Abstract
Previous studies from our laboratory using co-immunoprecipitation techniques suggested that the human lutropin receptor (hLHR) constitutively self-associates into dimers/oligomers and that agonist treatment of cells either increased hLHR dimerization/oligomerization and/or stabilized hLHR dimers/oligomers to detergent solubilization (Tao, Y. X., Johnson, N. B., and Segaloff, D. L. (2004) J. Biol. Chem. 279, 5904–5914). In this study, bioluminescence resonance energy transfer (BRET2) analyses confirmed that the hLHR constitutively self-associates in living cells. After subcellular fractionation, hLHR dimers/oligomers were detected in both the plasma membrane and endoplasmic reticulum (ER). Further evidence supporting the constitutive formation of hLHR dimer/oligomers in the ER is provided by data showing homodimerization of misfolded hLHR mutants that are retained in the ER. These mutants, when co-expressed with wild-type receptor, are shown by BRET2 to heterodimerize, accounting for their dominant-negative effects on cell surface receptor expression. Hormone desorption assays using intact cells demonstrate allosterism between hLHR protomers, indicating functional cell surface hLHR dimers. However, quantitative BRET2 analyses in intact cells indicate a lack of effect of agonist on the propensity of the hLHR to dimerize. Using purified plasma membranes, human chorionic gonadotropin was similarly observed to have no effect on the BRET2 signal. An examination of the propensity for constitutively active and signaling inactive hLHR mutants to dimerize further showed no correlation between dimerization and the activation state of the hLHR. Taken altogether, our data suggest that hLHR dimers/oligomers are formed early in the biosynthetic pathway in the ER, are constitutively expressed on the plasma membrane, and are not affected by the activation state of the hLHR.
The human lutropin receptor (hLHR)3 is a G protein-coupled receptor (GPCR) that plays a central role in reproductive physiology. In women, the LHR is expressed primarily in the ovary, where it mediates the actions of pituitary LH by stimulating the synthesis of androgens (which are mostly converted to estrogens) and ovulation. The hLHR also binds the nearly identical placental glycoprotein hormone hCG. As such, in pregnant women it rescues the corpus luteum, thus maintaining pregnancy. In males the hLHR is expressed predominantly in the testes, stimulating androgen biosynthesis in response to LH. The physiological importance of the LHR to reproductive physiology is underscored by disorders arising from mutations of the hLHR gene (1, 2). Thus, whereas constitutively active mutants (CAMs) of the hLHR are associated with gonadotropin-independent precocious puberty in males, loss-of-function mutations of the hLHR result in Leydig cell hypoplasia in males and infertility in females.
Structurally, the hLHR is composed of two structural domains. There is the canonical seven transmembrane serpentine region common to GPCRs as well as a large extracellular domain that is composed of numerous leucine-rich repeats, which bind LH or hCG with high affinity (3). The hLHR is most closely related to the other glycoprotein hormone receptors for follitropin and thyrotropin, and is a member of the family A or rhodopsin-like family of GPCRs (4). Agonist-occupied wild-type hLHR or hLHR CAMs activate the Gs, Gi/o, and Gq/11 families of G proteins (5, 6), with the primary actions of the hLHR being mediated by Gs.
A large body of work published in recent years supports the concept that GPCRs can form self-associated dimers and higher ordered oligomers within cells (for reviews see Refs. 7–10). In addition to GPCRs forming homodimers, distinct GPCRs can in some cases physically associate as heterodimers. The functional ramifications of GPCR dimerization are most clearly evident in heterodimers, where in many cases the pharmacological properties of heterodimers between two different GPCRs are unique as compared with those of each GPCR alone (11–19). In contrast, the role of GPCR homodimerization is less clear. It has been hypothesized that agonist binding may alter the dimerization state of the receptor. Although this question has been addressed in numerous studies, there is little consensus with some studies suggesting that agonist promotes GPCR dimer formation (20–25), others that agonist causes the dissociation of GPCR dimers (26), and yet others that the GPCR dimers are constitutively expressed and unaffected by agonist (27–30). As discussed in a recent review, unappreciated limitations to some of the methods used may have contributed to the apparently discrepant results (10).
