified with E. coli genomic DNA. The fibrillar morphology seen in Fig. 2A shows E. coli genomic DNA decorated by Lsr2, which appears to protect DNA from the DNase treatment. The decoration and protection of DNA by Lsr2 is consistent with previous reports using atomic force microscopy (15). Interestingly, class averages of near-linear segments of these fibrils suggested that intertwined protofilaments were wound in an approximate helical fashion around a core of DNA (Fig. 2B). Unwound protofibrils ≈45 Å in diameter, could be visualized in Cryo-EM of vitrified specimens of the Lsr2–DNA complex (Fig. 2C).
Fig. 2.
Electron microscopic structure of Lsr2/DNA complexes. (A) Micrograph of purified, recombinant Lsr2 embedded in uranyl-acetate (UA, negative stain) displaying a filamentous structure. (B) Five representative class averages of Lsr2 following reference-free classification of ≈700 boxed (244 × 244 Å) segments and isolated from the image shown in A and 2 other images. The fibrillar structure with helical winding is apparent. The diameter of the fibrils is ≈90 Å. (C) A cryoelectron micrograph revealing protofibrils of the Lsr2–DNA complex. Arrows indicate the protofibrils and lines have been placed to the immediate left of the protofibrils to further clarify their position. The diameter of the protofibrils is ≈45Å.
Effect of lsr2 Deletion and Overexpression on Hydrogen Peroxide Resistance.
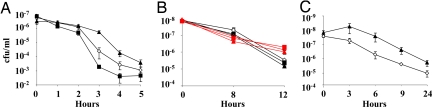
The ability of Lsr2 to protect DNA from oxidative damage in vitro suggested that Lsr2 might also protect live mycobacteria against ROI and RNI. Our laboratory and others have been unable to create M. tuberculosis knockouts of lsr2 (11), indicating that this gene is likely to be essential in M. tuberculosis. Fortunately, it is possible to knock lsr2 out of the related mycobacteria M. smegmatis. To test for altered susceptibility to ROI, we compared NJS22, an M. smegmatis mc2 155 lsr2 knockout stain, its parental mc2 155 control, and NJS22.1, the NJS22 knockout complemented with M. tuberculosis lsr2 on an overexpression plasmid, for their ability to survive in the presence of H2O2. Each strain was incubated in 5 mM H2O2 and aliquots were plated on solid culture media at different time points. We found that the lsr2 knockout was indeed more susceptible to H2O2 than the parental M. smegmatis control (P < 0.01) after 3 and 4 h of H2O2 treatment (Fig. 3A). Overexpression of M. tuberculosis lsr2 had the opposite effect, increasing the number of surviving cfu compared to the parental control at 3 and 4 h (P < 0.01). To test for altered susceptibility to RNI, we performed similar studies, this time treating the wild-type, knockout, and overexpression strains with sodium nitrate, which generates NO in acidified media (Fig. 3B). Unlike the case with H2O2 treatment, the viability of all 3 strains was equally affected. These results suggest that cellular Lsr2 protects live mycobacteria against ROI but not RNI.
Fig. 3.
Sensitivity of M. smegmatis and M. tuberculosis mutants to H2O2 and to NO. Five mM of H2O2, 10 mM of sodium nitrite, or 50 mM of sodium nitrite were added to cultures of each strain. Culture aliquots were removed at different time points and plated on solid media. Results are expressed as colony forming units (cfu) per milliliter of culture. (A) M. smegmatis strains treated with H2O2: (open diamonds) mc2 155 (control); (filled squares) NJS22 (lsr2 knockout); and (filled triangles) NJS22.1 (lsr2 knockout strain overexpressing lsr2 on an expression plasmid). (B) M. smegmatis strains treated with sodium nitrite. Red indicates treatment with 10 mM sodium nitrate: (red open diamonds) mc2 155; (red squares) NJS22; and (red triangles) NJS22.1. Black indicates treatment with 50 mM sodium nitrate: (open diamonds) mc2 155; (filled squares) NJS22; and (filled triangles) NJS22.1. (C) M. tuberculosis strains treated with H2O2: (open diamonds) H37Rv(pMV261) (control) (filled triangles); NJS18 (lsr2 overexpression strain). Error bars indicate plus and minus 1 standard deviation of a minimum of 3 experiments. The P-value at all time points (panel A) and at 3 and 4 h (panel B) was <0.05 in a Tukey's Studentize range.
