Abstract
The bystander effect refers to the biological response of a cell resulting from an event in an adjacent or nearby cell. Such effects depend on intercellular communication and amplify the consequences of the original event. These responses are of particular interest in the assessment of ionizing radiation risk because at public or occupational exposure levels not every cell receives a radiation track. Current radiation protection regulations and practices are based on the assumption of a linear increase in risk with dose, including low doses where not all cells are hit. Mechanisms that amplify biological effects are inconsistent with these assumptions. Evidence suggests that there are two different bystander effects in mammalian cells. In one type, a radiation track in one cell leads to damaging, mutagenic, and sometimes lethal events in adjacent, unhit cells. In the other type, a radiation track in one cell leads to an adaptive response in bystander cells, increasing resistance to spontaneous or radiation-induced events. This paper describes some of the data for radiation-induced bystander effects in vitro and correlates that data with in vitro and in vivo observations of risk at low doses. The data suggest that protective effects, including beneficial bystander effects, outweigh detrimental effects at doses below about 100 mGy, but that the reverse is true above this threshold.
Keywords: bystander effects, ionizing radiation, Trp53, cancer, mice
INTRODUCTION
Most of the practice of radiation protection is concerned with occupational exposures of people. However, increasing attention is being focused on public exposures of people, particularly from naturally occurring radon but also from industrial activity. Recently this attention has additionally been directed toward concerns about protection of nonhuman biota. All of these activities have in common a concern with exposure to low doses, typically at low dose rates. Current radiation principles and practices, employed worldwide at the recommendation of international advisory bodies such as the International Commission on Radiological Protection (ICRP), are based on the assumptions of the linear no-threshold (LNT) hypothesis, which states that at low doses risk is directly proportional to dose; that is, every dose, no matter how low, increases risk (ICRP, 1991).
Recently, a large body of evidence has rendered this assumption suspect. One of these findings has been the demonstration of bystander effects, defined as the biological response of a cell that results from an event that has occurred in an adjacent or nearby cell. Such effects imply the existence of a mechanism able to amplify the effects of a low dose, contradicting the assumption of a linear dose response. Bystander effects are often reported to result in detrimental effects to adjacent cells, a process that would imply a nonlinear increase in risk at low doses. For example, γ-radiation doses in the range 10–50 mGy can result in the production of extracellular factors, including reactive oxygen species, which amplify cell death. These effects persist for several generations in progeny cells and could imply a mechanism for induced genomic instability (Lyng et al., 2000, 2002; Seymour and Mothersill, 2000). Such effects have also been seen with low fluences of α-radiation, where not all cells receive an α-particle track. Bystander cells showed an increase in sister chromatid exchanges (Nagasawa and Little, 1992) and that effect was dependent on cell signals transmitted through connexin-mediated gap junctions (Azzam et al., 1998; Zhou et al., 2000). Recently, these damaging events were shown to be amplified in cells defective in DNA double-strand-break repair, the type of damage considered to be important in cancer risk (Nagasawa and Little, 2002; Nagasawa et al., 2003). In addition to these immediate effects on bystander cells, the surviving bystander cells also displayed genomic instability in succeeding cell generations, a hallmark of carcinogenesis (Seymour and Mothersill, 1997; Lorimore et al., 1998).
The cellular data described earlier (for a review see Mothersill and Seymour, 2001) would imply that low doses may produce a supralinear response and that the assumption of linearity with dose underestimates the actual risk (Zhou et al., 2001). Considerable additional data also indicate that, at low doses and dose rates of low Linear Energy Transfer (LET) radiation, the LNT assumption of linearity is invalid because risk is overestimated. All these other data show that low doses induce an adaptive response that reduces rather than increases risk at the molecular, cellular, and whole- animal levels (Olivieri et al., 1984; Azzam et al., 1992, 1994a, 1994b, 1996; Rigaud et al., 1993; Ishii et al., 1996; Redpath and Antoniono, 1998; Broome et al., 1999; Mitchel et al., 1999b), and does so at doses relevant to human occupational exposure. The mechanisms of these adaptive responses are the subject of intense investigation, but the discussion presented here implicates bystander effects as playing a large role in the adaptive response to radiation.