Previous studies have suggested that the LHR can self-associate into dimers/oligomers. Evidence pointing toward this goes back to as early as the 1980s when data from equilibrium sedimentation of detergent-solubilized LHR and radiation inactivation of LHRs on gonadal cells suggested that the LHR may be present in a dimeric or oligomeric form (31, 32). More recently, functional complementation studies further suggested the dimerization of the LHR (33, 34). Direct evidence in support of dimerization of the LHR has been obtained using fluorescent resonance energy transfer (FRET) (35–38) as well as our studies demonstrating specific co-immunoprecipitation of differentially tagged forms of the LHR (39). In this latter study it was shown that under basal conditions the hLHR physically self-associates into complexes of sizes consistent with dimers and higher ordered oligomers of the receptor. It was also observed that hCG pretreatment of cells prior to detergent solubilization led to an increase in the abundance of hLHR dimers/oligomers relative to monomers as detected by Western blotting. Although these results could be explained by an agonist-dependent induction of hLHR dimerization, it may also reflect a stabilization of preformed receptor dimers/oligomers to detergent solubilization when they are occupied by hCG.
To address this question, this work was undertaken utilizing bioluminescence resonance energy transfer (BRET) as an independent means to probe hLHR dimerization and to study the effects of receptor activation on dimerization. BRET is a highly sensitive means to assess protein-protein interactions in living cells, thus avoiding the detergent solubilization required for co-immunoprecipitation experiments (10, 40, 41). In the BRET paradigm, a given protein is fused to the energy donor Renilla luciferase (Rluc), which is co-expressed with the same or a different protein fused to an energy acceptor, typically a variant of green fluorescent protein (GFP). Addition of substrate for Rluc results in bioluminescence, which can also cause the excitation of GFP via resonance energy transfer only if the donor and acceptor are within 100 Å of each other, a distance consistent with the predicted size of a GPCR dimer (10, 40, 41). Although identical in principle, BRET2 is a second generation modification of BRET that achieves a greater spectral resolution between donor and acceptor emissions (10, 40, 41). Both have been widely used in recent years to examine homo- and heterodimerization of a number of different GPCRs (10). A limitation of BRET/BRET2 (as well as the related method of FRET) is that a positive signal from a single ratio of energy donor and acceptor is not necessarily reflective of the affinity of GPCR protomers to dimerize nor the extent of GPCR dimerization. This is because RET is dependent not only on the distance between the energy donor and acceptor, but on their relative orientations as well (10, 40, 41). A method of quantitative BRET analyses was therefore proposed by Mercier et al. (42) to permit the determination of the relative affinities of pairs of GPCR protomers to form homo- or heterodimers. Based on theoretical considerations of RET, this approach utilizes saturation curves (analogous to hormone-receptor binding curves) to calculate the half-maximal BRET signal (BRET50), which is related to the relative affinity of the protomers for each other (41, 42).
Using quantitative BRET2 analyses, we demonstrate that hLHR dimers can be detected under basal conditions in living cells, with constitutively expressed hLHR dimers/oligomers observed in both the plasma membrane as well as the endoplasmic reticulum. From a number of different experimental strategies, it is further concluded that dimerization/oligomerization of the hLHR is not affected by the activation state of the hLHR.
MATERIALS AND METHODS
Plasmids and Hormones—The hLHR cDNA was kindly given to us by Ares Advanced Technology (Ares-Serono Group, Randolph, MA). Mutants of the hLHR were made using standard techniques. The wild-type and mutant forms of the hLHR were all modified to contain a Myc epitope tag at the N terminus. Before use, the coding sequence of each construct was determined by the DNA Core of the University of Iowa. For the BRET2 studies, the cDNAs were subcloned into pRluc or pGFP2 vectors (PerkinElmer Life Sciences), which insert Renilla luciferase (Rluc) or GFP2, respectively, in-frame at the C terminus of the protein. The cDNA encoding KvLQT1 was used as described previously (43). Highly purified preparations of recombinant hCG and recombinant hLH were purchased from Dr. A. Parlow and the National Hormone and Pituitary Program, NIDDK, National Institutes of Health. hLH was iodinated as described previously for hCG (44). A crude preparation of hCG (used solely for the determination of nonspecific binding of 125I-hLH) was purchased from Sigma.
Cells and Transfections—Human embryonic kidney (HEK) 293 and 293T cells were obtained from the American Type Tissue Collection (Manassas, VA). Cells were maintained at 5% CO2 in growth media consisting of high glucose Dulbecco's modified Eagle's medium containing 50 μg/ml gentamicin, 10 mm HEPES, and 10% newborn calf serum. For experiments, cells were plated onto wells that had been precoated for 45 min with 0.1% gelatin in Dulbecco's phosphate-buffered saline, pH 7.1 (D-PBS), that was calcium- and magnesium-free. Cells were transiently transfected as described previously (45) and used for experiments 24 h after removing the mixture.
