Abstract
The regulation of photosynthetic acclimation to canopy density was investigated in tobacco canopies and in tobacco and Arabidopsis plants with part of their foliage experimentally shaded. Both species acclimated to canopy light gradients and partial shading by allocating photosynthetic capacity to leaves in high light and adjusting chloroplast organization to the local light conditions. An investigation was carried out to determine whether signalling mediated by photoreceptors, sugars, cytokinin, and nitrate is involved in and necessary for proper photosynthetic acclimation. No evidence was found for a role for sugars, or for nitrate. The distribution of cytokinins in tobacco stands of contrasting density could be explained in part by irradiance-dependent delivery of cytokinins through the transpiration stream. Functional studies using a comprehensive selection of Arabidopsis mutants and transgenics showed that normal wild-type responses to partial shading were retained when signalling mediated by photoreceptors or cytokinins was disrupted. This indicates that these pathways probably operate in a redundant manner. However, the reduction of the chlorophyll a/b ratio in response to local shade was completely absent in the Arabidopsis Ws-2 accession mutated in PHYTOCHROME D and in the triple phyAphyCphyD mutant. Moreover, cytokinin receptor mutants also showed a reduced response, suggesting a previously unrecognized function of phyD and cytokinins.
Keywords: Arabidopsis mutants, cytokinin, environmental signalling, photoreceptors, photosynthetic acclimation, tobacco
Introduction
An important ecophysiological question has been how plants perceive the light gradient imposed upon them by the proximity of neighbouring plants. Crowding leads to severe shading, especially of lower canopy layers, while upper leaves remain fully exposed to sunlight (Monsi and Saeki, 1953), inducing adaptive photosynthetic acclimation responses at the whole-plant, leaf, and chloroplast level (Niinemets, 2007). Mainly, such partial shading reduces photosynthetic capacity and hence nitrogen per unit area. Furthermore, it accelerates senescence of the shaded, oldest leaves, accompanied by reallocation of resources for the photosynthetic machinery from lower to upper leaves (Grindlay, 1997; Ono et al., 2001; Hirose, 2005; Terashima et al., 2005; Niinemets, 2007). Within the chloroplasts, shading leads to enhanced allocation of resources to light harvesting at the expense of photosynthetic capacity (Evans, 1993; Pons and Pearcy, 1994). This suite of traits is an example of phenotypic plasticity in response to neighbour proximity and was shown to be beneficial in terms of photosynthetic carbon gain by using model calculations (Grindlay, 1997; Anten et al., 2000; Pons and Anten, 2004; Hirose, 2005) and recently by using transgenic plants with delayed senescence (Boonman et al., 2006).
The perception of neighbouring plants and regulation of photosynthetic acclimation were shown to depend on photoreceptors and xylem-translocated cytokinin, and various other mechanisms were postulated (reviewed by Ono et al., 2001; Kull, 2002; Terashima et al., 2005). Photoreceptors perceive the quantity and spectral quality of light and regulate photosynthetic gene expression (Terzaghi and Cashmore, 1995; Chen et al., 2004). The phytochrome photoreceptors were shown to regulate senescence induced by the low red to far-red ratio of light (R:FR) at the bottom of dense sunflower canopies (Rousseaux et al., 1996, 2000). Reduced irradiance without a change in spectral composition of the light is sufficient to induce photosynthetic shade acclimation, with little additional effect of a change in R:FR (Pons and de Jong-van Berkel, 2004). All photoreceptor mutants studied to date showed normal acclimation to such spectrally neutral shade in the same way as the wild types (Walters et al., 1999; Weaver and Amasino, 2001), indicating they are not of major importance, or at least not the only players. Photoreceptors are characterized by partially redundant functions of different gene family members, possibly masking any phenotype when single mutations are studied (Franklin et al., 2003b; Chen et al., 2004). To date, a comprehensive study including higher order mutants encompassing all known members of the phytochrome, cryptochrome, and phototropin photoreceptor families is lacking.
Light may also be perceived independently of photoreceptors. For example, the redox state of components of the electron transport chain is used as a signal for light acclimation (Huner et al., 1998; Pfannschmidt et al., 1999). Another alternative pathway to regulate shade acclimations involves the xylem-transported phytohormone cytokinin (Pons and Bergkotte, 1996; Pons and Jordi, 1998; Pons et al., 2001; Boonman and Pons, 2007; Boonman et al., 2007). Measurements showed that cytokinin delivery to shaded leaves is decreased as a consequence of reduced stomatal conductance and consequently transpiration rates, resulting in a canopy density signal that regulates, at least in part, photosynthetic capacity and senescence (Boonman et al., 2007). Other xylem-transported compounds can also be considered potential signals, including nitrate, which is known to have signalling properties (Crawford, 1995; Stitt, 1999). Finally, foliar sugar concentrations are modulated in response to irradiance, and sugars repress photosynthetic gene expression (Sheen, 1990; Koch, 1996). It has therefore been proposed that they are involved in signalling in dense leaf canopies as well (Ono et al., 2001; Kull, 2002).
While it is clear that multiple signal transduction pathways are involved in canopy density perception, the relative importance of these routes for the regulation of acclimation at the level of whole plants or chloroplasts is not known. Two complementary approaches were used to clarify further the role of these various mechanisms. First, soluble sugars, nitrate, and a wide range of cytokinins were measured as putative signals along canopy height in tobacco stands of contrasting density. Vertical profiles of these compounds in tobacco have been reported before, but only in plants grown alone and for a limited number of cytokinins (Masclaux et al., 2000; Nordström et al., 2004). Whole-plant level acclimation to canopy density has been found previously in those tobacco stands, as demonstrated by a steeper decline in photosynthetic capacity from the top of the canopy downwards at the higher canopy density (Boonman et al., 2007). Here, the question was asked of whether the distribution of putative signals was consistent with a role in acclimation at both the whole-plant and chloroplast level. Secondly, acclimation was measured in artificially shaded tobacco and Arabidopsis leaves on plants of which the rest remained in high light, and found that this treatment mimics the responses of shaded leaves in a real canopy. This allowed the use of a large number of Arabidopsis mutants defective in photoreceptor, cytokinin, and sugar-mediated signalling. Double and higher order mutants corresponding to all known cytokinin receptors and all known photoreceptors, sugar-insensitive or hypersensitive mutants, and genotypes with constitutively lower or higher cytokinin content were included. The question was then asked of whether any of these pathways are necessary for proper acclimation to partial shading as found in dense canopies.