The relative importance of detrimental versus protective bystander effects for exposure to low doses of low-LET radiation is compared by examining data for neoplastic transformation in cells and for carcinogenesis in animals.
ADAPTIVE RESPONSES AND BYSTANDER EFFECTS IN CELLS
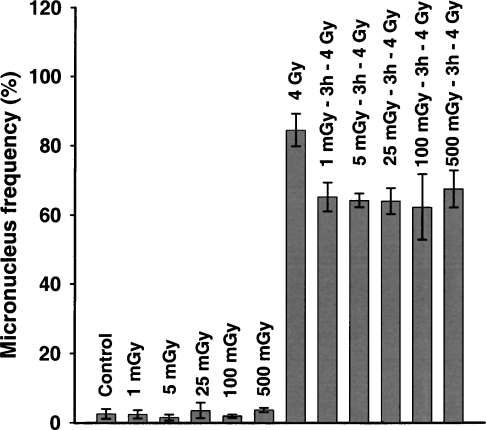
If adaptive responses that increase cellular resistance to radiation exist, and there is ample evidence that they do, then such responses should result in dose responses that deviate negatively from linearity. Adaptive responses that increase cellular ability for the repair of DNA double-strand breaks, as monitored by the formation of micronuclei after a large challenge exposure, indicate that this is true for both γ-photons and tritium β-particles (Broome et al., 2002; Figure 1). However, the data also indicate that the dose response for this protective effect reached a maximum at 1 mGy and then remained at that maximum between 1 and 500 mGy, when the dose is delivered at low-dose rate. Since a 60Co-γ-ray exposure of 1 mGy represents an average of one electron track per mammalian cell nucleus (Bond et al., 1988), this is the lowest exposure that a cell can receive. The results indicate, therefore, that the maximum protective effect occurred in response to the first radiation track, and that at higher doses, where each cell certainly received multiple tracks, there was no greater (or lesser) response. However, because of the random nature of radiation, a dose that gives an average of one track/cell does not actually deliver a track to each cell. A large fraction of the cells exposed to 1 mGy receive no track at all while a smaller fraction receive 2 or more tracks. Since higher doses (10–500 mGy) where cells certainly received multiple tracks did not result in any greater response than a dose of 1 mGy, the cells that received no tracks in the 1-mGy exposure must have been responding in the same way and to the same extent as the cells that actually received one or more tracks.
FIGURE 1.
Adaption of normal human fibroblast cells induced by exposure to various doses of 60Coγ-irradiation at 37°C, as measured by micronucleus frequency within binucleate cells. All cells were incubated for 3 h at 37°C after exposure to the adapting dose, and prior to irradiation with the 4 Gy (or sham) challenge dose. Control, unadapted cells were exposed to the 4 Gy challenge dose alone. Cells exposed to any of the adapting exposures prior to the 4 Gy challenge dose were significantly different from the cells exposed to the 4 Gy challenge alone; p < 0.05. Mean ± SD, n = 3. From Broome et al. (2002).
This result strongly suggests, therefore, that a different bystander effect also exists, which, instead of increasing damage or risk to bystander cells, protects those cells by increasing their capacity for DNA double-strand-break repair. Since reduced DNA double-strand-break repair capacity has been shown to elevate the detrimental type of bystander effects (Nagasawa et al., 2003), elevated repair capacity would be expected to counteract those detrimental effects.
The cells used in the experiment shown in Figure 1 were grown such that they were in contact with each other during the exposures. The signaling process for this adaptive response in bystander cells could therefore have been either through gap junctions or via signals secreted into the medium. We have recently repeated this experiment using skin fibroblast cells from white tail deer and found the same result (Ulsh et al., 2004). Those cells, however, were not in contact during the exposures, indicating an adaptive response signal transmitted via the culture medium.
