Abstract
The expensive brain hypothesis predicts an interspecific link between relative brain size and life-history pace. Indeed, animals with relatively large brains have reduced rates of growth and reproduction. However, they also have increased total lifespan. Here we show that the reduction in production with increasing brain size is not fully compensated by the increase in lifespan. Consequently, the maximum rate of population increase (rmax) is negatively correlated with brain mass. This result is not due to a confounding effect of body size, indicating that the well-known correlation between rmax and body size is driven by brain size, at least among homeothermic vertebrates. Thus, each lineage faces a ‘grey ceiling’, i.e. a maximum viable brain size, beyond which rmax is so low that the risk of local or species extinction is very high. We found that the steep decline in rmax with brain size is absent in taxa with allomaternal offspring provisioning, such as cooperatively breeding mammals and most altricial birds. These taxa thus do not face a lineage-specific grey ceiling, which explains the far greater number of independent origins of large brain size in birds than mammals. We also predict that (absolute and relative) brain size is an important predictor of macroevolutionary extinction patterns.
Keywords: encephalization, life history, speciation, intrinsic rate of increase, cognition
1. Introduction
The ‘expensive brain’ hypothesis proposes that the costs of an increase in brain size must be met by some combination of increasing the total energy turnover or a reduction in energy allocation to another expensive function such as maintenance or production (Isler & Van Schaik submitted). Among these responses, a trade-off with production (growth and reproduction) should be widespread, as its regulation is often responsive to environmental factors and thus easily modified by selection. This hypothesis explains why relatively large-brained mammals and birds often exhibit relatively slow development and maturation times and reduced fertility. However, low production must be compensated by increased reproductive lifespan to retain demographic viability. Although large-brained animals do indeed exhibit increased adult lifespans (Isler & Van Schaik submitted), the question arises as to whether the increased lifespan can continue to compensate for the delayed maturation and increased interbirth intervals as brain size increases further. One must expect that adult mortality cannot be reduced indefinitely in the face of inevitable accidents and environmental calamities in autonomous animals that are not supported by their conspecifics.
To answer the question whether the prolonging of lifespan can sufficiently compensate for reduced production in relatively large-brained animals, we compare brain size with the maximum rate of population increase (rmax), defined in Cole's (1954) equation as a combination of maximum reproductive lifespan and annual offspring production per female. It is known that rmax, which reflects a population's growth rate under optimal conditions, is negatively correlated with body size (Hennemann 1983). Here we ask whether in eutherian mammals this correlation is actually caused by the effect of brain size. An rmax–brain size trade-off would indicate that there is a maximum viable brain size (‘grey ceiling’) for any given lineage.
If this trade-off is indeed due to the energetic costs of maintaining and producing absolutely or relatively large brains, we predict that the rmax–brain size trade-off should weaken or even disappear when mothers receive help from conspecifics. Any allomaternal care, be it aimed at the mother or the offspring, and be it by the father or other conspecifics, allows for increased production and perhaps survival, and hence increased expected lifespan. This strong prediction will be tested for cooperatively breeding mammals and for the other class of homeothermic vertebrates, birds.
Birds are oviparous, and young of precocial birds must feed themselves immediately after hatching, so all energy provided by the parents must be put in the egg. Altricial birds have managed to overcome this limitation by evolving extensive provisioning during the hatchling period, which is shared between mothers, fathers and sometimes other conspecifics (81% of all bird species show biparental and 9% cooperative care, Cockburn 2006). In birds, precociality is the ancestral development mode, and altriciality has independently evolved multiple times (Ricklefs & Starck 1998). Consistent with our hypothesis, it has long been known that altricial birds have larger brains on average than precocials (e.g. Portmann 1947). More precisely, we predict that in altricial birds there is no negative relationship between rmax and brain mass, controlling for body mass effects.
2. Material and methods
We compiled data on fertility rates, age of first reproduction, maximum lifespan, body mass and brain mass of 536 eutherian mammals and 399 avian species from the literature. We obtained rmax by solving Cole's (1954) equation numerically. Details of data compilation and the comparative analyses including phylogenetic independent contrasts (IC) are given in the electronic supplementary material. In mammals, species were defined as precocial if the young open their eyes at birth or shortly thereafter. In birds, development modes were altricial, semi-altricial, semi-precocial and precocial (Iwaniuk & Nelson 2003).