Although lsr2 knockouts are not available in M. tuberculosis, lsr2 overexpression strains can be created in this species. We compared NJT18, an M. tuberculosis strain overexpressing lsr2 (11) to its empty vector control [H37Rv(pMV261)] for the ability to grow in the presence of H2O2 by culturing both strains in 7H9 media containing 2.5 mM H2O2 for various lengths of time and then plating on solid media. As in the M. smegmatis studies, the M. tuberculosis NJT18 strain overexpressing Lsr2 was less susceptible to the toxic effect of H2O2 (P > 0.01). Notably, the H37Rv(pMV261) control showed almost a half-log decrease in cfu after 3 h of incubation with H2O2, while the NJS18 did not show any decrease in cfu at this time point (Fig. 3C). In complementary experiments, we compared the ability of the 2 strains to grow in liquid media in the presence of H2O2 using the BACTEC 460 culture system (16). Growing cultures of the control strain were substantially inhibited in 40 mM H2O2 and were completely inhibited in 60 mM H2O2 (Fig. S2A). In contrast, the NJT18 strain overexpressing lsr2 grew normally in 40 mM H2O2, showed some growth inhibition in 60 mM H2O2, and was only completely inhibited in 80 mM H2O2 (Fig. S2B). Both strains were inhibited by identical concentrations of ciprofloxacin (Fig. S2 C and D), suggesting that survival differences were not general to all types of stress. Taken together, these studies strongly suggest that Lsr2 protects mycobacteria against H2O2-generated ROI.
Microarray Studies.
We have demonstrated that M. smegmatis strains with either lsr2 interruptions or deletions have significantly altered transcription patterns (11). We considered the possibility that overexpressing lsr2 could also alter transcription patterns in a way that would indirectly protect against ROIs. Microarray studies comparing the expression patterns of NJT18 versus H37Rv(pMV261) were performed. Of the 6 microarray replicas studied, a few showed upregulation of stress-response genes such as groES, Rv0692, Rv0690c above the cutoff of +2.0 in NJT18. However, none of these genes are known to be involved in detoxification, DNA repair, or have been shown to protect M. tuberculosis from ROI. Furthermore, only lsr2 expression was found to be significantly increased above this cutoff when the results of all 6 microarrays were analyzed together and expression changes were tested for statistical significance. These results strongly suggest that the increased resistance to H2O2 observed in NJT18 is because of a direct effect of the Lsr2 protein and not because of transcriptional changes in other protective genes.
Survival Differences of lsr2 Mutants Within Murine Macrophages.
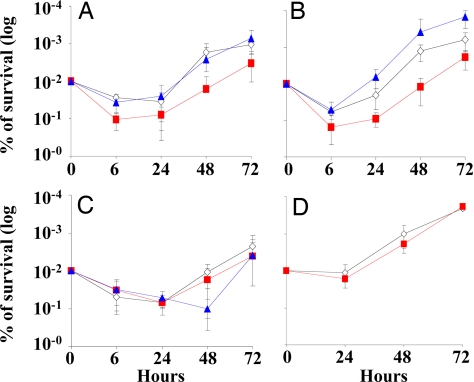
We examined the function of Lsr2 in the physiologically relevant conditions of macrophage infection to complement the in vitro studies. C57BL6 mouse bone marrow derived macrophages (BMM) were infected with the lsr2 overexpression strain NJT18 and its H37Rv(pMV261) control to determine the effect of lsr2 overexpression in M. tuberculosis on survival during an intracellular infection. No difference in intracellular growth was observed between the 2 strains (data not shown). We then repeated the intracellular infections with our panel of M. smegmatis strains so that we could study the effect of lsr2 deletion on intracellular survival (Fig. 4A). As in the case with M. tuberculosis, intracellular survival was not increased by lsr2 overexpression. However, deletion of lsr2 in strain NJS22 had a marked deleterious effect on survival. NJS22 survival decreased by approximately 1 log by 6 h after infection, while both M. smegmatis mc2 155 and the NJS22.1 complement were reduced only 3–4-fold (<0.05 in a Turkey's Studentize range). This difference remained statistically significant over all of the experimental time points. Cultures of each BMM supernatant at each time point before BMM lysis consistently produced cfu counts that were ≈100-fold lower than the lysed BMM cultures (data not shown), demonstrating that the observed intracellular growth differences were not artifacts of unequal extracellular growth.