Materials and methods
Plant material and growth conditions
The density experiment using tobacco (Nicotiana tabacum L.; cultivar Wisconsin 38) has been described elsewhere (Boonman et al., 2007). Briefly, an open stand (3.6 plants m−2) and a dense stand (35 plants m−2) were established in a greenhouse, grown for 11 weeks, and measurements were taken during the last 2 weeks. Leaves at three heights from at least three replicate individuals were harvested 70 d after sowing and immediately frozen in liquid nitrogen for biochemical analyses. In each stand, non-senescent leaves were taken from positions exposed to maximal, intermediate, or minimal irradiance. One week later, plants were moved to the laboratory for gas exchange and chlorophyll measurements. Tobacco and Arabidopsis plants for the partial shade experiments were grown in a climate-controlled growth chamber with a 20 °C light/16 °C dark cycle, a 16 h light period for tobacco, and a 9 h light period (short days) for Arabidopsis at a photosynthetic photon flux density (PPFD) of 200 mmol m−2 s−1, as described by Boonman et al. (2007).
Arabidopsis thaliana accession Columbia-0 (Col-0) was used for sugar and nitrate analysis, and mutants and transgenics were in the Col-0 background unless stated otherwise. The following cytokinin receptor mutants which show reduced cytokinin sensitivity were studied: ahk2-2tk, ahk3-3, the double mutant ahk2-2tkahk3-3 (Higuchi et al., 2004), and the cre1-2 line with a T-DNA insertion in the CRE1 gene (Inoue et al., 2001), as well as cre1-1 in the Landsberg erecta (Ler-0) background. The pleiotropic mutant amp1-1 that has constitutively elevated cytokinin levels (Chaudhury et al., 1993) was used together with transgenic 35S::CKX1, 35S::CKX2, 35S::CKX3, and 35S::CKX4 plants that have constitutively reduced cytokinin levels, through overexpression of cytokinin oxidase genes (Werner et al., 2003). The following mutants with altered sugar sensitivity were used: ctr1-1, defective in a central component of the ethylene signal transduction pathway and known to be sucrose insensitive (Kieber et al., 1993; León and Sheen, 2003), and the ethylene-insensitive and, by implication, sugar-hypersensitive mutants (León and Sheen, 2003) ein4-1 (Roman et al., 1995) and etr1-1 (Bleecker et al., 1988). In the Ler-0 background, the glucose-insensitive mutant gin2-1 (Jang et al., 1997) and the photoreceptor mutants phyAphyB (Reed et al., 1994), cry1cry2, which was a cross of the fha1 and hy4-1 mutant alleles (Ahmad et al., 1998; Weston et al., 2000), and hy2, defective in phytochrome chromophore synthesis (Koornneef et al., 1980; Parks and Quail, 1991), were used. The Wassilewskija (Ws-2) accession naturally harbours the phyD mutation (Aukerman et al., 1997). Also in the Ws background was phyAphyCphyD (Franklin et al., 2003a). Finally, phot1phot2 (Kinoshita et al., 2001) had a combined Ler/Ws background.
Partial shade treatment
To simulate light gradients as they occur in dense leaf canopies, single attached leaves were shaded for 6–7 d, while the rest of the plant remained in normal light. Previous work demonstrated that this treatment mimics changes induced by vertical light gradients in dense canopies (Boonman et al., 2007). Spectrally neutral shading was applied by enclosing the leaf in a mitten made of two paper sheets on the upper side and coarse mosquito mesh on the lower side. The sides were held apart by black foam so leaf expansion was not hindered by the treatment. The upper paper sheet had a fine grey print on the downward-facing side, resulting in a reduction of PPFD by 93% to ∼14 μmol m−2 s−1. Control leaves on different plants remained in growth light. The eighth true leaf counted from below which was still expanding was used in most cases, or a lower leaf number for those mutants that formed fewer leaves. In amp1-1 and ctr1-1, an expanding and exposed leaf was selected because leaf number could not be determined.
Humidity treatment
Transpiration rates of individual Arabidopsis leaves were reduced independently of irradiance by enclosing the leaf in a transparent cuvette flushed with humid air for 6 d as described by Boonman et al. (2007). Incident PPFD remained at 200 μmol m−2 s−1 and leaf temperature was equal to that of control leaves, which were enclosed in a cuvette flushed with growth chamber air.
Light quantity and light quality measurements in tobacco stands
PPFD in the dense tobacco canopy was measured on a horizontal plane in six positions at 10 cm height increments using a line sensor (AccuPAR Ceptometer PAR-80, Decagon, Pullman, WA, USA) and directly above the canopy using a quantum sensor (LI-185A; Li-Cor, Lincoln, NE, USA), to obtain relative PPFD. In the open stand, PPFD was measured using the LI-185A quantum sensor on the upper surface of leaves at various positions on six individual plants and also directly above the plants. R:FR gradients were measured with a line sensor with three R-sensitive sensors alternating with three FR-sensitive sensors. The R sensors were composed of a Hamamatsu G1118 GaAsP photodiode with a Schott RG-645 optical filter resulting in a peak sensitivity of ∼660 nm. The FR sensors consisted of a G1738 GaAsP photodiode with a Schott RG-9 optical filter resulting in a peak sensitivity of ∼730 nm. The sensors were calibrated against a spectroradiometer (LI-1800, LI-COR).
Leaf analyses
Photosynthetic capacity was measured as the light- and CO2-saturated rate of net photosynthesis (Amax) based on gas exchange, or as the light-saturated rate of electron transport based on chlorophyll fluorescence (ETRmax). In both species, Amax was measured in the laboratory using a gas-exchange measuring system with leaf chambers with a 69×67 mm window that was described previously (Pons and Welschen, 2002). An infrared gas analyser (LI-6262, Li-Cor) was used to measure CO2 and H2O partial pressure. Leaf temperature was kept at 25 °C, VPD was ∼1 kPa, CO2 partial pressure of the air entering the leaf chambers was 110 Pa, and PPFD was 1200 μmol m−2 s−1, which saturated photosynthesis. The leaf area enclosed in the chamber was measured. Net rates of photosynthesis were calculated according to von Caemmerer and Farquhar (1981). ETRmax of Arabidopsis leaves was measured in the growth room using a portable chlorophyll fluorometer with attached leaf-clip holder (Mini-PAM, Waltz, Effeltrich, Germany) that kept the leaf at 8 mm from the fibreoptic probe. Steady-state chlorophyll fluorescence (F′) was measured under saturating PPFD (600–1000 μmol m−2 s−1 for shaded and control leaves, respectively) using a halogen lamp which was assessed with a quantum sensor (LI-185A, Li-Cor). Maximal fluorescence (Fm′) was measured with a saturating light pulse of ∼5 mmol m−2 s−1. An air fan prevented any increase in leaf temperature. Photochemical yield (Y) was calculated as Y=(F′–Fm′)/Fm′ (Genty et al., 1989). Leaves were pre-induced at a PPFD of ∼600 μmol m−2 s−1 for at least 10 min and were placed in the measuring position for 2 min before readings were taken. A fresh 1 cm2 sample was taken for chlorophyll analysis with a spectrophotometer after extraction in dimethylformamide (Inskeep and Bloom, 1985). Leaf absorbance (α) was calculated as a function of leaf chlorophyll content (Chl, μmol m−2) following Evans (1993): α=Chl/(Chl+76). ETRmax was then calculated as: ETRmax=α×0.5×PPFD×Y (Genty et al., 1989). Acclimation at the chloroplast level was analysed by measuring the following parameters, Amax/Chl or ETRmax/Chl, calculated using the parameters described above, and the Chl a/b ratio. For soluble sugar analysis, leaf material was freeze-dried and ground, and extracted twice in 80% ethanol at 80 °C during 30 min. The extract was centrifuged and purified according to Bligh and Dyer (1959). Soluble sugars were determined in the supernatant with a spectrophotometer with anthrone as a colour reagent, and using glucose as a standard (Yemm and Willis, 1954). Nitrate was analysed in homogenized, dry material using salicylic acid as a reagent (Cataldo et al., 1975).