BYSTANDER EFFECTS AND SIGNAL RECEPTORS
Lymphocytes were the first human cell type where an adaptive response to low doses of radiation was demonstrated (Olivieri et al., 1984). If adaption at low doses involves bystander effect signals transmitted through the cell medium, then the response of cells depends not only on the ability of cells receiving a hit to send the signal but also on the ability of bystander cells to receive that signal. We have examined the influence of low doses on the ability of human lymphocytes to express cell-surface receptors for IL-2 and the ability of exposed cells to transmit signals to enhance receptor formation in bystander cells (Xu et al., 1996). Table 1 shows that low doses enhance the expression of such receptors and that the signals for enhanced expression can be transmitted to unexposed cells. Table 2 shows that those signals are secreted into the medium within the first 24 h after exposure, and by that time the irradiated cells alone are no longer able to induce the response in bystander cells.
TABLE 1.
Percentage of Cells (Mean ± S.D. for n = 7) Expressing IL-2Rα Receptors After Con A Stimulation*
| Time after Con A stimulation (h) | Control cells | Irradiated cells (10 mGy) | 50% control cells + 50% irradiated cells |
|---|---|---|---|
| 24 | 7.7 ± 4.1 | 17.8 ± 3.3 p <0.001 | 22.6 ± 4.8 p <0.0001 |
*Data from Xu et al. (1996).
TABLE 2.
Radiation-Induced (10 mGy) Release of IL-2Rα Receptor Stimulating Factor into the Medium†
| Control cells | Control cells + irradiated medium | 50% Control cells + 50% irradiated cells + control medium |
|---|---|---|
| 32.4 ± 3.2 | 40.9 ± 3.6 p <0.01 | 30.0 ± 4.2 n.s. |
†The numbers indicate the percentage (mean ± S.D. for n = 7) of cells expressing IL-2Rα after 48 h total incubation. Irradiated cells were incubated for 24 h prior to separation of those cells from their medium and for a further 24 h incubation with control cells. Data from Xu et al. (1996).
ADAPTIVE RESPONSES AGAINST DAMAGING BYSTANDER EFFECTS
If two independent types of bystander effects exist, one damaging and another protective, then it should be possible to show that the protective system can reduce the effects of the damaging system. Using doses where bystander effects have been shown to occur, such experiments have been reported by Iyer and Lehnert (2002a). They showed that medium taken from human lung fibroblasts exposed to 10 mGy of α-radiation increased clonogenic survival of other cells exposed to 100 mGy of α-particles. The same workers showed the same effect using the medium from cells exposed to 10 mGy of γ-radiation to protect other cells exposed to 1 Gy of γ-radiation (Iyer and Lehnert, 2002b). It has also been shown that signals generated by γ-irradiated cells can protect against the bystander effects produced by α-irradiated cells. Again, at doses where bystander effects occur after γ-radiation, Sawant et al. (2001) showed that prior exposure to 20 mGy of γ-radiation canceled out about half of the bystander effect that resulted from an γ-radiation exposure of rodent cells where only about 10% of the cell nuclei received an α-track.
LOW DOSES, BYSTANDER EFFECTS, AND RISK IN VITRO
For radiation protection purposes in humans, cancer is generally considered the risk of greatest concern. Most reports describing bystander effects, and their implied influence on dose-response curves, use surrogate measures of cancer risk such as DNA damage, chromosomal aberrations, mutations, and micronucleus formation. To properly translate information on bystander effects into risk, it is necessary to measure a more direct indicator of risk. Azzam et al. (1994b, 1996) have reported the effects of low doses of γ-radiation on both radiation-induced and spontaneous neoplastic transformation in rodent C3H 10T1/2 cells. Table 3 shows those results for spontaneous transformation. The data show that low doses reduce the risk of spontaneous neoplastic transformation in these cells. As was the case when micronucleus formation was measured (Figure 1), the dose response was flat (i.e., the maximum protective response was observed) for any dose given to the cells, from 1 to 100 mGy, indicating that, for this direct measure of cancer risk in cells, the maximum effect was also induced by the lowest exposure possible in a single cell. As was noted earlier, a large fraction of the cells exposed to 1 mGy receive no track and therefore the observed protective effect against neoplastic transformation must also, to a large extent, be due to bystander effects.