BRET2 Assay—HEK293T cells in 6-well plates were transiently co-transfected with vectors encoding Rluc fusion or GFP2 fusion proteins. In a given experiment, the total amount of plasmid transfected was made constant by the addition of empty vector. On the day of the experiment, cells were washed two times with calcium- and magnesium-free D-PBS and then detached from the well in 1 ml of D-PBS. Protein concentrations were measured, and then equal protein aliquots were distributed into microcentrifuge tubes and collected by gentle centrifugation. The cell pellets were resuspended in a small volume of D-PBS and transferred to a white-bottomed 96-well microplate (white Optiplate; PerkinElmer Life Sciences) such that all samples were of equal volume and protein concentration. Total fluorescence of the cell suspensions was measured using a POLARstar Optima plate reader (BMG Labtech, Offenburg, Germany), with an excitation filter at 485 nm and an emission filter at 520 nm, and was corrected for the fluorescence measured in cells transfected with empty vector only. The substrate Coelenterazine 400a (Biosynth; Zurich, Switzerland) was then added at a final concentration of 5 μm, and readings at 410/80 nm (reflecting the bioluminescence given off by Rluc) and 515/30 nm (reflecting the resonance energy transfer from Rluc to GFP2) were measured simultaneously. Bioluminescence readings were corrected for those obtained from cells transfected with empty vector only. The BRET2 ratio was calculated as the ratio of the light emitted by the receptor-GFP2 (515/30 nm) over the light emitted by the receptor-Rluc (410/80 nm). The BRET2 ratios reported were corrected by subtracting the ratios obtained when receptor-Rluc was expressed alone. Within a given experiment, each data point was obtained using duplicate wells of cells that were transfected and collected for BRET2 analyses. Data shown are the mean ± range of the duplicate determinations from one representative experiment (of at least three independent experiments).
BRET2 Titration Curves—For saturation curves, HEK293T cells in 6-well plates were co-transfected with a fixed concentration of Rluc fusion protein and increasing concentrations of a GFP2 fusion protein. When more than one curve was performed in a given experiment, the concentrations of plasmids encoding the Rluc fusion proteins were adjusted so that, after substrate addition, the bioluminescence values of the Rluc fusion proteins expressed alone were similar. Data were expressed as the net BRET2 ratio, calculated as described above, relative to the ratio of acceptor to donor. The data were plotted using GraphPad Prism (San Diego), and the Bmax and KD determinations were taken as the BRETmax and BRET50, respectively.
Subcellular Fractionation—HEK293T cells were co-transfected with hLHR-Rluc and hLHR-GFP2 or with KvLQT1-Rluc and hLHR-GFP2 as a negative control. On the day of the experiment, the cells were placed on ice, washed three times with ice-cold D-PBS, and scraped off the dish in ice-cold hypotonic lysis buffer (20 mm HEPES, pH 7.4, 2 mm EDTA, 2 mm EGTA, and 6 mm magnesium chloride) containing complete protease inhibitor mixture (Roche Diagnostics). Cells were homogenized with 50 strokes in a tight fitting Dounce homogenizer on ice. After centrifugation at 1000 × g for 5 min at 4 °C, the post-nuclear supernatant was collected and assayed for protein concentration. Each cell lysate was adjusted to 1 mg of protein in 463 μl of hypotonic lysis buffer and brought to contain a final sucrose concentration of 2 m and placed in the bottom of a 5-ml ultracentrifuge tube for a Beckman SW55 Ti rotor. A discontinuous step sucrose gradient was then made in each tube as follows. Applied above the sample, 237 μl of lysis buffer containing 1.9 m sucrose was added. Then 700 μl each of hypotonic lysis buffer containing 1.75, 1.5, 1.25, 1.0, 0.75, and 0.5 m sucrose were added. Samples were centrifuged 16 h at 50,000 rpm at 4 °C. Aliquots of 400 μl were taken from the top of each tube. BRET2 ratios were determined from 100-μl samples. Equal volume samples were also taken from each aliquot for Western blots (see below) to detect calnexin (a marker for the endoplasmic reticulum), Na+/K+-ATPase (a marker for the plasma membrane), or the myc-hLHR. Protein concentrations were measured in each aliquot. Western blots for myc-hLHR were also analyzed when the gels were run after applying equal amounts of protein to each well.
Western Blotting—Samples were diluted 1:6 into a 6-fold concentrated Laemmli sample buffer containing reducing agents (12% w/v SDS, 40% glycerol, 109 mm EDTA, 1.5 m Tris/HCl, 98 mg/ml dithiothreitol and 6% v/v β-mercaptoethanol), incubated 1 h at room temperature, fractionated by SDS-PAGE on 7.5% gels, and transferred to polyvinylidene difluoride membranes. Membranes were probed with anti-Myc monoclonal antibody 9E10 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), anti-calnexin polyclonal antibody (1:1000; StressGen, Ann Arbor, MI), or anti-Na+/K+-ATPase monoclonal antibody (1:250; Sigma). The membranes were then incubated with either horseradish peroxidase-conjugated sheep anti-mouse antibody (1:25,000; GE Healthcare) or donkey anti-rabbit antibody (1:100,000; GE Healthcare). The immunoreactive bands were visualized using the Amersham Biosciences ECL detection system (GE Healthcare).