Cytokinin analysis with micro LC-MS/MS
Cytokinins were analysed as previously described (Corbesier et al., 2003; Boonman et al., 2007). Frozen leaf samples were ground in liquid nitrogen, transferred into Bieleski solution (Bieleski, 1964), and extracted overnight at –20 °C. For each cytokinin compound determined, 10 pmol of 2[H3]dihydrozeatin, 2[H3]dihydrozeatin riboside, 2[H3]dihydrozeatin 9-glucoside, 2[H3]dihydrozeatin riboside 5′-monophosphate, 2[H6]N6-(Δ2isopentenyl)adenine, 2[H6]N6-(Δ2isopentenyl)adenosine, 2[H6]N6-(Δ2isopentenyl) adenine glucoside, and 2[H6]N6-(Δ2isopentenyl)adenosine 5′-monophosphate (OLCHEMIM Ltd, Olomouc, Czech Republic) were added as internal standards. Cytokinins were purified by a combination of solid-phase and immunoaffinity chromatography using a broad-spectrum anti-isoprenoid cytokinin immunoaffinity column (OLCHEMIM) as described (Redig et al., 1996). O-Glucosides were not collected. Cytokinins were quantified by micro-liquid chromatography-positive electrospray-tandem mass spectrometry (micro-LC MS/MS) in multiple reactant monitoring mode (Prinsen et al., 1998). The chromatograms obtained were processed using Masslynx software (Micromass, Manchester, UK).
Statistical analyses
Analysis of covariance (ANCOVA) was used to study the effects of stand density and relative height on the distribution of various parameters in the tobacco canopies. The effects of partial shade were analysed with a Student's t-test. High light control levels of ETRmax and Chl a/b of mutants were compared with those of their respective wild types using a one-way analysis of variance (ANOVA) followed by Tukey's b-test. Data were log-transformed when it improved homogeneity.
Results
Effects of canopy density on tobacco
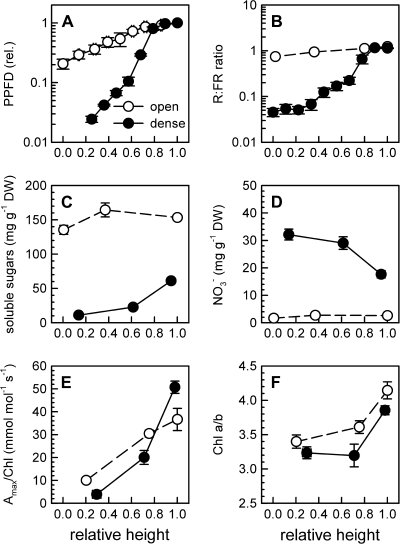
Tobacco plants growing in a dense stand were exposed to a much steeper gradient in irradiance (Fig. 1A) and R:FR (Fig. 1B) when compared with the open stand. Besides these primary signals, canopy density also affected the distribution of soluble sugars (Fig. 1C), nitrate (Fig. 1D), and cytokinins (Table 1). Soluble sugars accumulated to much higher levels in open stand plants at all canopy positions (Fig. 1C), whereas nitrate accumulated much more in dense stand plants and was only present at low concentrations in the open stand at all positions (Fig. 1D). In the dense stand, only upper leaves had significant sugar concentrations, and intermediate and lower leaves exposed to deep canopy shade contained almost no sugar (Fig. 1C). In contrast, nitrate accumulated in the lower leaves in dense stand plants, opposite to the light gradient (Fig. 1D). Eleven different cytokinins were detected using micro-LC MS/MS (Table 1). In general, the dominant cytokinins were cis-zeatin riboside monophosphate (ZRP) and isopentenyl adenosine monophosphate (iPRP), and concentrations were highest in the upper leaves and declined towards the bottom in both stands. High canopy density reduced the concentration of cis-ZRP in upper leaves by ∼50%. Trans-Z-type cytokinins were only detected in upper leaves in both stands, and dihydrozeatin (DHZ)-type cytokinins were below or close to the detection limit in all samples. For one of the cytokinins, isopentenyl adenosine (iPR), a significant height by density interaction was observed when only upper and intermediate leaves were considered (P <0.05; ANCOVA), that was consistent with the irradiance gradient. That is, iPR concentrations were reduced more strongly from the top of the canopy downwards in the dense stand than in the open stand (Table 1).
Fig. 1.
Canopy density effects on putative signal parameters and photosynthetic acclimation in tobacco. Shown are PPFD (A), R: FR (B), soluble sugars (C), and nitrate (D), photosynthetic capacity per unit chlorophyll (Amax/Chl) (E) and the chlorophyll a/b ratio (Chl a/b) (F) measured in open (3.6 plants m−2) and dense (35 plants m−2) tobacco canopies at three heights representative for maximal, intermediate, and minimal irradiance in each stand. Data are means±SE, n=6–12. Note the log-scale on the y-axis in A and B.
Table 1.