3. Results
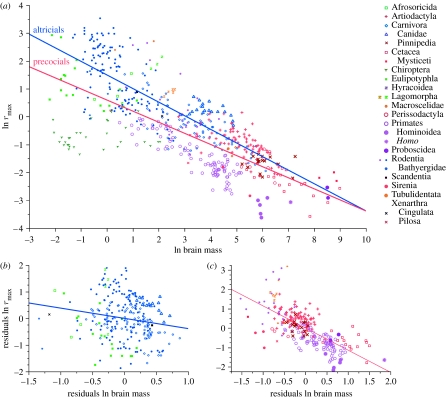
In mammals, rmax is negatively correlated with body mass, as expected. However, rmax shows a much stronger negative correlation with brain size (figure 1; for bivariate regressions, see the electronic supplementary material), also after controlling for the effect of body mass (table 1; multiple regressions). Indeed, once the effect of brain mass is taken into account, the effect of body mass on rmax is positive, rather than negative (table 1; in precocials and most of the orders). This relationship is not a statistical artefact that arises because the size of organs is actually a better estimate of body size than body mass itself (Harvey & Krebs 1990), as it is only observed for brain size, but not for other organs (see the electronic supplementary material). In conclusion, consistent with the expensive brain hypothesis, among mammals rmax is largely driven by brain size, not body size.
Figure 1.
(a) Least-squares regression of ln rmax on ln brain mass in eutherian mammals (n=536, Homo excluded). The regressions of the residuals of ln rmax on the residuals of ln brain mass are shown for (b) altricials and (c) precocials separately. Full statistical details are given in the electronic supplementary material.
Table 1.
Multiple least-squares regressions of rmax in mammals and birds (ln rmax as dependent variable, ln brain mass and ln body mass as independent variables, Homo excluded), for both species-level data (raw) or IC. (Significant effects are shown in italics.)
| brain mass | body mass | ||||||
|---|---|---|---|---|---|---|---|
| method | n | r2 | p-value | slope | p-value | slope | |
| mammals | |||||||
| all species | raw | 536 | 0.596 | <0.0001 | −0.955 | <0.0001 | +0.389 |
| IC | 535 | 0.130 | <0.0001 | −0.428 | 0.353 | +0.054 | |
| altricials | raw | 249 | 0.597 | 0.005 | −0.385 | 0.439 | −0.079 |
| IC | 248 | 0.116 | 0.032 | −0.357 | 0.951 | −0.006 | |
| precocials | raw | 256 | 0.618 | <0.0001 | −1.157 | <0.0001 | +0.534 |
| IC | 255 | 0.162 | <0.0001 | −0.519 | 0.068 | +0.115 | |
| terrestrial Carnivora | raw | 98 | 0.481 | 0.191 | −0.267 | 0.385 | −0.116 |
| IC | 97 | 0.256 | 0.0006 | −0.649 | 0.183 | +0.154 | |
| non-canid Carnivora | raw | 79 | 0.673 | <0.0001 | −0.893 | 0.039 | +0.264 |
| IC | 78 | 0.360 | <0.0001 | −0.976 | 0.014 | +0.325 | |
| birds | |||||||
| all species | raw | 388 | 0.352 | <0.0001 | −0.433 | 0.398 | +0.042 |
| IC | 387 | 0.132 | 0.294 | −0.082 | 0.0007 | −0.167 | |
| altricials | raw | 137 | 0.532 | 0.264 | +0.115 | <0.0001 | −0.306 |
| IC | 136 | 0.123 | 0.216 | −0.168 | 0.742 | −0.032 | |
| semi-altricials | raw | 77 | 0.670 | 0.649 | −0.070 | <0.0001 | +0.362 |
| IC | 76 | 0.332 | 0.586 | +0.085 | <0.0001 | −0.443 | |
| semi-precocials | raw | 42 | 0.326 | 0.0002 | −1.986 | 0.0005 | +1.201 |
| IC | 41 | 0.315 | 0.0007 | −1.772 | 0.002 | +1.074 | |
| precocials | raw | 132 | 0.259 | <0.0001 | −0.854 | 0.0004 | +0.368 |
| IC | 131 | 0.161 | 0.0002 | −0.561 | 0.158 | +0.115 | |
Given that these results support the expensive brain hypothesis, we can examine the prediction that species or lineages that increase their brain size without further reducing rmax must have significant allomaternal inputs. In mammals, this seems likely for members of the family of Canidae, most of which are biparental or cooperative breeders and which show both relatively large brains and high rmax (cf. figure 1). Within this family there is no correlation, and if this family is excluded, the negative effect of brain size on rmax among the remaining carnivores becomes much stronger (table 1).