TABLE 3.
Influence of Low γ-Doses on the Risk of Spontaneous Malignant Transformation in C3H 10T1/2 Cells†
| Treatment | Transformation frequency (×10–3) |
|---|---|
| Control | 1.7 |
| 1.0 mGy | 0.53 |
| 10 mGy | 0.42 |
| 100 mGy | 0.53 |
†Data from Azzam et al. (1996).
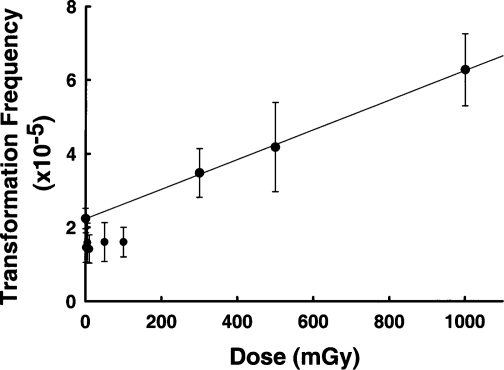
A similar finding of reduced transformation has been reported by Redpath and coworkers (Redpath and Antoniono, 1998; Redpath et al., 2001), who used human hybrid cells to examine spontaneous neoplastic transformation after low doses of γ-radiation. However, in addition to doses between 1 and 100 mGy, those workers also examined higher doses up to 1 Gy (Figure 2). Those data clearly show that the protective effect was lost and transformation increased above control at doses between 100 and 300 mGy. Both protective bystander effects (Azzam et al., 1996; Broome et al., 2002) and detrimental bystander effects (Lyng et al., 2000; Seymour and Mothersill, 2000) have been shown to result from γ-radiation exposure at doses between 1 and 500 mGy. The data in Figure 2 therefore suggest the existence of a transition dose point between 100 and 300 mGy, below which protective effects, including protective bystander effects, predominate, and above which detrimental effects overwhelm the protective effects.
FIGURE 2.
Transformation frequency of human fibroblast cells exposed to various doses of γ-radiation. The line shows a linear regression analysis of data for control cells and data for cells receiving ≥300 mGy. Data for exposed cells receiving 100 mGy or less were not included in the regression analysis. Data redrawn from Redpath et al. (2001).
LOW DOSES, BYSTANDER EFFECTS, AND RISK IN VIVO
Ultimately, to impact on radiation protection assumptions and practices, the relative influence of protective and detrimental bystander effects must be tested in vivo. Mitchel et al. (1999b) examined the influence of a low dose (100 mGy) on the frequency and latency of γ-radiation-induced myeloid leukemia in genetically normal mice. This dose was within the range of γ-radiation doses shown to result in protective cellular bystander effects for both micronucleus formation and neoplastic transformation (Azzam et al., 1996; Broome et al., 2002) but also detrimental bystander effects (Lyng et al., 2000, 2002; Seymour and Mothersill, 2000). Those data showed that this low dose did not change the frequency of radiation-induced leukemia but did significantly increase latency. The protective effect persisted for all leukemias that appeared over the lifespan of the mice. That result suggested that the protective type of bystander effect preferentially slows the progression of genomic instability rather than reducing the frequency of initiating mutations. Since detrimental bystander effects have been linked to increased genomic instability, the data suggest that protective effects have partly suppressed bystander-induced detrimental effects.
