Abstract
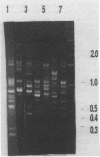
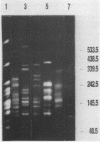
A polymerase chain reaction (PCR) technique was applied to the fingerprinting of different strains of Acinetobacter baumannii from a cluster of patients infected or colonized with the incriminated pathogen. The DNA was extracted by boiling and was subjected to PCR amplification by using the core sequence of the M13 phase as a single primer. The amplified products were separated by agarose gel electrophoresis and were detected by staining with ethidium bromide. In 1990, 49 multiresistant A. baumannii strains were isolated from 13 patients from the same intensive care unit of the Charité Hospital; 45 of these outbreak isolates obtained from 12 patients showed the same PCR patterns, indicating an epidemiological relatedness of these strains. Four strains isolated from the same patient belonged to another genetic group, as revealed by a distinct amplification pattern. Another single subtype of A. baumannii was identified as the causative agent in patients during a second outbreak at a different intensive care unit in the same hospital. Seventeen isolates recovered from 10 immunocompromised patients had the same amplification patterns, which were distinct from all other PCR profiles. Five strains were obtained from two other hospitals; three isolates from the hospital of Magdeburg, Germany, had identical PCR patterns which, however, could be clearly distinguished from the patterns of all other strains. The remaining two isolates displayed individual patterns of amplified fragments. PCR fingerprinting may provide a useful and particularly rapid identification technique for epidemiological investigations of nosocomial infections.
Full text
PDF
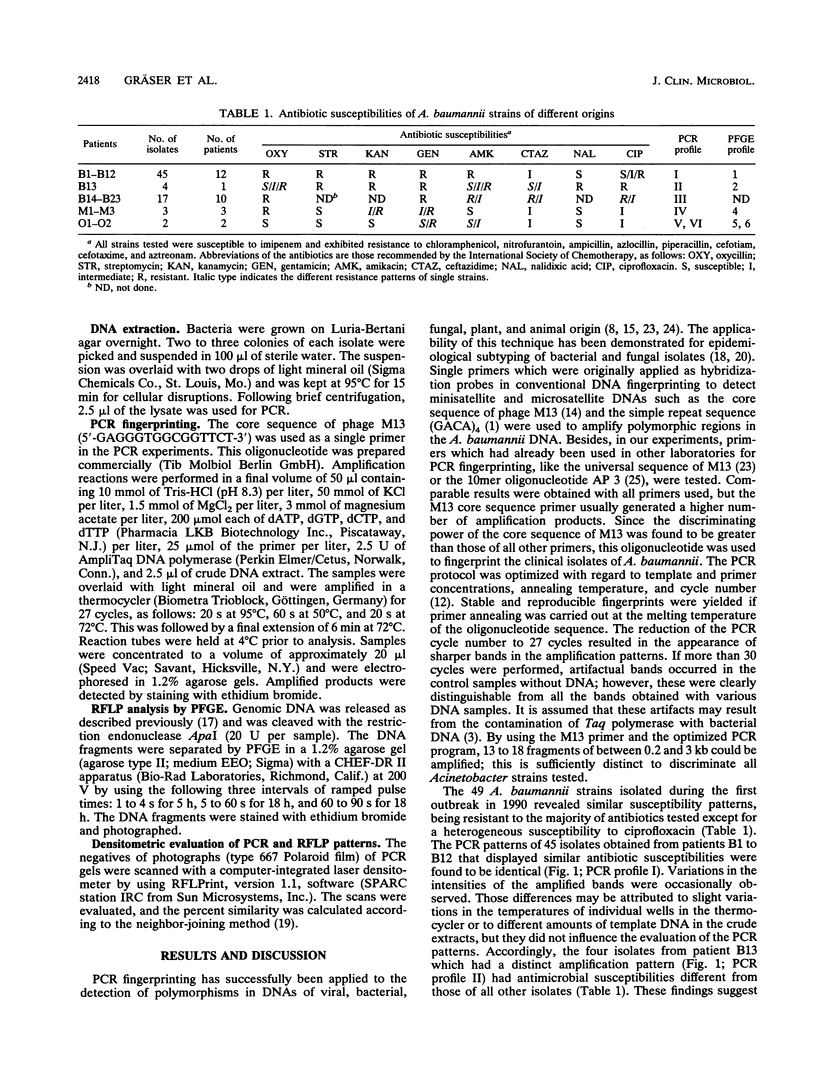
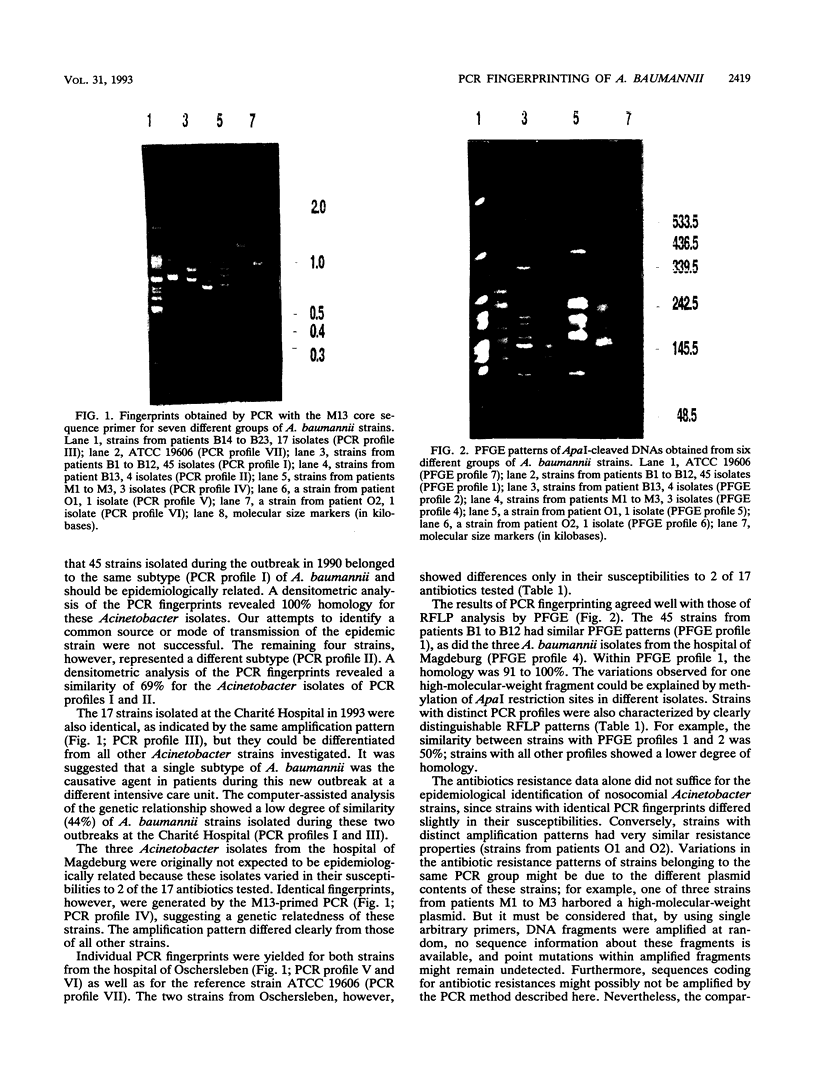

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Müller C. R., Epplen J. T. DNA finger printing by oligonucleotide probes specific for simple repeats. Hum Genet. 1986 Nov;74(3):239–243. doi: 10.1007/BF00282541. [DOI] [PubMed] [Google Scholar]