Fig. 4.
Survival of M. smegmatis mutants within macrophages. Bone marrow derived macrophages (BMM) from (A) wild-type C57BL/6 mice; (B) NOS−/− mice; (C) Phox−/− mice; and (D) wild-type C57BL/6 mice were infected with (open diamonds) M. smegmatis mc2 155 (control) (red squares); NJS22 (lsr2 knockout); and (blue triangles) NJS22.1 (lsr2 knockout strain overexpressing lsr2 on an expression plasmid). In panel D, the BMM were treated with 500 μM of Fenbufen 1 h before infection and again 48 h after infection. For each strain, the cfu at each time point are expressed relative to the cfu at time 0. Results from a minimum of 3 replicates are shown for each time point. Error bars indicate plus and minus 1 standard deviation. The P-values between wild-type and lsr2 knockout strains for time points 48 and 72 h (panel A); and time points 24, 48, and 72 h (panel B) were <0.05 in a Turkey's Studentize range. The P-values between wild-type and lsr2 knockout strains were not significantly different in panels C and D at all time points tested.
Macrophages produce both ROI and RNI to defend against intracellular M. tuberculosis. Our in vitro studies had suggested that lsr2 deletion only alters susceptibility to ROI. Therefore, we repeated the intracellular infection studies, this time using BMM derived from NOS−/− mice (which cannot produce RNI) and Phox−/− mice (which cannot produce ROI), to identify which protective pathways were interacting with Lsr2 during intracellular infections. The lsr2 knockout strain survived less well than the control strain after infecting NOS−/− derived BMM (Fig. 4B). This pattern was similar to the one produced when the strains infected wild-type BMM. In contrast, the lsr2 knockout strain had similar numbers of cfu compared to the control strain when BMM derived from Phox−/− mice were infected (Fig. 4C). These results indicate that Lsr2 protects against ROI but not against RNI. We then repeated the BMM infection experiments, this time depleting the C57BL6 derived BMM of ROI by treating with Fenbufen. Fenbufen has recently been shown to scavenge oxygen radicals in vitro and within human leukocytes (17). Fenbufen has a half life of ≈20 h; therefore, BMM were treated 1 h before infection and 48 h after infection. Importantly, treatment with Fenbufen eliminated the growth differences between the lsr2 knockout and control strain, reproducing the results obtained by infecting BMM from Phox−/− mice (Fig. 4D). Together, these results provide strong evidence that Lsr2 protects against ROI but not RNI during intracellular infection.
Interestingly, we noted that overexpression of lsr2 (by NJT22.1) permitted an enhanced level of intracellular growth compared to the control in the NOS−/− BMM (Fig. 4B). These results suggest that increased Lsr2 expression in M. smegmatis can enhance protection against ROI during intracellular infection, but only when RNI are not present. However, the significance of this finding is unclear because the analogous growth advantage was not seen when NOS−/− BMM were infected with M. tuberculosis overexpressing Lsr2 (data not shown). On the other hand, lsr2 overexpression resulted in decreased intracellular survival compared to both the knockout and control strains in the Phox−/− derived BMM. It is possible that lsr2 overexpression is deleterious under intracellular conditions where it is not needed to provide protection against ROI.