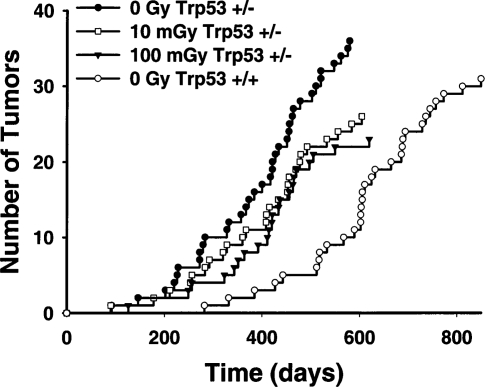
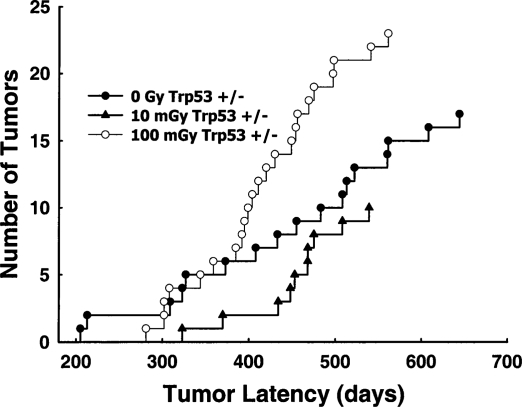
Mitchel et al. (2003) have also examined the effects of low γ-radiation doses on spontaneous cancer formation in cancer-prone (Kemp et al., 1994) Trp53 heterozygous mice (Trp53 +/−) over the duration of their natural life span. Figure 3 shows that exposure of 8-week-old Trp53 +/− mice to a single60Co-γ dose of either 10 or 100 mGy at low dose rate (0.5 mGy/min) significantly increased latency for spontaneous lymphomas (p <10–4, Wilcoxon two-sided probability) but had no significant effect on lymphoma frequency (p >0.05, chi-squared test). Spinal osteosarcomas leading to paralysis were also monitored in the same mice. Figure 4 shows that while 10 mGy significantly reduced risk by increasing spontaneous osteosarcoma latency (p = 0.005), increasing the dose to 100 mGy was detrimental and significantly decreased latency (p <0.04), again with no significant influence on osteosarcoma frequency (p >0.05). These data indicate that there is a dose threshold for a transition from protective effects to detrimental effects. While that threshold appears to be tumor-type- (i.e., cell-type)-dependent and may also be influenced by Trp53 status, the threshold occurs around 100 mGy. This value is remarkably similar to the value observed for in vitro neoplastic transformation in human cells (Redpath and Antonino, 1998) and is also consistent with the observations in rodent cells (Azzam et al., 1996). The data indicate that, in vivo, protective effects, including protective bystander effects, must predominate at doses below this threshold. Since the magnitude of detrimental bystander effects, appears to be related to DNA double-strand-break repair capacity (Nagasawa et al., 2003), the elevated capacity for DNA double-strand-break repair observed at those doses (Broome et al., 2002) must outweigh the detrimental effects. Above the dose threshold, detrimental bystander effects due to increasing DNA double-strand breaks must overwhelm those protective effects. None of the data are consistent with an assumption of a linear response with dose at low doses.
FIGURE 3.
Latency of lymphomas appearing in unexposed Trp53 normal mice (Trp53 +/+, n = 159 mice total ) and in Trp53 heterozygous (Trp53 +/−) mice exposed to various doses of radiation (0 Gy, n = 174; 10 mGy, n = 167; 100 mGy, n = 168). From Mitchel et al. (2003).
FIGURE 4.
The latency of spinal osteosarcomas associated with paralysis in Trp53 heterozygous (Trp53 +/−) mice exposed to 0, 10, or 100 mGy, given at low-dose rate. Group sizes are given in Figure 3. From Mitchel et al. (2003).