Hormone Desorption Experiments—HEK293 cells were seeded on 24-well culture plates that had been coated with gelatin as described above. Cells were transfected as described above and used for experiments 24 h after removing the mixture. On the day of the experiment, the cells were washed two times with binding medium consisting of warm Waymouth's MB752/1 containing 50 μg/ml gentamicin and 1 mg/ml bovine serum albumin. The cells were then incubated 1 h at room temperature in binding medium containing a saturating concentration of 125I-hLH (final concentration 1000 ng/ml) with or without an excess of unlabeled crude hCG (final concentration 50 IU/ml) to determine nonspecific binding. The end of the binding assay was defined as t = 0 relative to the subsequent desorption phase of the experiment. One group of cells was used to determine the maximal binding at t = 0. These cells were set on ice, washed three times with ice-cold Hanks' balanced salt solution modified to contain 50 μg/ml gentamicin and 1 mg/ml bovine serum albumin, solubilized in 0.5 n NaOH, transferred to plastic test tubes with cotton swabs, and counted in a gamma counter. Another group of cells was used to determine the time course of desorption of the prebound 125I-hLH. These cells were washed three times with warm binding medium, incubated in binding medium containing hCG (final concentration 500 ng/ml), hLH (1000 ng/ml), or vehicle only and incubated for increasing times at room temperature. At a given time point, the cells were placed on ice, and the 125I-hLH released into the medium was determined by collecting the medium and precipitating intact hormone with trichloroacetic acid. To determine the amount of 125I-hLH remaining bound to the cells, the cells were washed and solubilized, and the bound radioactivity was counted as described for the t = 0 time point.
RESULTS
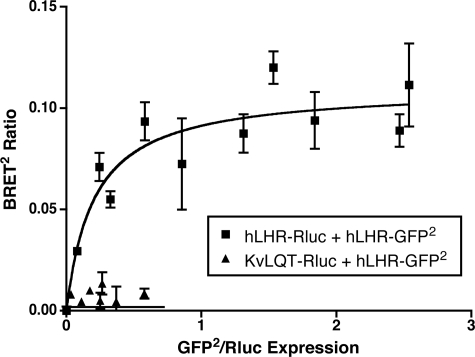
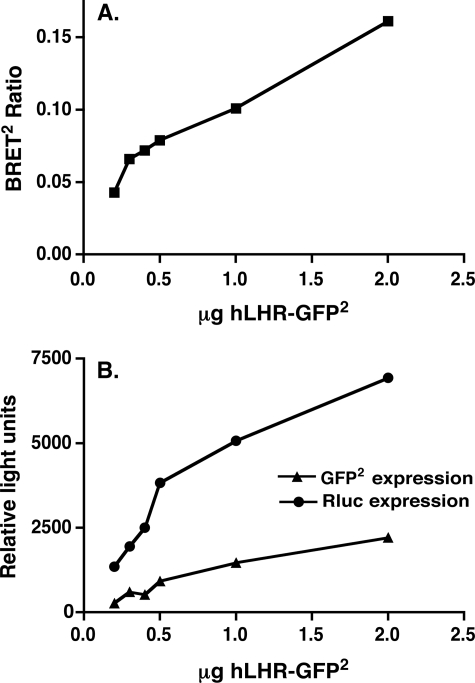
To determine whether hLHR self-association could be detected in living cells, we examined whether specific BRET2 signals would be observed in cells co-transfected hLHR(wt)-Rluc and hLHR(wt)-GFP2. As shown in Fig. 1, titration curves were performed in which cells were transfected with a subsaturating and fixed concentration of the energy donor hLHR(wt)-Rluc and increasing concentrations of the energy acceptor hLHR(wt)-GFP2. As the ratio of energy acceptor to donor was increased, the BRET2 ratio increased, as would be predicted by molecules that were clustered together and not randomly distributed (42, 46). At the higher ratios of hLHR(wt)-Rluc to hLHR(wt)-GFP2, the BRET2 signal reached a plateau, indicative of a saturable interaction between the energy donor and acceptor (42). As a negative control, cells were co-transfected with the plasma membrane cardiac voltage-gated K+ channel KvLQT1 fused to Rluc and hLHR(wt)-GFP2. Little or no detectable BRET2 ratios were detected under those conditions, suggesting that the BRET2 signals observed between hLHR(wt)-Rluc and hLHR(wt)-GFP2 are because of the physical interaction between the hLHR portions of the fusion proteins. To ensure that the BRET2 observed between hLHR(wt)-Rluc and hLHR(wt)-GFP2 was not because of spurious bystander interactions between the two molecules, we examined the effect of decreasing the protein expression levels of the receptor, while maintaining the ratio of hLHR(wt)-Rluc/hLHR(wt)-GFP2 constant. As shown in Fig. 2, the BRET2 ratio remained detectable at low levels of receptor expression, consistent with what would be predicted by specific clustering of molecules and not random collisions (46).