Canopy density effects on the cytokinin distribution in tobacco
| Cytokinin (pmol g−1 fresh weight) | ||||||
| Dense canopy | Open canopy | |||||
| Low* | Intermediate | High | Low | Intermediate | High | |
| cis-Z | 0.282±0.114 | 0.246±0.009 | 0.217±0.040 | 0.390±0.117 | 0.386±0.139 | 0.392±0.096 |
| cis-ZR | 0.198±0.079 | 0.156±0.028 | 0.176±0.014 | 0.146±0.028 | 0.177±0.079 | 0.205±0.007 |
| cis-ZRP | 0.631±0.095 | 1.315±0.143 | 3.140±1.181 | 0.908±0.094 | 1.763±0.679 | 7.661±1.361 |
| trans-Z | nd | nd | 0.129±0.056 | nd | nd | 0.166±0.047 |
| trans-ZR | nd | nd | 0.081±0.016 | nd | nd | nd |
| trans-ZRP | nd | nd | 1.430±0.566 | nd | nd | 1.279±0.143 |
| DHZ | nd | nd | nd | nd | nd | 0.067±0.028 |
| DHZRP | nd | 0.028±0.008 | 0.242±0.097 | nd | nd | 0.570±0.095 |
| iP | 0.342±0.037 | 0.333±0.051 | 0.266±0.028 | 0.212±0.015 | 0.261±0.019 | 0.369±0.102 |
| iPR | 0.227±0.035 | 0.236±0.019 | 0.632±0.059 | 0.179±0.008 | 0.284±0.046 | 0.409±0.020 |
| iPRP | 1.063±0.227 | 2.777±0.115 | 11.39±2.275 | 1.376±0.286 | 2.844±1.067 | 12.04±0.682 |
Values represent means±SE, n=3.
DHZ, dihydrozeatin; DHZRP, dihydrozeatin riboside monophosphate; iP, isopentenyl adenine; iPR, isopentenyl adenosine; iPRP, isopentenyl adenosine monophosphate; nd, not detected; Z, zeatin; ZR, zeatin riboside; ZRP, zeatin riboside monophosphate.
Positions correspond to relative heights in Fig. 1C, D.
Chloroplast acclimation to canopy density was evident in the tobacco stands because Amax/Chl (Fig. 1E) and to a lesser extent Chl a/b (Fig. 1F) both declined more strongly from the top of the canopy downwards in the dense stand as compared with the open stand. Both parameters showed significant height by density interactions when only the upper and intermediate leaves were considered (P <0.05; ANCOVA).
Partial shading of tobacco and Arabidopsis plants
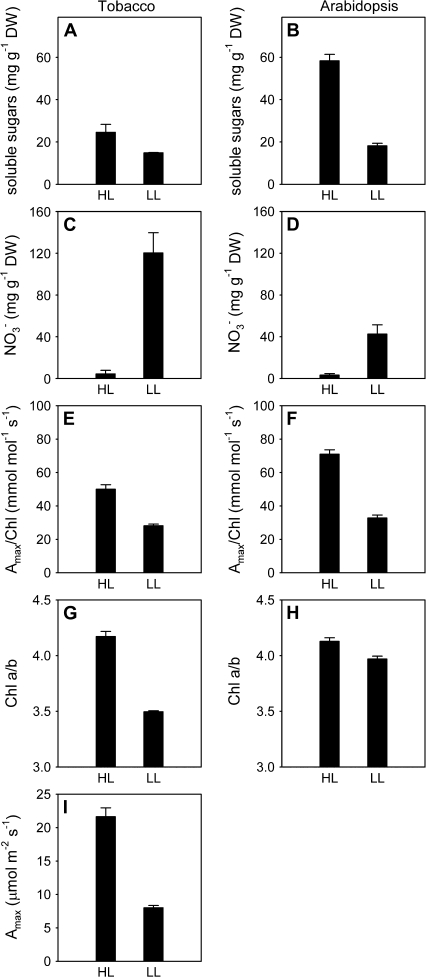
In order to obtain a model system that could easily be used in the lab, partial shade was applied to mimic the effects of canopy light gradients. When a single leaf was shaded (93% spectrally neutral shade) on tobacco or Arabidopsis plants remaining in the light, a decline in soluble sugar concentration (Fig. 2A, B) and accumulation of nitrate (Fig. 2C, D) were again observed. This treatment also induced acclimation within chloroplasts, as indicated by the reduction in Amax/Chl (Fig. 2E, F) and Chl a/b (Fig. 2G, H). Furthermore, in both species, Amax was reduced in the shaded leaves (Fig. 2I, J), as was described previously for a similar experiment using Arabidopsis (Boonman et al., 2007). Thus, photosynthetic acclimation at the whole-plant and chloroplast level as observed in tobacco canopies was mimicked by the partial shade treatment in both tobacco and Arabidopsis.
Fig. 2.
Effects of shading a single leaf of a plant on putative signals and photosynthetic acclimation in tobacco (A, C, E, G, H, I) and Arabidopsis (B, D, F, H, J). Shown are soluble sugars (A, B), nitrate (C, D), photosynthetic capacity per unit chlorophyll (Amax/Chl) (E, F), chlorophyll a/b ratio (Chl a/b) (G, H), and photosynthetic capacity per unit area (Amax) (I). One attached leaf was shaded (PPFD ∼14 μmol m−2 s−1; low light, LL) for 5–6 d and compared with a leaf on a different plant that remained in the light (PPFD ∼200 μmol m−2 s−1; high light, HL). Data are means±SE, n=2–6. *, P <0.05 (Student's t-test).
Arabidopsis mutants and transgenics
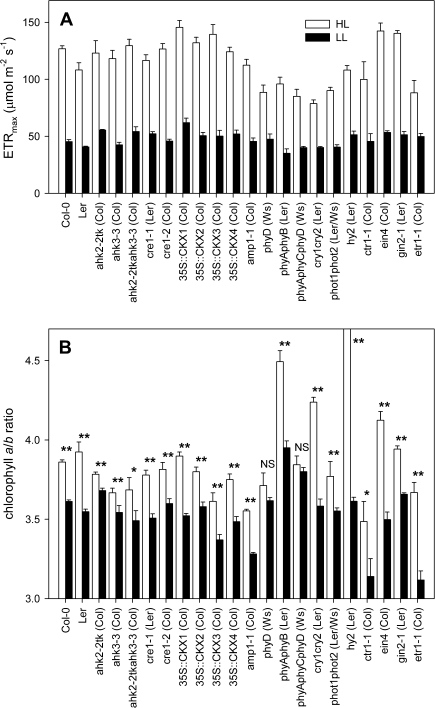
In order to study the role of signalling mediated by cytokinin, photoreceptors, or sugars, the partial shade treatment was applied to a wide range of Arabidopsis mutants and transgenics defective in signalling mediated by these pathways. In all genotypes tested, ETRmax was reduced in shaded leaves the same way as in the wild types (Fig. 3A). These data suggest that there is apparently a large degree of redundancy in the regulatory mechanisms controlling photosynthetic capacity in response to irradiance. Photoreceptor mutants in particular did show variation with respect to ETRmax in control leaves, but ETRmax in low light was remarkably similar between genotypes. ETRmax was reduced by 64% in Col-0 and 62% in Ler (Table 2). Several mutants and transgenics had a significantly lower ETRmax in high light controls than their respective wild types (P <0.05, ANOVA) and these also showed the smallest relative reductions upon partial shading (Table 2): phyD (–46%), phyAphyCphyD (–53%), cry1cry2 (–49%), and etr1-1 (–44%). ETRmax/Chl, a parameter pertaining to chloroplast-level acclimation, was also significantly reduced in all genotypes (Supplementary Fig. S1A available at JXB online), as well as Chl a/b in most cases (Fig. 3B). ETRmax/Chl was reduced to a rather similar level in all genotypes, and the reduction of ETRmax/Chl correlated well with the reduction of ETRmax. Chl a/b was reduced by 6.4% in Col-0 and 9.6% in Ler (Table 2). Relatively large decreases in Chl a/b were observed in cry1cry2 and etr1-1, as well as ein4 (all ∼15%). Chl a/b of hy2 in high light was exceptionally high (6.22±0.198), as was found previously for the similar hy1-1 mutant (Walters et al., 1999), but normally reduced in response to local shade. However, Chl a/b did not change significantly in response to partial shade in the triple photoreceptor mutant phyAphyCphyD and the phyD mutant (Fig. 3B). Also, only small reductions were observed in the cytokinin receptor mutants ahk2-2tk (–2.7%), ahk3-3 (–3.4%), and ahk2-2tkahk3-3 (–5.3%) (Table 2). These findings identify a new role for phytochromes and cytokinins in the response of Chl a/b to light availability.
