Abstract
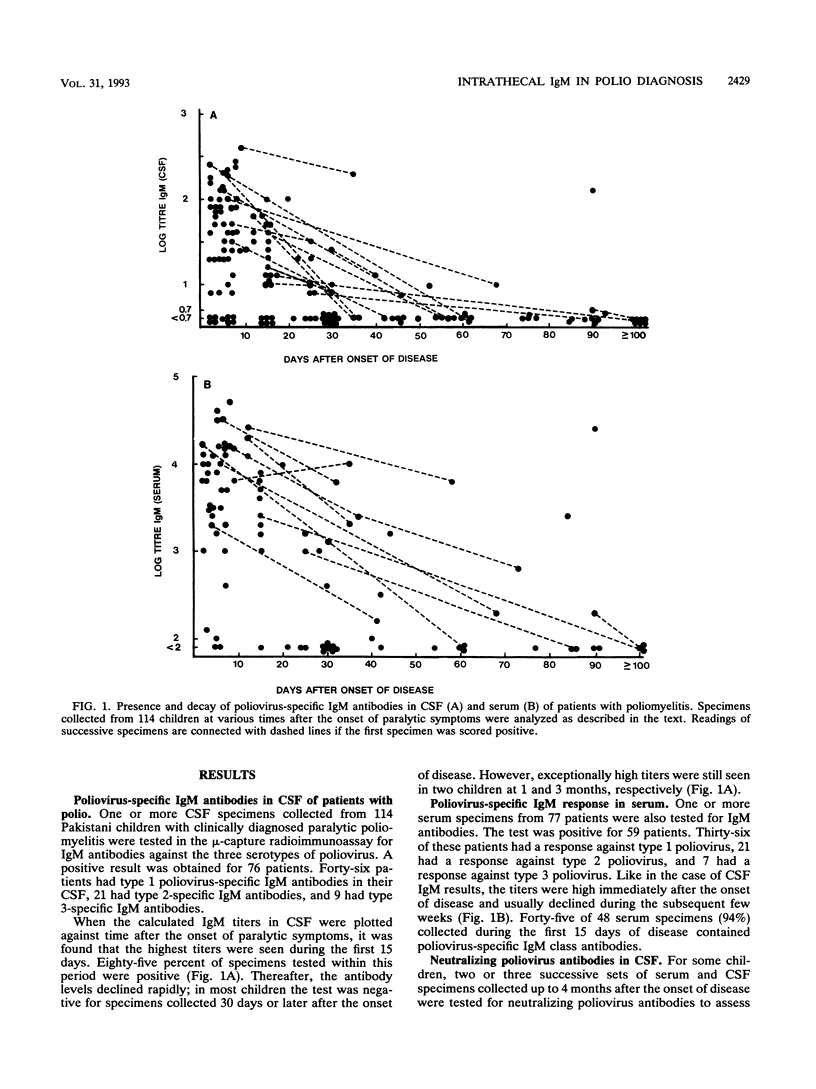
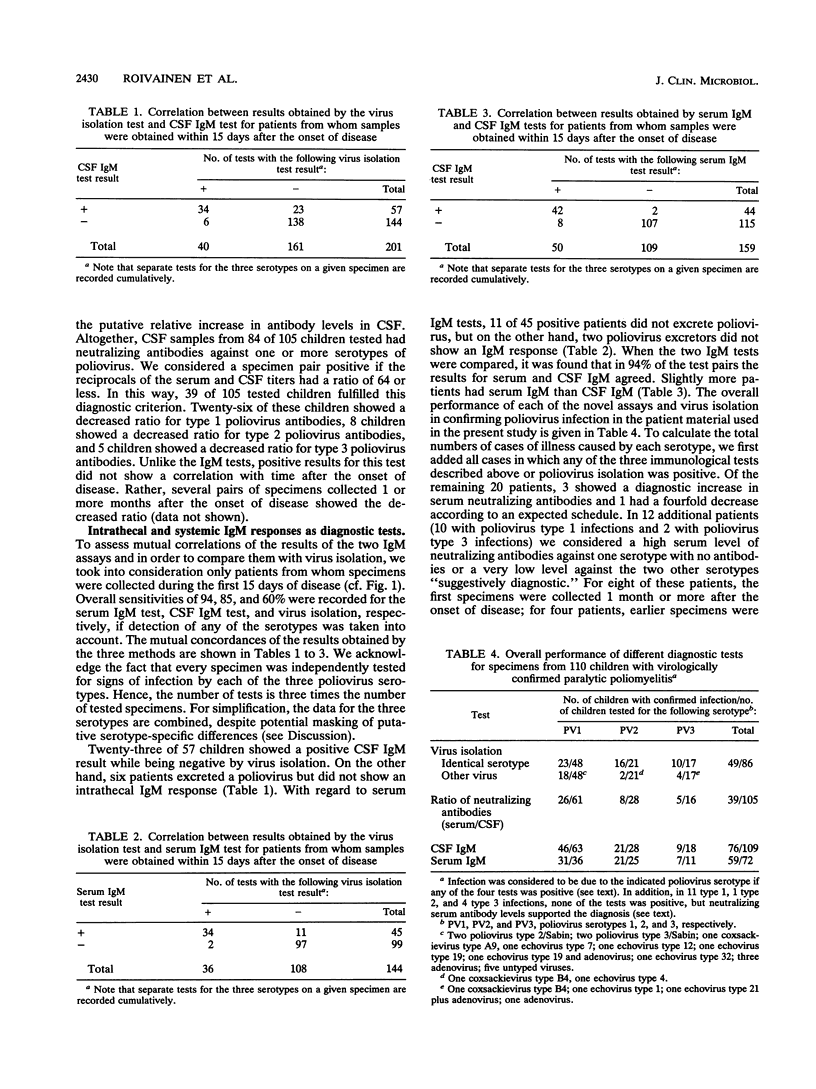
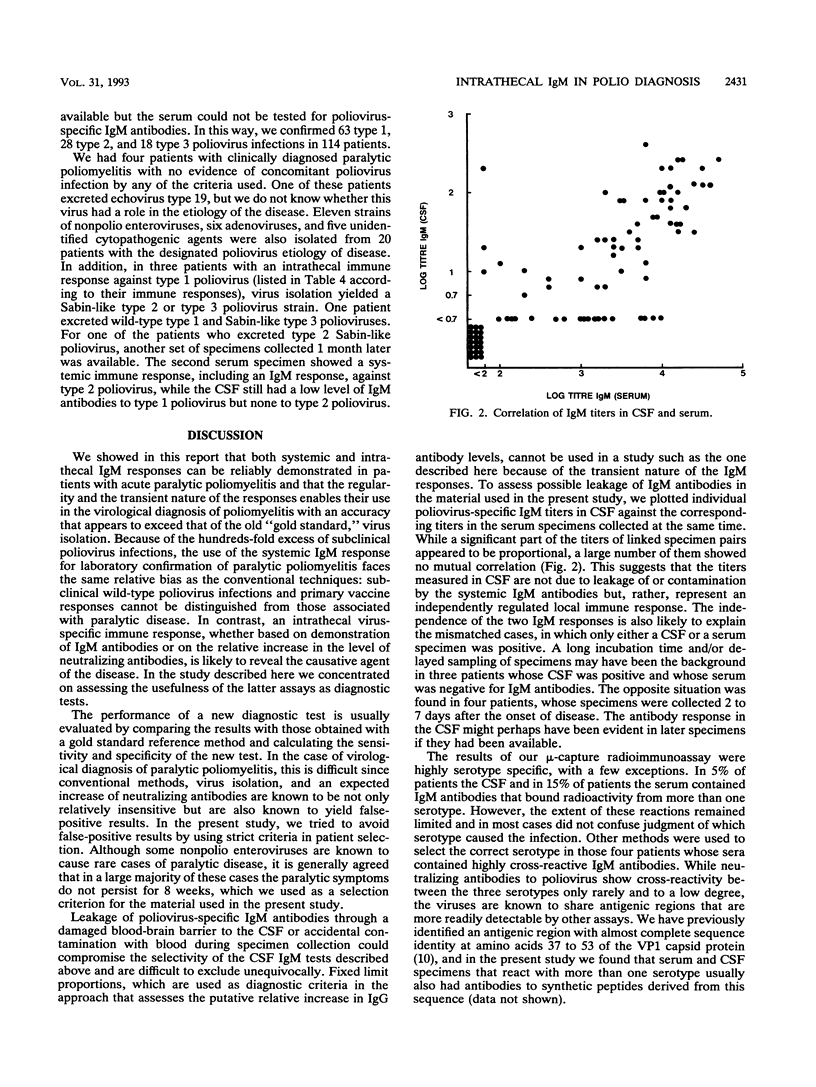
The intrathecal immune response in 114 patients with clinically diagnosed acute poliomyelitis was studied by measuring poliovirus-specific immunoglobulin M (IgM) antibodies in cerebrospinal fluid (CSF) by a mu-capture immunoassay and by assessing the ratio between levels of poliovirus-neutralizing antibodies in serum and CSF. Fecal specimens were used for attempts to isolate the causative agents. Eighty-five percent of CSF specimens collected during the first 15 days of disease contained virus-specific IgM antibodies. Forty-five of 48 tested children (94%) also showed virus-specific IgM responses in their sera. Later on, the antibody levels decreased, and positive results after 30 days of onset of paralytic symptoms were rare. If the presence of poliovirus-specific IgM antibodies in the CSF was considered diagnostic, more cases were confirmed by this test than by virus isolation. A relative increase in poliovirus-neutralizing antibodies in the CSF was observed in about one-third of the cases; in all but three cases the increase was observed together with the presence of virus-specific IgM antibodies. A systemic virus-specific response can be seen and poliovirus can be isolated from a subclinically infected individual suffering from a concomitant poliomyelitis-like disease, while positive results by the two methods demonstrating an intrathecal immune response are likely to indicate a true causal relationship between infection and disease. Demonstration of poliovirus-specific IgM antibodies in the CSF thus appears to be a sensitive and specific method for laboratory confirmation of clinically diagnosed poliomyelitis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P., van Steenis G., van Wezel A. L., Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S540–S544. doi: 10.1093/clinids/6.supplement_2.s540. