Abstract
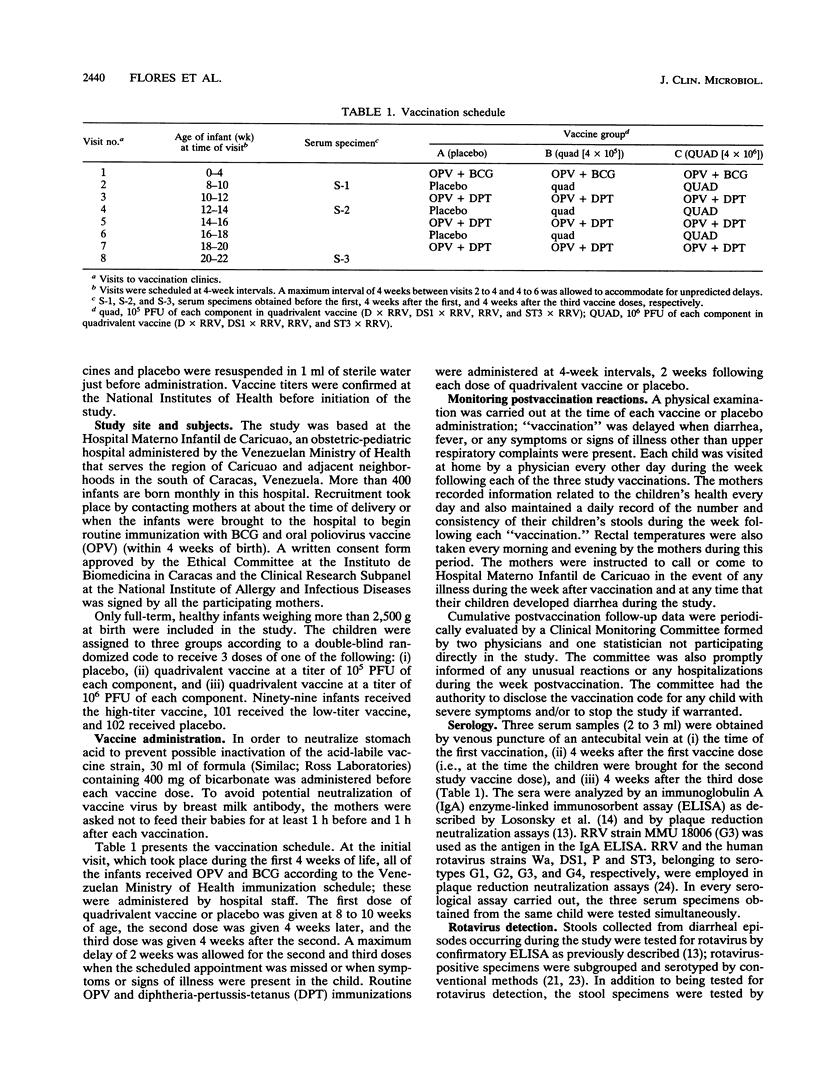
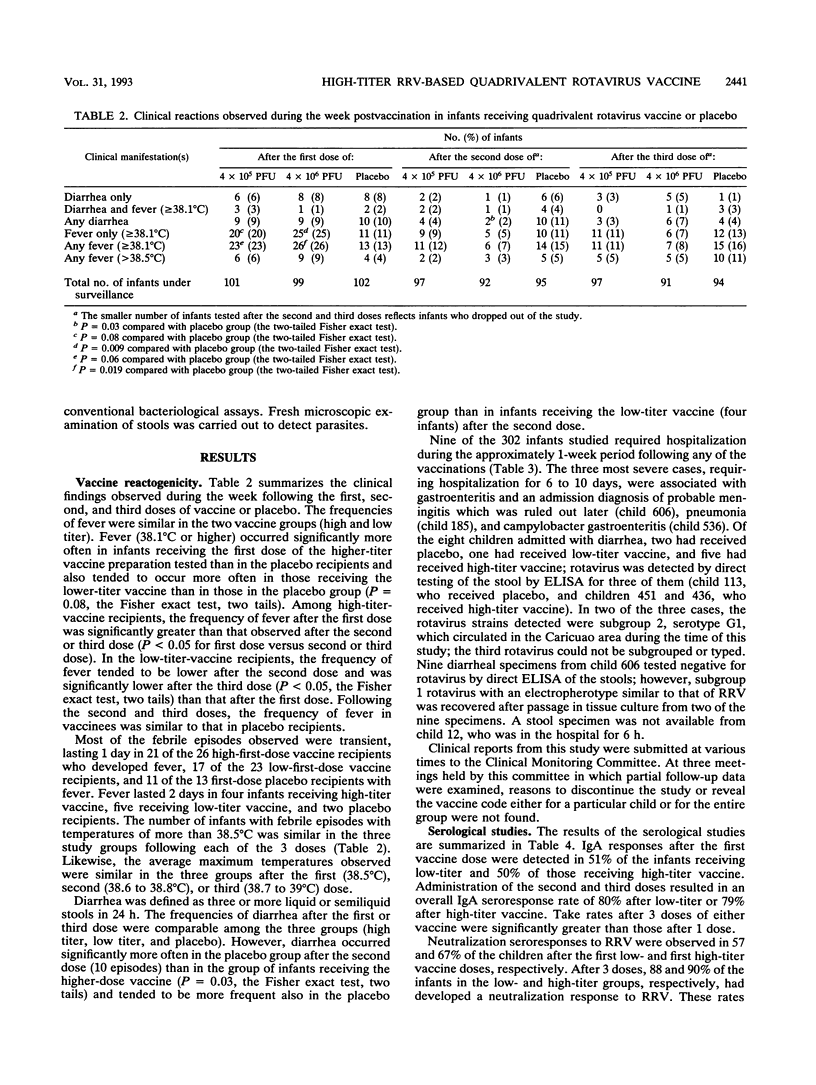
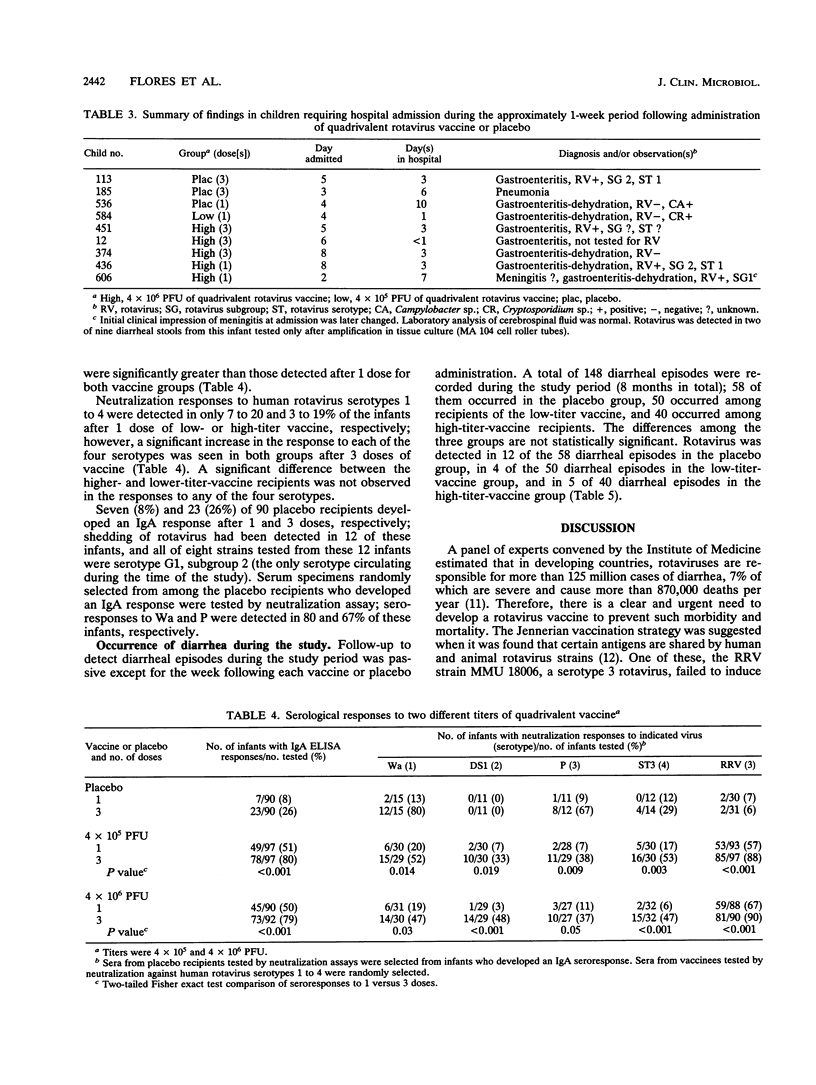
We evaluated the reactogenicity and antigenicity of a quadrivalent rotavirus vaccine composed of serotype 3 rhesus rotavirus (RRV) and three single-gene-substitution reassortants of RRV and human strain D (D x RRV, serotype 1), DS1 (DS1 x RRV, serotype 2), or ST3 (ST3 x RRV, serotype 4) in a double-masked study with 302 infants in Caracas, Venezuela. Three doses of the quadrivalent vaccine composed of either 10(5) PFU (low titer) or 10(6) PFU (high titer) of each component were administered to 99 and 101 infants, respectively, at 4-week intervals starting at the second month of age; 102 infants received a placebo. Postvaccination reactions were monitored by home visits every other day during the week postvaccination. The vaccine was associated with the occurrence of mild, short-lived febrile episodes in 26 and 23% of the recipients after the first doses of high- or low-titer vaccine, respectively, in comparison with 13% of the infants receiving the placebo. Febrile reactions occurred less frequently in vaccinees after the second or third dose than after the initial dose. The vaccine was not significantly associated with diarrhea or any additional symptom or sign. Serum specimens obtained shortly before the first, 4 weeks after the first, and 4 weeks after the third dose of vaccine or placebo were tested by an immunoglobulin A enzyme-linked immunosorbent assay and by neutralization assays. Seroresponses occurred significantly more often after 3 doses than after a single