Fig. 3.
Effects of shading a single leaf of a plant on photosynthetic acclimation in Arabidopsis mutants and transgenics disrupted in signalling mediated by cytokinin, photoreceptors, or sugars. Shown are the light-saturated rate of electron transport per unit area (ETRmax) (A) and chlorophyll a/b ratio (Chl a/b) (B). One attached leaf was shaded (PPFD ∼14 μmol m−2 s−1; low light, LL) for 6 d and compared with a leaf on a different plant that remained in the light (PPFD ∼200 μmol m−2 s−1; high light, HL). The Chl a/b of hy2 in HL was 6.22±0.198. Shading induced significant (P <0.05; Student's t-test) decreases in ETRmax in all genotypes tested. Data are means±SE, n=6. In B, NS, not significant; *, P < 0.1; **, P <0.05 (Student's t-test).
Table 2.
Decease in ETRmax and CHL a/b in shaded leaves (low light; LL) relative to leaves remaining in high light (HL). Single attached leaves were shaded of Arabidopsis mutants and transgenics, and their respective wild types
| Genotype | Decrease in LL relative to HL (%) | |
| ETRmax | Chl a/b | |
| Col-0 | 64.3 | 6.4 |
| Ler | 62.4 | 9.6 |
| ahk2-2tk (Col) | 54.9 | 2.7 |
| ahk3-3 (Col) | 64.0 | 3.4 |
| cre1-1 (Ler) | 55.2 | 7.2 |
| cre1-2 (Col) | 63.8 | 5.6 |
| ahk2-2tkahk3-3 (Col) | 58.2 | 5.3 |
| 35S::CKX1 (Col) | 57.4 | 9.7 |
| 35S::CKX2 (Col) | 61.7 | 5.8 |
| 35S::CKX3 (Col) | 64.1 | 6.7 |
| 35S::CKX4 (Col) | 58.1 | 7.1 |
| amp1-1 (Col) | 59.5 | 7.7 |
| Ws/phyD | 46.4 | 2.6 |
| phyAphyB (Ler) | 63.5 | 12.1 |
| phyAphyCphyD (Ler) | 52.8 | 1.1 |
| cry1/cry2 (Ler) | 48.9 | 15.5 |
| phot1phot2 (Ler/Ws) | 55.0 | 5.8 |
| hy2 (Ler) | 52.4 | 41.9 |
| ctr1-1 (Col) | 54.5 | 9.9 |
| ein4 (Col) | 62.5 | 15.2 |
| gin2-1 (Ler) | 63.4 | 7.2 |
| etr1-1 (Col) | 43.5 | 15.0 |
For further description see Fig. 3
Chloroplast-level acclimation controlled by transpiration rate in Arabidopsis
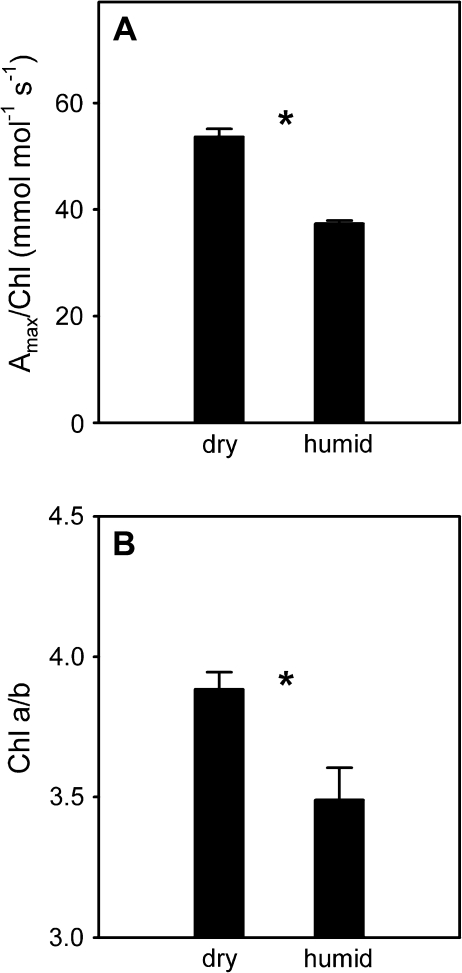
Because of the finding that cytokinin receptor mutants showed less responsiveness to partial shade in terms of Chl a/b, chloroplast-level acclimation in Arabidopsis in response to manipulation of the transpiration rate independently of light was analyzed. This humid air treatment was previously shown to reduce cytokinin content and activity (Boonman et al., 2007). Indeed, Amax/Chl (Fig. 4A) and Chl a/b (Fig. 4B), parameters associated with chloroplast-level acclimation, were reduced in response to humid air.
Fig. 4.
Effects of experimental reduction of the transpiration rate on chloroplast-level acclimation in Arabidopsis. One attached leaf was placed in a cuvette flushed with humid air or with growth chamber air (dry) as a control for 6 d. Shown are photosynthetic capacity per unit chlorophyll (Amax/Chl) (A) and chlorophyll a/b ratio (Chl a/b) (B). Data are means±SE, n=6. *P <0.05; (Student's t-test).