Discussion
M. tuberculosis has developed a number of strategies to survive within the hostile environment of host phagocytes. This includes the ability to prevent phagolysosome maturation (18, 19), and an ability to resist ROI and RNI (20). Proteins such as KatG and AhpC-AhpD appear to detoxify ROI and RNI and are important components of the M. tuberculosis oxidative stress response (21–23). M. tuberculosis also secretes superoxide dismutase encoded by sodA (24) which may protect against ROI, although conclusive data on this function are lacking in M. tuberculosis. Various cell wall components (25, 26), reductases (27, 28), and the genes responsible for mycothiol production (29) also appear to play a protective role. Lsr2 appears to use a different mechanism to protect mycobacteria against oxidative stress that requires direct protein-DNA binding. Many DNA binding proteins locally protect DNA from ROI in vitro, indeed this phenomenon is the basis for DNA foot printing studies (30); thus, one could question the physiological relevance of the in vitro protection which we observed. However, Lsr2 differs from classical DNA binding proteins in its ability to bind long DNA sequences with relative nonspecificity, to oligomerize, and to regulate expression of a large number of genes (11, 15). These features, along with its small size and high isoelectric point suggest that Lsr2 is a member of the bacterial histone-like family of proteins. Histones and histone-like proteins bind to DNA through electrostatic interactions between the highly positively charged regions of the protein and the negative charges present on the phosphate groups of the DNA backbone (31). Studies in eukaryotes have shown that histones can significantly protect DNA against hydroxyl radical-induced DNA strand breaks by binding DNA and organizing it into higher order chromatin structures (32). The mechanisms by which nucleosomal histones protect DNA from oxidant damage are still unclear (33, 34). Protection may be related to alterations in DNA conformation, or the histones may be preferentially oxidized over DNA and become sacrificial targets when they are closely associated with the chromosome (33). Here, we show that a prokaryotic histone-like protein can also have the ability to protect DNA from oxidative damage even though it does not form nucleosomes or bind iron. Our biochemical and structural results suggest that Lsr2 protects DNA by acting as a physical barrier against ROI.
Our work was complicated by the fact that Lsr2 appears to have an important role in global transcriptional regulation (11). Although we showed that Lsr2 overexpression has a minimal effect on transcriptional control in M. tuberculosis, M. smegmatis lsr2 knockouts do have altered transcriptional responses (11). This past observation made it difficult for us to definitively differentiate between the direct and indirect effects of lsr2 deletion in the BMM infection studies. However, the overexpression and in vitro studies and the electron microscopic results presented here all strongly suggest a direct role for Lsr2 in ROI protection. Our work was also limited by the inability of our group and others to knock out lsr2 from the M. tuberculosis genome. However, we were able to perform BMM infection studies using an M. smegmatis lsr2 knockout strain. M. tuberculosis and M. smegmatis lsr2 have 86% sequence identity and we showed that the M. tuberculosis lsr2 gene could fully complement the phenotype of the M. smegmatis knockout in our BMM infection studies. Essentiality complicates functional investigations; however, it also points to the importance of this protein to M. tuberculosis. Essential proteins clearly make good drug targets. However, Lsr2 is not just an essential protein, but also one that helps protect against a major host defense mechanism. Our results suggest that Lsr2 could be an outstanding drug target whose inhibition could help to control M. tuberculosis in both extracellular and intracellular compartments.
Experimental Procedures
Growth studies were performed by incubating the mycobacterial strains in liquid 7H9 media containing H2O2 and then plating onto solid 7H10 media or using the BACTEC 460 system as described previously (16) with either H2O2 or antibiotic added to the cultures when the growth index had reached 200. The microarray studies were performed as described (35), using 6 separate RNA samples and a dye flip protocol. Growth within macrophages was studied using BMM from C57BL/6, Nos2 tm1Lau/J, or Ncf1m1J/J (p47phox) knockout mice. Bone marrow cells were differentiation into macrophages, infected with mycobacterial strains at a multiplicity of infection of 3, and then incubated with amikacin for 1 h. At different time points, infected BMM were lysed and serial dilutions were plated to measure mycobacterial cfu. DNA protection assays were performed by incubating linear or plasmid DNA with 2 μM of Lsr2 protein or BSA followed by treatment with FeSO4 and H2O2 or control buffer. Lsr2 was disassociated from DNA by dialyzing against 50 mM phosphate (pH 7.4), 800 mM NaCl. Spin-trapping experiments and EPR measurements were performed as described previously (36, 37), X-band EPR spectra were recorded at room temperature on a Bruker EleXsys E500 spectrometer fitted with a high sensitivity SHQ cavity. All spectra were recorded ≈3 min after the addition of Fe(II). Lsr2-iron binding was measured using a Hitachi F-4500 Fluorescence Spectrophotometer. Transmission electron microscopy and image processing were performed by embedding the purified Lsr2 in uranyl acetate and examining at 52,000 magnification in a FEI Tecnai12 microscope operated at 120 kV. A complete description of the experimental procedures is included in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants AI043268, AI060014, and American Heart Association Grant 0535249N. A.K.M. was supported by a Marsden grant and N.Z. and M.R. by doctoral fellowships from the German Academic Exchange Service (DAAD) and University of Auckland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810126106/DCSupplemental.