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Ussery M. A. Antibody capture immunoassay detection of japanese encephalitis virus immunoglobulin m and g antibodies in cerebrospinal fluid. J Clin Microbiol. 1982 Dec;16(6):1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykers T. I., Brown K. L., Gundersen C. B., Beaty B. J. Rapid diagnosis of LaCrosse encephalitis: detection of specific immunoglobulin M in cerebrospinal fluid. J Clin Microbiol. 1985 Nov;22(5):740–744. doi: 10.1128/jcm.22.5.740-744.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Stenvik M., Kinnunen E. Diagnosis of poliomyelitis by demonstration of intrathecal synthesis of neutralizing antibodies. J Infect Dis. 1986 May;153(5):998–999. doi: 10.1093/infdis/153.5.998. [DOI] [PubMed] [Google Scholar]
- Julkunen I., Ukkonen P., Stenvik M., Hovi T., Renkonen L., Mäkelä O. Proportions of immunoglobulin isotypes in paralytic poliomyelitis and after vaccination. J Clin Immunol. 1987 Jul;7(4):319–326. doi: 10.1007/BF00915554. [DOI] [PubMed] [Google Scholar]
- Kahlon J., Chatterjee S., Lakeman F. D., Lee F., Nahmias A. J., Whitley R. J. Detection of antibodies to herpes simplex virus in the cerebrospinal fluid of patients with herpes simplex encephalitis. J Infect Dis. 1987 Jan;155(1):38–44. doi: 10.1093/infdis/155.1.38. [DOI] [PubMed] [Google Scholar]
- Openshaw H., Lieberman J. S. Vaccine-related poliomyelitis: serum IgM and cerebrospinal fluid antibodies. West J Med. 1983 Mar;138(3):420–422. [PMC free article] [PubMed] [Google Scholar]