Discussion
No evidence for the regulation of canopy density acclimation by sugars or nitrate
The proximity of neighbouring vegetation is primarily associated with reductions in irradiance and R:FR in the lower canopy layers (Fig. 1A, B). In this study, an investigation was carried out to determine to what extent light signals and various alternative signals are required for the photosynthetic acclimation shown by plants in response to the proximity of neighbours. The sugar and nitrate data on tobacco and Arabidopsis argue against a role for these compounds. High sugar concentrations repress the expression of photosynthetic genes while, conversely, low sugar concentrations induce their expression (Sheen, 1990; Koch, 1996; Yu, 1999; Smeekens, 2000). In the tobacco canopies as well as in the partially shaded plants, the highest sugar concentrations were found in the control leaves in high light (Figs 1, 2) that had the highest photosynthetic capacity (Boonman et al., 2007), and levels were reduced in the shade, opposite to what would be expected. Sugar levels were also found to be decreased in shaded sunflower leaves (Ono et al., 2001). Hence, sugar accumulation probably reflects photosynthetic activity in a leaf canopy. There is a possibility though that individual sugars show different responses to partial shade than shown by the bulk soluble sugar level, and this may have an impact on sugar signalling.
Nitrate stimulates its own assimilation (Crawford, 1995; Stitt, 1999) and may thus contribute to the synthesis of photosynthetic enzymes and other proteins, as well as provide a nitrogen source. However, the upper leaves in the tobacco canopy and the control leaves in the partial shading experiments that had the highest photosynthetic capacity (Boonman et al., 2007) showed the lowest nitrate concentrations, while nitrate accumulated to high levels in shaded leaves (Figs 1, 2). These findings are also opposite to what would be expected if nitrate signalling played a role in regulating photosynthetic capacity. The lower photosynthetic capacity of shaded leaves does not appear to be the result of limited nitrogen supply, since the highest nitrate concentrations were found there. It is possible that nitrate delivery rates, rather than foliar concentrations, function in the regulation of photosynthetic capacity distribution in leaf canopies. It is known that nitrate in shaded leaves is mostly localized in the vacuole and not metabolically active (Aslam et al., 1976; Granstedt and Huffaker, 1982), but further studies are required to unravel any role for nitrate.
The mutants ctr1-1, ein4, etr1-1, and gin2-1 with altered sugar responsiveness retained the capacity for photosynthetic acclimation to partial shade in hte same way as the wild types (Fig. 3). Even though a role cannot be ruled out, these data also do not provide evidence in support of an important role for sugar signalling. There were quantitative differences from the wild types though: both ein4 and etr1-1, which are insensitive to the plant hormone ethylene and are sugar hypersensitive, showed a stronger reduction of Chl a/b upon shading than the Col-0 background, which may be related to either of these signals. Furthermore, etr1-1 had a lower ETRmax and Chl a/b in high light than the wild type. These findings are in agreement with previous reports showing that Arabidopsis etr1-1 had a lower rate of photosynthesis per unit leaf area (Tholen et al., 2004), while ethylene-insensitive tobacco was also shown to have a lower investment of foliar nitrogen in electron transport and Rubisco, and a lower Chl a/b ratio as the result of sugar hypersensitivity (Tholen et al., 2007).
Regulation by photoreceptors of allocation of photosynthetic capacity
Previous studies have demonstrated that several PHYTOCHROME and CRYPTOCHROME mutants showed wild-type acclimation to shading or darkening (Walters et al., 1999; Weaver and Amasino, 2001). Here, it has been shown that also in double and triple photoreceptor mutants including PHOTOTROPIN mutants, a normal wild-type reduction of photosynthetic capacity occurred when part of the foliage was shaded (Fig. 3A). Although the change in capacity in response to shade still occurred in all photoreceptor mutants, there was a reduction in capacity in light-exposed leaves relative to the wild types (Fig. 3A), and chlorophyll contents were lower (Supplementary Fig. S1B at JXB online). This probably reflects the essential role of the photoreceptors in green leaf development (Sullivan and Deng, 2003). Studies in which R:FR was manipulated have shown that phytochrome photoreceptors are involved in the induction of leaf senescence in dense canopies (Rousseaux et al., 1996; Rousseaux et al., 2000; Pons and de Jong-van Berkel, 2004). Whether Cry or Phot photoreceptors are involved is not known at present, although low blue light perceived by these photoreceptors can be used by plants as a neighbour proximity signal in morphological shade avoidance (Ballaré et al., 1991; Pierik et al., 2004). A possible explanation for these findings is that photoreceptors operate in a functionally redundant manner, such that double or triple photoreceptor mutants are still able to regulate photosynthetic capacity in response to partial shade. Moreover, these results emphasize that there are alternative signalling pathways that may act independently of photoreceptors.
Regulation by cytokinins of allocation of photosynthetic capacity
A forceful indication that plants perceive irradiance also independently of photoreceptors was provided by experiments in which the transpiration rate was reduced without a reduction in irradiance (Pons and Bergkotte, 1996). In this treatment, the leaf was surrounded by humid air in a transparent cuvette, which proved to be sufficient to reduce photosynthetic capacity. Shading is accompanied by reduced stomatal conductance and leaf transpiration rates in a canopy (Boonman et al., 2007). Root-borne cytokinins carried in the transpiration stream are therefore delivered more to light-exposed leaves than to shaded leaves, and regulate photosynthetic capacity accordingly (Pons et al., 2001; Boonman et al., 2007). Also at the whole-plant level, the transpiration rate controls the cytokinin transport rate from roots to shoots (Aloni et al., 2005). In experiments where part of the foliage of Arabidopsis plants was shaded, it was shown that a reduced transpiration rate decreased the concentration and activity of cytokinins, while applied cytokinins partially rescued the shade-induced decline in photosynthetic capacity (Boonman et al., 2007). The mutant data presented here (Fig. 3A) suggest that, similar to photoreceptor-mediated signalling, cytokinin signalling is not absolutely required for the regulation of photosynthetic capacity. The evidence outlined above does, however, support a role for cytokinins in canopy density acclimations, but as one of multiple, redundantly operating mechanisms.
Cytokinin distribution in tobacco canopies
The distribution of cytokinins in the tobacco canopies is the result of import through the xylem (see above), as well as a multitude of other factors, including local synthesis (Singh et al., 1992; Nordström et al., 2004) and possibly breakdown (Werner et al., 2001) and transport through the phloem (Hoad, 1995). Cytokinin concentrations in both stands were highest in the upper leaves (Table 1), which can be explained by their higher transpiration rates. Furthermore, it has been shown that Z-type cytokinins are synthesized particularly in young, upper leaves in tobacco (Singh et al., 1992; Nordström et al., 2004), which contributes to the high ZRP concentrations there. Possibly there is also synthesis of iP-type cytokinins in upper leaves, but this has not been explored. The surprisingly large quantity of cis-ZRP in upper leaves in the open stand may be the result of transport from dormant buds through the phloem, as well as transport from the roots through the xylem, which has been observed in other species (Vonk and Davelaar, 1981; Mader et al., 2003). Notably, transgenic plants with reduced cis-ZRP were chlorotic (Miyawaki et al., 2006). In accordance, it was found that upper leaves in the open stand had a higher cis-ZRP concentration (Table 1), as well as higher chlorophyll contents per unit area, than upper leaves in the dense stand (673.1 μmol Chl m−2 and 399.4 μmol Chl m−2, respectively).