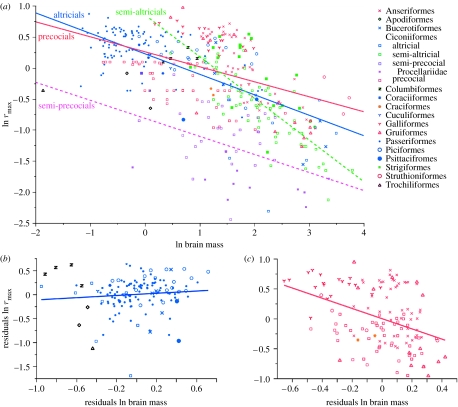
In birds, we find that rmax is negatively correlated with brain size only in precocials and semi-precocials, but not in altricials or semi-altricials (figure 2; table 1). In addition, as in mammals, the independent effect of body mass on rmax is positive rather than negative in precocial and semi-precocial birds (table 1).
Figure 2.
(a) Least-squares regression of ln rmax on ln brain mass in birds (n=389). The regressions of the residuals of ln rmax on the residuals of ln brain mass are shown for (b) altricials and (c) precocials separately. Full statistical details are given in the electronic supplementary material.
In all groups analysed, the correlation between rmax and brain size is much more driven by fertility rate than reproductive lifespan (table 2).
Table 2.
Partial correlation coefficients between life-history components of rmax (maximum reproductive period (MaxRP=max lifespan−age at first reproduction) and fertility) and (a) rmax or (b) brain mass, partialling out body mass and the other life-history variable. (Partial correlation coefficients (r) and their p-values for species-level data are given; significant values are shown in italics.)
| altricials | precocials | ||||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| (a) rmax | |||||
| mammals | maxRP | 0.018 | 0.783 | −0.276 | <0.0001 |
| fertility | 0.765 | <0.0001 | 0.918 | <0.0001 | |
| birds | maxRP | −0.049 | 0.566 | −0.034 | 0.704 |
| fertility | 0.837 | <0.0001 | 0.862 | <0.0001 | |
| (b) brain mass | |||||
| mammals | maxRP | 0.281 | <0.0001 | 0.044 | 0.482 |
| fertility | −0.189 | 0.003 | −0.601 | <0.0001 | |
| birds | maxRP | 0.068 | 0.428 | 0.217 | 0.015 |
| fertility | 0.120 | 0.158 | −0.239 | 0.007 | |
4. Discussion
The observed trade-off between the maximum rate of population increase (rmax) and both absolute and relative brain size supports the notion that this trade-off is caused by an energetic constraint, especially since it disappears in lineages where the mother's energetic burden during reproduction is alleviated through helpers. Thus, our results fully support the expensive brain hypothesis, which predicts that relatively large brains can evolve only when either energy input increases (Isler & Van Schaik 2006b) or there is an allocation shift from another expensive body function, such as production, or the size of an expensive tissue, such as the digestive tract in primates (Aiello & Wheeler 1995) or the pectoral muscle in birds (Isler & Van Schaik 2006a). To our knowledge, this framework is the only one that accounts for the well-known correlation between life-history patterns and brain size (reviewed in Deaner et al. 2003; Barrickman et al. 2008), while at the same time incorporating the energetic consequences of lifestyles that are influenced by ecological conditions of habitat and diet.