dose of either vaccine. Immunoglobulin A responses were observed in 80 and 79% of the infants after 3 doses of high- or low-titer vaccine, respectively. Most of the infants tested developed a neutralization response to RRV after 3 doses of the high- (90%) or low-(88%) titer vaccine. Neutralization response rates to human rotavirus serotypes 1 to 4 after 3 doses were similar in both vaccine and 87 of 90 receiving the high-titer vaccine developed seroresponses, as detected by any of the assays employed. The study indicates that 3 doses of quadrivalent vaccine at a titer of 10(6) PFU of each component offered no advantage over the lower-titer preparation for use in efficacy trials.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Smith V. E., Sander D. S., Pax K. A., Schiff G. M., Ward R. L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990 Nov;162(5):1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Christy C., Madore H. P., Pichichero M. E., Gala C., Pincus P., Vosefski D., Hoshino Y., Kapikian A., Dolin R. Field trial of rhesus rotavirus vaccine in infants. Pediatr Infect Dis J. 1988 Sep;7(9):645–650. doi: 10.1097/00006454-198809000-00009. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mol P., Zissis G., Butzler J. P., Mutwewingabo A., André F. E. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986 Jul 12;2(8498):108–108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., White L., Garcia D., Vilar M., Cunto W., Gonzalez R., Urbina C., Boher J. Comparison of reactogenicity and antigenicity of M37 rotavirus vaccine and rhesus-rotavirus-based quadrivalent vaccine. Lancet. 