Canopy density also had a significant effect on one of the active cytokinins, iPR, that was qualitatively correlated to the light gradient, i.e. iPR concentrations were reduced more strongly from upper canopy leaves downwards at high stand density compared with low density. The significant height by density effect was found when considering only the upper and intermediate leaves, consistent with the previous finding that Amax and transcript levels of the small subunit of Rubisco also changed most prominently in mid-canopy leaves (Boonman et al., 2007). The oldest, lower leaves were already starting to senesce and therefore showed less effect of canopy density in this experiment. Interestingly, iPR was one of three cytokinins that could be detected in the xylem sap collected from intact, transpiring plants, with the others being iP and iPRP (Boonman et al., 2007). The canopy density effect on iPR can therefore be explained by the gradient in transpiration rate, and hence cytokinin delivery, that is ultimately controlled by the light gradient. Accordingly, a previous study on bean plants grown at contrasting densities demonstrated that in lower canopy layers, cytokinin concentrations were more reduced in the dense stand compared with the open stand (Pons et al., 2001). Combined with the known stimulation of photosynthetic capacity by cytokinins (Chory et al., 1994), these data support the model that cytokinin import controlled by transpiration rates provides leaves with a canopy density signal that regulates photosynthetic acclimation to the light gradient (Boonman and Pons, 2007).
Regulation of chloroplast-level acclimation by phytochromes and cytokinins
Chl a/b showed no change in response to partial shading in the phyAphyCphyD mutant and in the phyD mutant, and reduced effects compared with the wild type were observed in the cytokinin receptor mutants ahk2-2tkahk3-3, ahk2-2tk, and ahk3-3 (Fig 3B). However, another parameter pertaining to chloroplast-level acclimation, ETRmax/Chl, was reduced in these mutants in the same way as in the wild types (Supplementary Fig. S1A at JXB online). The lower Chl a/b normally observed in shaded leaves reflects the greater abundance of light-harvesting complex II (LHCII) that contains Chl b, relative to core Chl of photosystem II (PSII) and the chlorophyll associated with PSI which is only Chl a (Evans and Seemann, 1989; Anderson et al., 1995). The results suggest that cytokinins, phyD, and possibly phyA and phyC, are involved in the regulation of the abundance of LHCII. It should be noted that the Ws-2 accession was used, which has the phyD mutation, but also many other polymorphisms compared with Col-0 and Ler, so the possibility that other genes also affect photosynthetic acclimation of this accession cannot be excluded.
The possibility that cytokinins are involved is exciting since experimental reduction of transpiration rates was sufficient to induce chloroplast-level acclimation in Arabidopsis (Fig. 4) and a range of other species (Pons and Bergkotte, 1996; Pons and Jordi, 1998; Pons et al., 2001). Furthermore, ahk3-3, ahk2-2tkahk3-3, and 35S::CKX3 had a reduced Chl a/b in high light compared with the wild type (Fig. 3B), which suggests that cytokinin has an impact on this aspect of chloroplast organization during development. It shows that Chl a/b is not only under control of the local light environment, but is also affected systemically, as is the case with the allocation of photosynthetic capacity in canopies. The effect of the local transpiration rate on Chl a/b further suggests that it is mediated by the action of cytokinins carried in the transpiration stream according to the model described above.
Conclusions
The regulation of photosynthetic acclimation to canopy density at the whole-plant level involves photoreceptors and xylem-transported cytokinins operating in a redundant manner, because neither mechanism can fully explain the distribution of photosynthetic capacity in response to partial shade. No evidence was found for a role for sugars or nitrate, although their involvement could not be excluded completely. A possible role for phyD and cytokinin in regulating Chl a/b as part of the acclimation at the chloroplast level has been identified. The distribution of cytokinins in tobacco leaf canopies could be explained in part by the effect of irradiance on delivery of cytokinins via the transpiration stream. This mechanism is involved in acclimation at both the whole-plant and chloroplast levels.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. ETRmax per unit chlorophyll (A) and chlorophyll level per unit area (B) of Arabidopsis mutants and transgenics.
Supplementary Material
Acknowledgments
We gratefully acknowledge Yvonne de Jong-van Berkel and Sevgi Öden for skilled technical assistance. We thank two anonymous reviewers for their helpful suggestions.
References
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature. 1998;392:720–723. doi: 10.1038/33701. [DOI] [PubMed] [Google Scholar]
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany. 2005;56:1535–1544. doi: 10.1093/jxb/eri148. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Chow WS, Park Y-I. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynthesis Research. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Hikosaka K, Hirose T. Nitrogen utilisation and the photosynthetic system. In: Marshall B, Roberts JA, editors. Leaf development and canopy growth. Sheffield: Sheffield Academic Press; 2000. pp. 171–203. [Google Scholar]
- Aslam M, Oaks A, Huffaker RC. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiology. 1976;58:588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. The Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant, Cell and Environment. 1991;14:57–65. [Google Scholar]
- Bieleski RL. The problem of halting enzyme action when extracting plant tissues. Analytical Biochemistry. 1964;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bligh EJ, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boonman A, Anten NPR, Dueck TA, Jordi WJRM, van der Werf A, Voesenek LACJ, Pons TL. Functional significance of shade-induced leaf senescence in dense canopies: an experimental test using transgenic tobacco. American Naturalist. 2006;168:597–607. doi: 10.1086/508633. [DOI] [PubMed] [Google Scholar]
- Boonman A, Pons TL. Canopy light gradient perception by cytokinin. Plant Signaling and Behaviour. 2007;2:489–491. doi: 10.4161/psb.2.6.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJM, Voesenek LACJ, Pons TL. Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiology. 2007;143:1841–1852. doi: 10.1104/pp.106.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis. 1975;6:71–80. [Google Scholar]
- Chaudhury AM, Letham DS, Craig S, Dennis ES. amp1—a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. The Plant Journal. 1993;4:907–916. [Google Scholar]
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annual Review of Genetics. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins) Plant Physiology. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Prinsen E, Jacqmard A, Lejeune P, Van Onckelen H, Périlleux C, Bernier G. Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. Journal of Experimental Botany. 2003;54:2511–2517. doi: 10.1093/jxb/erg276. [DOI] [PubMed] [Google Scholar]
- Crawford NM. Nitrate: nutrient and signal. The Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I Canopy characteristics. Australian Journal of Plant Physiology. 1993;20:55–67. [Google Scholar]
- Evans JR, Seemann JR. The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Briggs WR, editor. Photosynthesis. New York: Liss; 1989. pp. 183–205. [Google Scholar]