The rmax–brain size trade-off indicates that there is a maximum viable brain size for a species (its grey ceiling), beyond which viable populations cannot be sustained. The rate rmax represents the ability of a species to recover from population crashes due to starvation, disease or other evolutionary disasters, and therefore indexes the risk of local extinction. In species with low rmax, temporarily high rates of mortality are not easily buffered, so genetically based adaptation to environmental changes is hindered owing to the very limited room for selective mortality. Although it is impossible to pinpoint the exact value of this grey ceiling for any given lineage, it should depend on the stability of the habitat and the species' ability to buffer itself from such fluctuations, and thus its lifestyle. We assume that extant great ape species are very close to the absolute minimum viable rmax, and thus to the grey ceiling for primates. A similar value may apply to cetaceans, although valid estimates of maximum lifespan are notoriously difficult to obtain for these animals. In other lineages that are neither arboreal nor oceanic, the threshold may be considerably higher, as they may more often suffer from periodic population crashes.
These analyses demonstrate that at least in the precocial mammals and birds examined here, brain size, rather than body size, drives the value of rmax, and therefore a species' extinction risk. Thus, we propose that the historical pattern of species extinctions, generally attributed to large body size (Brook & Bowman 2005; but see Pimm et al. 1988), is instead at least partly driven by large brain size. Despite substantial benefits of enhanced cognitive abilities (e.g. Sol et al. 2007), we therefore predict that during mass extinctions large-brained taxa are especially vulnerable. On a macroevolutionary time scale, homoeothermic vertebrates tend to increase their brain size (but not in reptiles: Jerison 1969). Owing to the rmax–brain size trade-off, reproductive capacity decreases at the same time, leading taxa to a ‘drift’ towards ever-lower rmax. Over evolutionary time, we therefore also predict that lineages will tend to evolve towards a maximum sustainable brain size, and that every clear increase in brain size beyond their grey ceiling is accompanied by a significant change in lifestyle (usually accompanied by the emergence of a new lineage).
But what change of lifestyle would allow the evolution of larger brained lineages? Our results show that, as predicted by the expensive brain hypothesis, allomaternal energy inputs during offspring production are one critically important factor. In lineages in which mothers are helped, such as altricial birds or canid carnivores, the rmax–brain size trade-off is not found. This means that allomaternal care enables species to increase their brain size without compromising their demographic viability. More generally, we propose that extensive allomaternal care will allow brain size, and thus also cognitive abilities, to increase relative to their independently breeding relatives when conditions favour this.
This also explains why there are many lineages of birds that independently evolved relatively large brains (Nealen & Ricklefs 2001), but only a few in mammals (for phylogenetic analyses, see the electronic supplementary material). For 127 bird families from 23 orders, the upper 10 per cent quantile of brain mass residuals contains 13 families in seven different orders (Bucerotiformes, Psittaciformes, Piciformes, Strigiformes, Passeriformes and Ciconiiformes), all of which are altricial or semi-altricial. On the other hand, for 109 eutherian mammal families from 18 orders, the upper 15 per cent quantile of brain mass residuals contain 16 families in only two orders (Cetacea and Primates). In the absence of systematic comparisons, we draw attention to one spectacular example, Homo sapiens (see Van Schaik & Isler submitted). Humans have evolved allomaternal provisioning of offspring and allocare among adults, especially for the benefit of reproducing females (Hrdy 2005), and increased brain size approximately threefold relative to their sister group, the genus Pan.
Acknowledgments
We thank Georgina Mace, Robert Martin and Alexandra Müller for contributing data to our compilations. Financial support was provided through Swiss National Science Foundation grant no. 3100A0-117789.
Footnotes
One contribution of 10 to a Special Feature on ‘Brain evolution’.