1990 Aug 11;336(8711):330–334. doi: 10.1016/0140-6736(90)91876-c. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Gothefors L., Wadell G., Juto P., Taniguchi K., Kapikian A. Z., Glass R. I. Prolonged efficacy of rhesus rotavirus vaccine in Swedish children. J Infect Dis. 1989 Apr;159(4):753–757. doi: 10.1093/infdis/159.4.753. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Madore H. P., Christy C., Pichichero M., Long C., Pincus P., Vosefsky D., Kapikian A. Z., Dolin R. Field trial of rhesus rotavirus or human-rhesus rotavirus reassortant vaccine of VP7 serotype 3 or 1 specificity in infants. The Elmwood, Panorama, and Westfall Pediatric Groups. J Infect Dis. 1992 Aug;166(2):235–243. doi: 10.1093/infdis/166.2.235. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Blanco M., Vilar M., Garcia D., White L., Gonzalez R., Kapikian A. Z., Flores J. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. J Clin Microbiol. 1990 Mar;28(3):553–558. doi: 10.1128/jcm.28.3.553-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosham M., Letson G. W., Wolff M., Reid R., Gahagan S., Adams R., Callahan C., Sack R. B., Kapikian A. Z. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. J Infect Dis. 1991 Mar;163(3):483–487. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- White L., Perez I., Perez M., Urbina G., Greenberg H., Kapikian A., Flores J. Relative frequency of rotavirus subgroups 1 and 2 in Venezuelan children with gastroenteritis as assayed with monoclonal antibodies. J Clin Microbiol. 1984 Apr;19(4):516–520. doi: 10.1128/jcm.19.4.516-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