FIGURE 1.
BRET2 saturation curves examining homodimerization of the hLHR in living cells. HEK293T cells were transiently transfected with a fixed concentration of hLHR(wt)-Rluc or KvLQT1-Rluc and increasing concentrations of hLHR(wt)-GFP2. Data shown are the mean ± range of duplicate net BRET2 ratios as a function of GFP2/Rluc expression taken from one experiment that is representative of at least 20 independent experiments.
FIGURE 2.
BRET2 signal resulting from co-expression of hLHR(wt)-Rluc and hLHR(wt)-GFP2 is not a function of random self-association. HEK293T cells were transiently transfected with varying total amounts of a fixed ratio (1:5) of hLHR(wt)-Rluc and hLHR(wt)-GFP2. A, data shown are the net BRET2 ratios as a function of the total amount of plasmid transfected. B, expression of hLHR(wt)-GFP2 (as measured by fluorescence prior to substrate addition) and hLHR(wt)-Rluc (as measured by luminescence) are shown. The data are from one experiment are representative of at least four independent experiments.
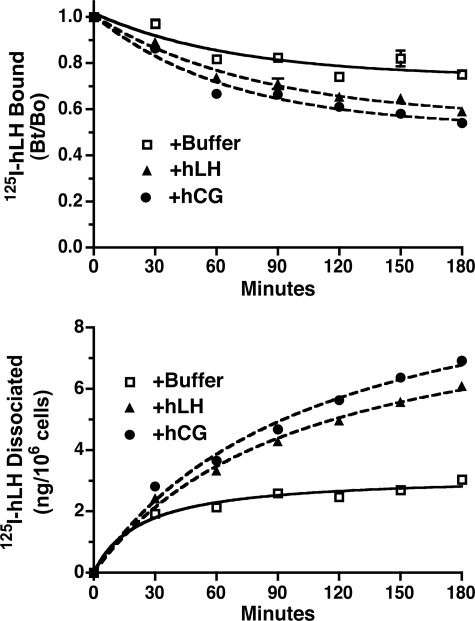
The following experiment was then performed to independently confirm the formation of hLHR dimer/oligomers and to determine whether hLHR dimers/oligomers were present on the cell surface in a functional form. This experiment is based on the observation that changes in the rate of hormone desorption reflect allosteric regulation of one receptor protomer by another receptor protomer within a GPCR dimer (47, 48). In our study, cells that had been transfected with hLHR were allowed to bind 125I-hLH. After removing the unbound hormone, the cells were incubated for increasing times with buffer only, with hLH, or with hCG. As shown in Fig. 3, the presence of hLHR or hCG during the desorption phase of the experiment promoted an increased rate of dissociation of prebound 125I-hLH. This was observed by a more rapid decrease in the 125I-LH remaining bound to cells (Fig. 3, top panel) as well as a more rapid increase in the release of 125I-LH into the media (as measured by acid-precipitable cpm in the media, Fig. 3, bottom panel) when LH or hCG was present. The ability of unlabeled hLH and hCG to allosterically modulate the dissociation of 125I-LH further supports the conclusion that hLHR dimers are present on the cell surface and demonstrates that these dimers are functional with respect to hormone binding.
FIGURE 3.
Allosteric modulation of hormone dissociation indicates the presence of hLHR dimers on the cell surface. HEK293 cells transiently transfected with a relatively low level of the hLHR(wt) were allowed to bind a saturating concentration of 125I-hLH (1 μg/ml final concentration). After washing, the cells were incubated for the indicated times at room temperature with buffer only or with a saturating concentration of hLH (1 μg/ml final concentration) or hCG (500 ng/ml final concentration). At the end of the incubation period, the amount of 125I-hLHR remaining specifically bound to the cells was determined (top panel), and the amount of acid-precipitable counts/min in the medium was determined (bottom panel). Data shown are the mean ± S.E. of triplicate determinations within one representative experiment. Experiments entailing only top panel were performed five times and those entailing top and bottom panels were performed two times.