- Beck-Sagué C. M., Jarvis W. R., Brook J. H., Culver D. H., Potts A., Gay E., Shotts B. W., Hill B., Anderson R. L., Weinstein M. P. Epidemic bacteremia due to Acinetobacter baumannii in five intensive care units. Am J Epidemiol. 1990 Oct;132(4):723–733. doi: 10.1093/oxfordjournals.aje.a115714. [DOI] [PubMed] [Google Scholar]
- Bouvet P. J., Grimont P. A. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- Bouvet P. J., Jeanjean S., Vieu J. F., Dijkshoorn L. Species, biotype, and bacteriophage type determinations compared with cell envelope protein profiles for typing Acinetobacter strains. J Clin Microbiol. 1990 Feb;28(2):170–176. doi: 10.1128/jcm.28.2.170-176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson Y., Tran Van Nhieu G., Ginot L., Bouvet P., Schill H., Driot L., Meyran M. Nosocomial outbreaks due to amikacin-resistant tobramycin-sensitive Acinetobacter species: correlation with amikacin usage. J Hosp Infect. 1990 Jan;15(1):83–93. doi: 10.1016/0195-6701(90)90024-i. [DOI] [PubMed] [Google Scholar]
- Böttger E. C. Frequent contamination of Taq polymerase with DNA. Clin Chem. 1990 Jun;36(6):1258–1259. [PubMed] [Google Scholar]
- Cefai C., Richards J., Gould F. K., McPeake P. An outbreak of Acinetobacter respiratory tract infection resulting from incomplete disinfection of ventilatory equipment. J Hosp Infect. 1990 Feb;15(2):177–182. doi: 10.1016/0195-6701(90)90128-b. [DOI] [PubMed] [Google Scholar]
- Crowhurst R. N., Hawthorne B. T., Rikkerink E. H., Templeton M. D. Differentiation of Fusarium solani f. sp. cucurbitae races 1 and 2 by random amplification of polymorphic DNA. Curr Genet. 1991 Nov;20(5):391–396. doi: 10.1007/BF00317067. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992 Oct;30(10):2680–2685. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouby A., Carles-Nurit M. J., Bouziges N., Bourg G., Mesnard R., Bouvet P. J. Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J Clin Microbiol. 1992 Jun;30(6):1588–1591. doi: 10.1128/jcm.30.6.1588-1591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey B., Hall J. Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol. 1989 May;171(5):2528–2532. doi: 10.1128/jb.171.5.2528-2532.