BYSTANDER EFFECTS, RISK, AND HIGH-LET RADIATION IN VIVO
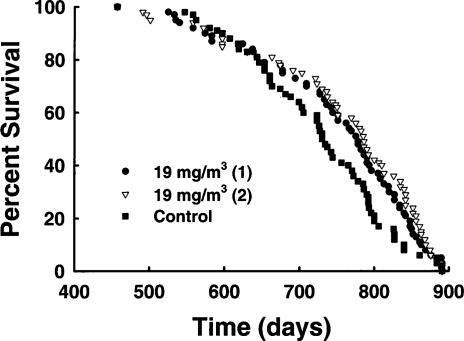
Less information is available concerning the in vivo influence of bystander effects from low doses of high-LET exposure. Mitchel et al. (1999a) reported that rats chronically exposed to α-radiation from inhaled natural uranium ore dust lived longer than control rats (Figure 5) even though many of the exposed animals (22/126) developed malignant tumors late in life (mainly lung cancers) while the controls did not (1/63). This result suggests that low doses of high-LET radiation produce protective effects, but that detrimental effects appear as dose increases, an outcome that exactly parallels the results seen with low-LET radiation exposure of mice. Cohen (1995) correlated residential radon levels with lung cancer rates for a large portion of U.S. counties and found a strong negative correlation. An area of Japan with high natural radon levels was shown to have lower cancer rates, including lung cancer, than the average for all of Japan (Mifune et al., 1992) or the average for a control area with low natural radon levels (Suzuki et al., 1994). These results also suggest that, in both rats and humans, protective bystander effects may predominate. However, it is useful to consider the difference in the minimum possible dose to a single cell from a low-LET radiation like a γ-ray (about 1 mGy) and from a high-LET radiation like an α-particle (200–300 mGy). If the process of carcinogenesis after high-LET radiation exposure is the same as after low-LET radiation exposure, then similar dose thresholds for the transition from protective to detrimental effects might be expected. Since these thresholds appear to be in the 100–300 mGy range for low-LET exposures, and this is also the dose range for a single α-track in a cell, it is possible that variable results (protective or detrimental) will be observed for bystander-induced changes in risk from high-LET radiation. This variability may be amplified if the dose threshold for high-LET exposure is also cell-type dependent, as it appears to be after low-LET exposure.
FIGURE 5.
Survival of Sprague Dawley rats inhaling natural uranium ore dust aerosol for 4.2 h/day, 5 days/week for 65 weeks. The figure shows survival of two separate groups of animals inhaling ore dust aerosol at 15 mg/m3 in air (22 malignant tumors total/126 rats total) and the control group (one malignant tumor/63 rats) treated in the same way without the ore dust aerosol. A nose-only inhalation system was used. Data from Mitchel et al. (1999).
CONCLUSIONS
Low doses can induce both protective and detrimental bystander effects, both of which lead to nonlinear responses with dose. Signaling for these effects can be transmitted via gap junctions or by factors released into the medium. Bystander effects induced by low-LET radiation are associated with protective-type adaptive responses to both radiation-induced and spontaneous malignant transformation. At doses below about 100 mGy, these effects reduce rather than increase cancer risk in cells and animals, suggesting that protective effects outweigh detrimental effects in that dose range. Above that dose threshold, detrimental effects outweigh protective effects. The threshold is cell-type dependent. For high-LET radiation, the influence on risk is less clear. Animal data suggest that, as dose increases, protective and then detrimental effects can be observed as is the case with low-LET radiation. Some human data suggest that low doses of high-LET alpha radiation reduce risk. However, a single alpha track is in the range of the threshold dose between reduced and increased risk, as determined from observations with low-LET radiation. Since the threshold is also cell-type dependent, it is conceivable that experimental data using single α-tracks could either increase or decrease risk.