Supplementary Material
“Why are there so few smart mammals (but so many smart birds)?” containing an Appendix with additional description of methods and results
References
- Aiello L.C., Wheeler P. The expensive-tissue hypothesis: the brain and the digestive-system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. doi:10.1086/204350 [Google Scholar]
- Barrickman N.L., Bastian M.L., Isler K., Van Schaik C.P. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J. Hum. Evol. 2008;54:568–590. doi: 10.1016/j.jhevol.2007.08.012. doi:10.1016/j.jhevol.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Brook B.W., Bowman D. One equation fits overkill: why allometry underpins both prehistoric and modern body size-biased extinctions. Popul. Ecol. 2005;47:137–141. doi:10.1007/s10144-005-0213-4 [Google Scholar]
- Cockburn A. Prevalence of different modes of parental care in birds. Proc. R. Soc. B. 2006;273:1375–1383. doi: 10.1098/rspb.2005.3458. doi:10.1098/rspb.2005.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L. The population consequences of life-history phenomena. Q. Rev. Biol. 1954;29:103–137. doi: 10.1086/400074. doi:10.1086/400074 [DOI] [PubMed] [Google Scholar]
- Deaner R., Barton R., Van Schaik C.P. Primate brains and life histories: renewing the connection. In: Kappeler P., Pereira M., editors. Primate life histories and socioecology. University of Chicago Press; Chicago, IL: 2003. pp. 233–265. [Google Scholar]
- Harvey P.H., Krebs J.R. Comparing brains. Science. 1990;249:140–146. doi: 10.1126/science.2196673. doi:10.1126/science.2196673 [DOI] [PubMed] [Google Scholar]
- Hennemann W.W. Relationship among body mass, metabolic rate and the intrinsic rate of natural increase in mammals. Oecologia. 1983;56:104–108. doi: 10.1007/BF00378224. doi:10.1007/BF00378224 [DOI] [PubMed] [Google Scholar]
- Hrdy S.B. Evolutionary context of human development: the cooperative breeding model. In: Carter C.S., Anhert L., Grossmann K.E., Hrdy S.B., Lamb M.E., Porges S.W., Sachser N., editors. Attachment and bonding: a new synthesis. MIT Press; Cambridge, MA: 2005. pp. 9–32. [Google Scholar]
- Isler K., Van Schaik C.P. Costs of encephalisation: the energy trade-off hypothesis tested on birds. J. Hum. Evol. 2006a;51:228–243. doi: 10.1016/j.jhevol.2006.03.006. doi:10.1016/j.jhevol.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Isler K., Van Schaik C.P. Metabolic costs of brain size evolution. Biol. Lett. 2006b;2:557–560. doi: 10.1098/rsbl.2006.0538. doi:10.1098/rsbl.2006.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler, K. & Van Schaik, C. P. Submitted. The expensive brain: a framework for explaining evolutionary changes in brain size. [DOI] [PubMed]
- Iwaniuk A.N., Nelson J.E. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 2003;81:1913–1928. doi:10.1139/z03-190 [Google Scholar]
- Jerison H.J. Brain evolution and dinosaur brains. Am. Nat. 1969;103:575–588. doi:10.1086/282627 [Google Scholar]
- Nealen P.M., Ricklefs R.E. Early diversification of the avian brain : body relationship. J. Zool. 2001;253:391–404. doi:10.1017/S095283690100036X [Google Scholar]
- Pimm S.L., Jones H.L., Diamond J. On the risk of extinction. Am. Nat. 1988;132:757–785. doi:10.1086/284889 [Google Scholar]
- Portmann A. Etudes sur la cérébralisation chez les oiseaux. III. Cérébralisation et mode ontogenetique. Alauda. 1947;15:161–171. [Google Scholar]
- Ricklefs R., Starck J. The evolution of the developmental mode in birds. In: Starck J., Ricklefs R., editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press; Oxford, UK: 1998. pp. 366–380. [Google Scholar]
- Sol D., Székely T., Liker A., Lefebvre L. Big-brained birds survive better in nature. Proc. R. Soc. B. 2007;274:763–769. doi: 10.1098/rspb.2006.3765. doi:10.1098/rspb.2006.3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaik, C. P. & Isler, K. Submitted. Was allomaternal care a precondition for brain enlargement in early Homo? J. Hum. Evol.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Why are there so few smart mammals (but so many smart birds)?” containing an Appendix with additional description of methods and results