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Bassam B. J., Caetano-Anollés G., Gresshoff P. M., Oliver S. P. Subtyping of Streptococcus uberis by DNA amplification fingerprinting. J Clin Microbiol. 1992 May;30(5):1347–1350. doi: 10.1128/jcm.30.5.1347-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Guillou M. L., Bergogne-Berezin E., Vieu J. F. A study of the relationships between antibiotic resistance phenotypes, phage-typing and biotyping of 117 clinical isolates of Acinetobacter spp. J Hosp Infect. 1990 Jul;16(1):49–58. doi: 10.1016/0195-6701(90)90048-s. [DOI] [PubMed] [Google Scholar]
- Klare I., Heier H., Claus H., Witte W. Environmental strains of Enterococcus faecium with inducible high-level resistance to glycopeptides. FEMS Microbiol Lett. 1993 Jan 1;106(1):23–29. doi: 10.1111/j.1574-6968.1993.tb05930.x. [DOI] [PubMed] [Google Scholar]
- Mazurier S. I., Audurier A., Marquet-Van der Mee N., Notermans S., Wernars K. A comparative study of randomly amplified polymorphic DNA analysis and conventional phage typing for epidemiological studies of Listeria monocytogenes isolates. Res Microbiol. 1992 Jun;143(5):507–512. doi: 10.1016/0923-2508(92)90097-8. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Acinetobacter baumannii serotyping for delineation of outbreaks of nosocomial cross-infection. J Clin Microbiol. 1989 Dec;27(12):2713–2716. doi: 10.1128/jcm.27.12.2713-2716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Spohr M. Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. baumannii, A. haemolyticus, genospecies 3, and genospecies 6). Antimicrob Agents Chemother. 1989 Sep;33(9):1617–1619. doi: 10.1128/aac.33.9.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Petersen C., McClelland M. Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res. 1991 Jan 25;19(2):303–306. doi: 10.1093/nar/19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ygout J. F., Housset B., Derenne J. P., Daguet G. L. Hospital-acquired Acinetobacter baumanii pneumonitis. Lancet. 1987 Apr 4;1(8536):802–802. doi: 10.1016/s0140-6736(87)92822-4. [DOI] [PubMed] [Google Scholar]