REFERENCES
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Radiation-induced radioresistance in a normal human skin fibroblast cell line. In: Sugahara T, Sagan LA, Aoyama T, editors. Low Dose Irradiation and Biological Defense Mechanisms. Amsterdam: Elsevier Science Publishers B.V.; 1992. pp. 291–294. [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Réponse adaptative au rayonnement ionisant des fibroblastes de peau humaine. Augmentation de la vitesse de réparation de l’ADN et variation de l’expression des gènes. J Chim Phys. 1994a;91:931–936. [Google Scholar]
- Azzam EI, Raaphorst GP, Mitchel REJ. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T 1/2 mouse embryo cells. Radiat Res. 1994b;138:528–531. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T 1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Bond VP, Feinendegen LE, Booz J. What is a ‘low dose’ of radiation? Int J Radiat Biol. 1988;53:1–12. doi: 10.1080/09553008814550361. [DOI] [PubMed] [Google Scholar]
- Broome EJ, Brown DL, Mitchel REJ. Adaption of human fibroblasts to radiation alters biases in DNA repair at the chromosome level. Int J Radiat Biol. 1999;75:681–690. doi: 10.1080/095530099140014. [DOI] [PubMed] [Google Scholar]
- Broome EJ, Brown DL, Mitchel REJ. Dose responses for adaption to low doses of 60Co-γ and 3H-β radiation in normal human fibroblasts. Radiat Res. 2002;158:181–186. doi: 10.1667/0033-7587(2002)158[0181:drfatl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission for Radiological Protection) Recommendations of the International Commission for Radiological Protection 1990 1991. Publication 60, Annals of the ICRPPergamon Press; Oxford [Google Scholar]
- Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res. 1996;146:582–585. [PubMed] [Google Scholar]
- Iyer R, Lehnert BE. Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res. 2002a;157:3–7. doi: 10.1667/0033-7587(2002)157[0003:apiiit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res. 2002b;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Wheldon T, Balmain A. P53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nature Genet. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Kadhim MA, Pocock DA, Papworth D, Stevens DL, Goodhead DT, Wright EG. Chromosomal instability in the descendants of unirradiated surviving cells after alpha-particle irradiation. Proc Natl Acad Sci USA. 1998;95:5730–5773. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Brit J Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: A possible mechanism for bystander-induced genomic instability? Radiat Res. 2002;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mifune M, Sobue T, Arimoto H, Komoto Y, Kondo S, Tanooka H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn J Cancer Res. 1992;83:1–5. doi: 10.1111/j.1349-7006.1992.tb02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ, Heinmiller B, Jackson JS. Inhaled uranium ore dust and lung cancer risk in rats. Health Phys. 1999a;76:145–155. doi: 10.1097/00004032-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, McCann RA, Boreham DR. Adaptive response modification of latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999b;152:273–279. [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced bystander effects: Past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat Res. 2002;508:121–129. doi: 10.1016/s0027-5107(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int J Radiat Biol. 2003;79:35–41. [PubMed] [Google Scholar]
- Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentration of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Antoniono RJ. Induction of an adaptive response against spontaneous neoplastic transformation in vitro by low-dose gamma radiation. Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, Christie C, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: Evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rigaud O, Papadopoulo D, Moustacchi E. Decreased deletion mutation in radioadapted human lymphoblasts. Radiat Res. 1993;133:94–101. [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Metting NF, Hall EJ. Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat Oncol Investig. 1997;5:106–110. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Honjo S, Kawamura H, Koishi F, Suzuki T, Hirohata T. Cancer mortality in low radon spa area. Jpn J Cancer Res. 1994;85:1063–1066. doi: 10.1111/j.1349-7006.1994.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsh B, Miller S, Boreham D, Mallory F, Mitchel REJ, Morrison D. Cytogenetic dose-responses in the cells of three ungulate species exposed to high and low doses of ionizing radiation. J Environ Radioact. 2004;74:73–81. doi: 10.1016/j.jenvrad.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Greenstock CL, Trivedi A, Mitchel REJ. Occupational levels of radiation exposure induce surface expression of interleukin-2 receptor in stimulated human peripheral blood lymphocytes. Radiat Environ Biophys. 1996;35:89–93. doi: 10.1007/BF02434030. [DOI] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK. Radiation risk to low fluences of alpha particles may be greater than we thought. Proc Natl Acad Sci USA. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]