- Franklin KA, Davis SJ, Stoddart WM, Vierstra RD, Whitelam GC. Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. The Plant Cell. 2003a;15:1981–1989. doi: 10.1105/tpc.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiology. 2003b;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Granstedt RC, Huffaker RC. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiology. 1982;70:410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindlay DJC. Towards an explanation of crop nitrogen demand based on the optimization of leaf nitrogen per unit area. Journal of Agricultural Science. 1997;128:377–396. [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T. Development of the Monsi–Saeki theory on canopy structure and function. Annals of Botany. 2005;95:483–494. doi: 10.1093/aob/mci047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoad GV. Transport of hormones in the phloem of higher plants. Plant Growth Regulation. 1995;16:173–182. [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiology. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. The Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K-I. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana L. Heynh. Zeitschrift für Pflanzenphysiologie. 1980;100:147–160. [Google Scholar]
- Kull O. Acclimation of photosynthesis in canopies: models and limitations. Oecologia. 2002;133:267–279. doi: 10.1007/s00442-002-1042-1. [DOI] [PubMed] [Google Scholar]
- León P, Sheen J. Sugar and hormone connections. Trends in Plant Science. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Mader JC, Emery RJN, Turnbull CGN. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiologia Plantarum. 2003;119:295–308. [Google Scholar]
- Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots to nitrogen management and leaf senescence. Planta. 2000;211:510–518. doi: 10.1007/s004250000310. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsi M, Saeki T. Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany. 1953;14:22–52. [Google Scholar]
- Niinemets Ü. Photosynthesis and resource distribution through plant canopies. Plant, Cell and Environment. 2007;30:1052–1071. doi: 10.1111/j.1365-3040.2007.01683.x. [DOI] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proceedings of the National Academy of Sciences, USA. 2004;101:8039–8044. doi: 10.1073/pnas.0402504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Nishi Y, Watanabe A, Terashima I. Possible mechanisms of adaptive leaf senescence. Plant Biology. 2001;3:234–243. [Google Scholar]
- Parks BM, Quail PH. Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. The Plant Cell. 1991;3:1177–1186. doi: 10.1105/tpc.3.11.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF. Photosynthetic control of chloroplast gene expression. Nature. 1999;397:625–628. [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, Visser EJW. Canopy studies on ethylene-insensitive tobacco indentify ethylene as a novel element in blue light and plant–plant signalling. The Plant Journal. 2004;38:310–319. doi: 10.1111/j.1365-313X.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- Pons TL, Anten NPR. Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Functional Ecology. 2004;18:802–811. [Google Scholar]
- Pons TL, Bergkotte M. Nitrogen reallocation in response to partial shading of a plant: possible mechanisms. Physiologia Plantarum. 1996;98:571–577. [Google Scholar]
- Pons TL, de Jong-van Berkel Y. Species-specific variation in the importance of the spectral quality gradient in canopies as a signal for photosynthetic resource partitioning. Annals of Botany. 2004;94:725–732. doi: 10.1093/aob/mch197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Jordi W. Induction of leaf senescence and shade acclimation in leaf canopies—variation with leaf longevity. In: Lambers H, Poorter H, Van Vuuren MMI, editors. Inherent variation in plant growth. Physiological mechanisms and ecological consequences. Leiden: Backhuys Publishers; 1998. pp. 121–137. [Google Scholar]
- Pons TL, Jordi W, Kuiper D. Acclimation of plants to light gradients in leaf canopies; evidence for a possible role for cytokinins transported in the transpiration stream. Journal of Experimental Botany. 2001;52:1563–1574. doi: 10.1093/jexbot/52.360.1563. [DOI] [PubMed] [Google Scholar]
- Pons TL, Pearcy RW. Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiologia Plantarum. 1994;92:636–644. [Google Scholar]
- Pons TL, Welschen RAM. Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. Plant, Cell and Environment. 2002;25:1367–1372. [Google Scholar]
- Prinsen E, Van Dongen W, Esmans EL, van Onckelen HA. Micro and capillary liquid chromatography–tandem mass spectrometry: a new dimension in phytohormone research. Journal of Chromatography A. 1998;826:25–37. [Google Scholar]
- Redig P, Schmülling T, van Onckelen HA. Analysis of cytokinin metabolism in ipt transgenic tobacco by liquid chromatography–tandem mass spectrometry. Plant Physiology. 1996;112:141–148. doi: 10.1104/pp.112.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiology. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchez RA. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiologia Plantarum. 1996;96:217–224. [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchez RA. Basal leaf senescence in a sunflower (Helianthus annuus) canopy: responses to increased R/FR ratio. Physiologia Plantarum. 2000;110:477–482. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. The Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Letham DS, Palni LMS. Cytokinin biochemistry in relation to leaf senescence. VIII. Translocation, metabolism and biosynthesis of cytokinins in relation to sequential leaf senescence in tobacco. Physiologia Plantarum. 1992;86:398–406. [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Stitt M. Nitrate regulation of metabolism and growth. Current Opinion in Plant Biology. 1999;2:178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Deng XW. From seed to seed: the role of photoreceptors in Arabidopsis development. Developmental Biology. 2003;260:289–297. doi: 10.1016/s0012-1606(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Terashima I, Araya T, Miyaza SI, Sone K, Yano S. Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Annals of Botany. 2005;95:507–519. doi: 10.1093/aob/mci049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi WB, Cashmore AR. Light-regulated transcription. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:445–474. [Google Scholar]
- Tholen D, Pons TL, Voesenek LACJ, Poorter H. Ethylene insensitivity results in down-regulation of Rubisco expression and photosynthetic capacity in tobacco. Plant Physiology. 2007;144:1305–1315. doi: 10.1104/pp.107.099762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Voesenek LACJ, Poorter H. Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia×hybrida. Plant Physiology. 2004;134:1803–1812. doi: 10.1104/pp.103.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vonk CR, Davelaar E. 8-14C-Zeatin metabolites and their transport from leaf to phloem exudate of Yucca. Physiologia Plantarum. 1981;52:101–107. [Google Scholar]
- Walters RG, Rogers JJM, Shephard F, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the role of photoreceptors. Planta. 1999;209:517–527. doi: 10.1007/s004250050756. [DOI] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiology. 2001;127:876–886. [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston E, Thorogood K, Vinti G, López-Juez E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light perception mutants. Planta. 2000;211:807–815. doi: 10.1007/s004250000392. [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM. Cellular and genetic responses of plants to sugar starvation. Plant Physiology. 1999;121:687–693. doi: 10.1104/pp.121.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.