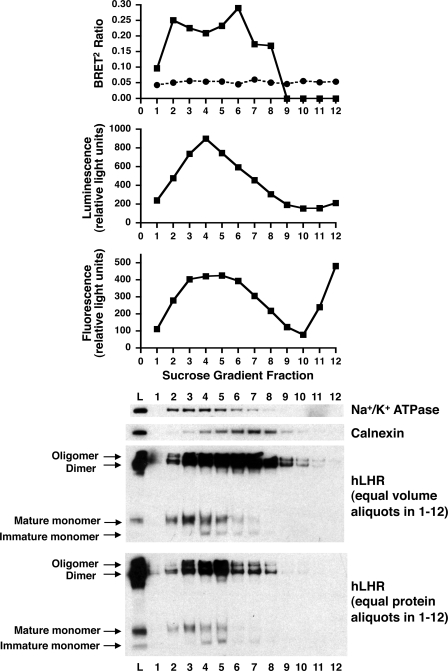
Although the hormone desorption assays confirm the presence of hLHR dimers/oligomers on the cell surface, one cannot exclude the possible intracellular localization of hLHR dimers/oligomers as well. In this respect, the BRET2 data from the above experiments do not permit the determination of the subcellular localization of the source of the energy transfer. To address this question, subcellular fractionation studies were performed. Cells were co-transfected with hLHR(wt)-Rluc and hLHR(wt)-GFP2 and then the cells were lysed, and the post-nuclear membranes were fractionated by centrifugation in a discontinuous sucrose gradient. As shown in Fig. 4, the most highly enriched plasma membranes (as indicated by Na+/K+-ATPase expression) were in fractions 2–5, and the most highly enriched ER membranes (as indicated by calnexin expression) were in fractions 4–8. The BRET2 data indicate resonance energy transfer emanating from both plasma membrane-enriched fractions as well as ER-enriched fractions (Fig. 4, top panel). Little or no BRET2 signal was observed when cells coexpressing hLHR(wt)-Rluc and KvLQT1-GFP2 were subjected to the same fractionation, again confirming the specificity of the BRET2 data arising from hLHR self-association. The BRET2 data obtained from the cell fractionation studies indicate that self-associated hLHR dimers/oligomers are present not only on the cell surface but in the ER as well.
FIGURE 4.
Subfractionation of membranes reveals constitutive hLHR dimers/oligomers in the plasma membrane and the ER. HEK293T cells were transiently transfected with myc-hLHR-Rluc and myc-hLHR-GFP2. Cell membranes lysates were applied to the bottom of a sucrose gradient as described under “Materials and Methods” and resolved by floatation during centrifugation. Equal volume fractions were analyzed (top to bottom) for hLHR dimerization as determined by BRET2, hLHR-Rluc expression as determined by luminescence, hLHR-GFP2 expression as determined by fluorescence, Na+/K+-ATPase expression as determined by Western blotting, and calnexin expression as determined by Western blotting. The bottom two panels depict hLHR expression as determined by Western blotting, in the membrane lysate prior to fractionation (L) and each of the fractions. Equal volume aliquots (next to bottom panel) or equal protein aliquots (bottom panel) of the fractions were evaluated. Data shown are from one experiment representative of at least three independent experiments.
The subcellular membrane fractions were also assayed for hLHR expression, as determined by Western blotting. Because all hLHR constructs used in this study contained a Myc epitope tag at the N terminus, migration of the hLHR in the SDS gels was ascertained by probing Western blots with anti-Myc antibody. Therefore, these blots do not distinguish between the Rluc and GFP2 fusion proteins of the hLHR(wt), but rather reflect both forms of hLHR(wt). As with the BRET2 analyses and the Western blotting for plasma membrane and ER markers, a Western blot was performed for the hLHR using equal volume aliquots of each fraction (Fig. 4, next to bottom panel). In this view, the intensity of hLHR bands would reflect both the relative abundance of the hLHR as well as the membrane protein concentration in the fraction. To obtain an assessment of the relative abundance of the hLHR independent of fluctuations of membrane protein content between fractions, a Western blot was also performed in which equal protein aliquots of each fraction were resolved on the SDS gels (Fig. 4, bottom panel). In both cases, bands were revealed that had previously been shown to correspond to intracellularly localized immature, monomeric hLHR (39, 49), cell surface localized mature monomeric hLHR (39, 49), dimeric hLHR (39), and oligomeric hLHR (39). As would be expected, mature monomeric and immature monomeric forms of the hLHR co-fractionated with markers for the plasma membrane and ER, respectively. The Western blots reveal high molecular weight hLHR dimers and oligomers in plasma membrane and ER. These data are in agreement with the BRET2 data from the same experiment, further suggesting that hLHR dimerization initially occurs within the ER.