- Reiber H., Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991 Jul;37(7):1153–1160. [PubMed] [Google Scholar]
- Roivainen M., Hyypiä T., Piirainen L., Kalkkinen N., Stanway G., Hovi T. RGD-dependent entry of coxsackievirus A9 into host cells and its bypass after cleavage of VP1 protein by intestinal proteases. J Virol. 1991 Sep;65(9):4735–4740. doi: 10.1128/jvi.65.9.4735-4740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Närvänen A., Korkolainen M., Huhtala M. L., Hovi T. Antigenic regions of poliovirus type 3/Sabin capsid proteins recognized by human sera in the peptide scanning technique. Virology. 1991 Jan;180(1):99–107. doi: 10.1016/0042-6822(91)90013-2. [DOI] [PubMed] [Google Scholar]
- Roivainen M., Piirainen L., Hovi T. Persistence and class-specificity of neutralizing antibody response induced by trypsin-cleaved type 3 poliovirus in mice. Vaccine. 1993;11(7):713–717. doi: 10.1016/0264-410x(93)90254-u. [DOI] [PubMed] [Google Scholar]
- Sever J. L., Krebs H., Ley A., Barbosa L. H., Rubinstein D. Diagnosis of subacute panencephalitis. The value and availability of measles antibody determinations. JAMA. 1974 Apr 29;228(5):604–606. doi: 10.1001/jama.228.5.604. [DOI] [PubMed] [Google Scholar]
- Ukkonen P., Granström M. L., Räsänen J., Salonen E. M., Penttinen K. Local production of mumps IgG and IgM antibodies in the cerebrospinal fluid of meningitis patients. J Med Virol. 1981;8(4):257–265. doi: 10.1002/jmv.1890080406. [DOI] [PubMed] [Google Scholar]
- van Wezel A. L., Hazendonk A. G. Intratypic serodifferentiation of poliomyelitis virus strains by strain-specific antisera. Intervirology. 1979;11(1):2–8. doi: 10.1159/000149005. [DOI] [PubMed] [Google Scholar]