References
- 1.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 2.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 1997;78:237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson TM, de Lisle GW, Collins DM. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol Microbiol. 1995;15:1009–1015. doi: 10.1111/j.1365-2958.1995.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52:1291–1302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee PP, et al. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J. 2008;27:224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 8.Zhao G, et al. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem. 2002;277:27689–27696. doi: 10.1074/jbc.M202094200. [DOI] [PubMed] [Google Scholar]
- 9.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Pandit SB, Srinivasan N, Chatterji D. Proteomics analysis of carbon-starved Mycobacterium smegmatis: induction of Dps-like protein. Protein Eng. 2002;15:503–512. doi: 10.1093/protein/15.6.503. [DOI] [PubMed] [Google Scholar]
- 11.Colangeli R, et al. Transcriptional regulation of multi-drug tolerance and antibiotic-induced responses by the histone-like protein Lsr2 in M. tuberculosis. PLoS Pathog. 2007;3:e87. doi: 10.1371/journal.ppat.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozzi M, et al. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 13.Papinutto E, et al. Structure of two iron-binding proteins from Bacillus anthracis. J Biol Chem. 2002;277:15093–15098. doi: 10.1074/jbc.M112378200. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Poole LB, Hantgan RR, Kamio Y. An iron-binding protein, Dpr, from Streptococcus mutans prevents iron-dependent hydroxyl radical formation in vitro. J Bacteriol. 2002;184:2931–2939. doi: 10.1128/JB.184.11.2931-2939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JM, et al. Lsr2 of Mycobacterium tuberculosis is a DNA-bridging protein. Nucleic Acids Res. 2008;36:2123–2135. doi: 10.1093/nar/gkm1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colangeli R, et al. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol Microbiol. 2005;55:1829–1840. doi: 10.1111/j.1365-2958.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- 17.Costa D, Moutinho L, Lima JL, Fernandes E. Antioxidant activity and inhibition of human neutrophil oxidative burst mediated by arylpropionic acid non-steroidal anti-inflammatory drugs. Biol Pharm Bull. 2006;29:1659–1670. doi: 10.1248/bpb.29.1659. [DOI] [PubMed] [Google Scholar]
- 18.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom BR. Tuberculosis. Washington, DC: American Society for Microbiology; 1994. [Google Scholar]
- 21.Manca C, Paul S, Barry CE, III, Freedman VH, Kaplan G. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect Immun. 1999;67:74–79. doi: 10.1128/iai.67.1.74-79.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi S, Ehrt S. Dihydrolipoamide acyltransferase is critical for Mycobacterium tuberculosis pathogenesis. Infect Immun. 2006;74:56–63. doi: 10.1128/IAI.74.1.56-63.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Xie QW, Nathan C. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol Cell. 1998;1:795–805. doi: 10.1016/s1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- 24.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE., III Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:6630–6634. doi: 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katti MK, et al. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar P, et al. Rv3303c of Mycobacterium tuberculosis protects tubercle bacilli against oxidative stress in vivo and contributes to virulence in mice. Microbes Infect. 2006;8:2855–2862. doi: 10.1016/j.micinf.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Senaratne RH, et al. 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol. 2006;59:1744–1753. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 29.Buchmeier NA, Newton GL, Fahey RC. A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J Bacteriol. 2006;188:6245–6252. doi: 10.1128/JB.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain SS, Tullius TD. Footprinting protein-DNA complexes using the hydroxyl radical. Nat Protoc. 2008;3:1092–1100. doi: 10.1038/nprot.2008.72. [DOI] [PubMed] [Google Scholar]
- 31.Loyola A, Reinberg D. Histone deposition and chromatin assembly by RSF. Methods. 2003;31:96–103. doi: 10.1016/s1046-2023(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 32.Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa A, et al. Guanine-specific DNA damage induced by gamma-irradiated histone. Biochem J. 2005;388:813–818. doi: 10.1042/BJ20050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enright HU, Miller WJ, Hebbel RP. Nucleosomal histone protein protects DNA from iron-mediated damage. Nucleic Acids Res. 1992;20:3341–3346. doi: 10.1093/nar/20.13.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voskuil MI, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dikalov SI, Mason RP. Reassignment of organic peroxyl radical adducts. Free Radic Biol Med. 1999;27:864–872. doi: 10.1016/s0891-5849(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki I, Piette LH. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990;265:13589–13594. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.