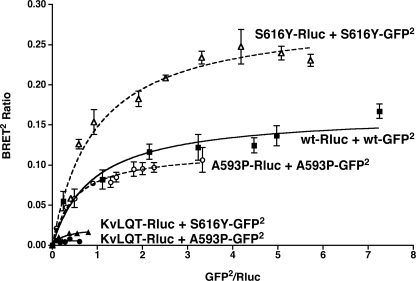
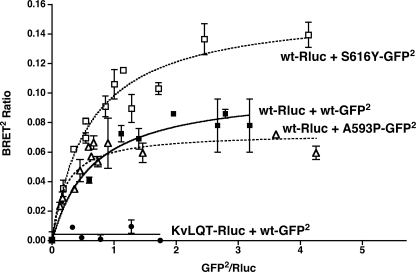
The data presented in Fig. 4 demonstrate that hLHR dimers/oligomers are constitutively found in both plasma membrane and ER fractions. Further evidence supporting the conclusion that dimerization of the hLHR occurs initially in the ER comes from the following experiments examining two loss-of-function hLHR mutants, S616Y and A593P, each of which have been shown previously to be misfolded and retained in the ER with little or no mutant receptor making it to the cell surface (39, 50). As shown in Fig. 5, each of these two mutants exhibit specific and saturable homodimerization as determined by BRET2 saturation curves. It has been shown previously that co-expression of S616Y or A593P with the WT hLHR causes a reduction in the cell surface expression of the WT receptor presumably because of dimerization of the misfolded mutant with the WT receptor because the two can be co-immunoprecipitated together (39). BRET2 saturation curves of a fixed concentration of hLHR(wt)-Rluc coexpressed with increasing concentrations of each of the misfolded mutants fused to GFP2 reveal specific and saturable heterodimerization between the mutants and WT receptor (Fig. 6). These data show that misfolded mutants of the hLHR that are retained in the ER undergo specific homodimerization and that the ER-localized misfolded mutant can form heterodimers with the hLHR.
FIGURE 5.
Homodimerization of ER-retained misfolded mutants of the hLHR. For the hLHR(wt) and the two hLHR misfolded mutants shown, HEK293T cells were transfected with a fixed amount of Rluc fusion protein (adjusted so that the expression of receptor-Rluc in the absence of receptor-GFP2 was the same for all constructs) and increasing concentrations of the corresponding GFP2 fusion protein. As a negative control, KvLQT1-Rluc (matched as above) was co-transfected with increasing concentrations of hLHR(wt)-GFP2. Data shown are the corrected BRET2 signals (mean ± range of duplicate determinations) as a function of GFP2/Rluc expression. The constructs shown were analyzed within the same experiment. The data shown are from one experiment that is representative of at least three independent experiments.
FIGURE 6.
Heterodimerization of ER-retained misfolded mutants of the hLHR with hLHR(wt). HEK293T cells were transfected with a fixed amount of hLHR(wt)-Rluc and increasing concentrations of hLHR(S16Y)-GFP2 or hLHR(A593P)-GFP2. Data shown are the corrected BRET2 signals as a function of GFP2/Rluc expression. The constructs shown were analyzed within the same experiment. The data shown are the mean ± range of duplicate determinations from one experiment that is representative of at least three independent experiments.
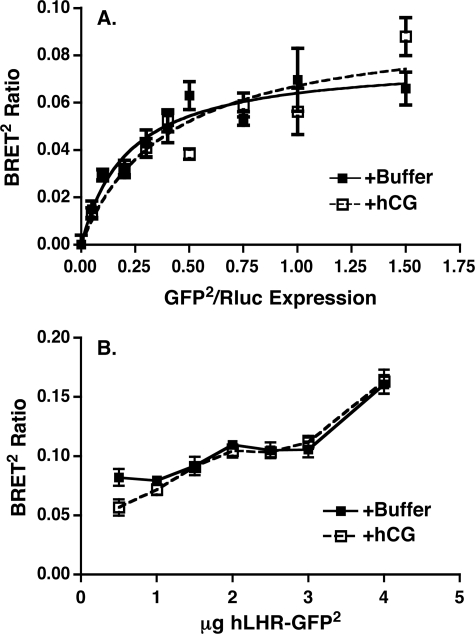
The data presented thus far establish that self-association of the hLHR is a constitutive process initiated in the ER and that functional hLHR dimers/oligomers are present on the cell surface of living cells. To ascertain if activation of the hLHR results in alterations in hLHR dimerization, the following studies were performed. First, we examined the effects of hCG on BRET2 between WT hLHR donor and acceptor fusion proteins. In one scenario, the BRET2 ratios were determined under conditions of a fixed concentration of donor and increasing concentrations of acceptor (Fig. 7A). The resulting BRET2 saturation curves from cells incubated with or without hCG appeared similar. In the experiment shown, the hCG incubation was performed at room temperature to minimize internalization of the hormone-occupied receptor. Using the donor/acceptor ratio yielding the half-maximal BRET2 shown, the effects of a 15-min incubation with hCG at 37C were also examined, but again no changes in BRET2 were observed. In the other scenario, we examined the effects of a room temperature incubation of the cells with hCG on BRET2 ratios under conditions where the ratio of hLHR donor and acceptor fusion proteins were held constant, but the total receptor expression levels were varied. Again, no differences with hCG stimulation were observed (Fig. 7B).
FIGURE 7.
Lack of effect of hCG on BRET2 analyses in intact cells. A, HEK293T cells were transiently transfected with a fixed concentration of hLHR(wt)-Rluc and increasing concentrations of hLHR(wt)-GFP2. On the day of the experiment, cells were incubated with buffer only or with hCG (1000 ng/ml final concentration) for 20 min at room temperature and then analyzed for BRET2. Data shown are the net BRET2 signals (mean ± range of duplicate determinations) as a function of GFP2/Rluc expression taken from one experiment that is representative of at least four independent experiments. B, HEK293T cells were transiently transfected with decreasing total amounts of a fixed ratio (1:5) of hLHR(wt)-Rluc and hLHR(wt)-GFP2. On the day of the experiment, the cells were incubated with buffer only or with hCG (1000 ng/ml final concentration) for 20 min at room temperature and then analyzed for BRET2. Data shown are the net BRET2 signals (mean ± range of duplicate determinations) as a function of the total amount of plasmid transfected taken from one experiment that is representative of at least four independent experiments.
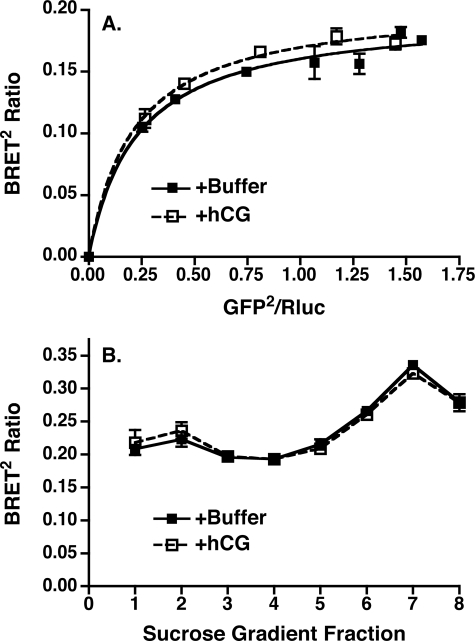
Although hCG was without effect on BRET2 ratios determined in intact cells, it remained possible that the BRET2 signal arising from intracellularly located hLHR dimers/oligomers might have obscured hCG-mediated effects on cell surface hLHR. Therefore, we examined the effects of hCG on total membrane lysates prepared from cells co-expressing a fixed concentration of hLHR(wt)-Rluc and increasing concentrations of hLHR(wt)-GFP2, focusing on the lower expression ratios of GFP2/ Rluc where it would be more likely to observe an agonist-mediated change in the BRET2 signal. As shown in Fig. 8A, incubation of the membranes with a saturating concentration of hCG for 15 min at 37 °C was without effect on the BRET2 ratios. Agonist addition was similarly without effect on the specific BRET2 ratios measured in membranes fractionated by sucrose density centrifugation, even in fraction 2, which is highly enriched in plasma membranes (Fig. 8B). It should be noted that 125I-hCG binding was readily detected in intact cells whose media contained sucrose at the same concentration as fraction 2. Therefore, at least for the purified plasma membrane fraction, it is highly unlikely that sucrose was adversely affecting the binding of hCG. Therefore, the data examining BRET2 arising from self-association of the WT hLHR in both living cells as well as isolated membranes suggest the absence of an effect of hCG on this phenomenon.
FIGURE 8.
Lack of effect of hCG on BRET2 analyses in isolated membranes. A, HEK293T cells were transiently transfected with a fixed concentration of hLHR(wt)-Rluc and increasing concentrations of hLHR(wt)-GFP2. On the day of the experiment, total cell membrane lysates were prepared from each group of cells. Membranes were incubated with buffer only or with hCG (1000 ng/ml final concentration) for 15 min at 37 °C and then analyzed for BRET2. Data shown are the net BRET2 signals (mean ± range of duplicate determinations) as a function of GFP2/Rluc expression taken from one experiment that is representative of at least three independent experiments. B, HEK293T cells were transiently transfected with hLHR(wt)-Rluc and hLHR(wt)-GFP2. On the day of the experiment, total cell membrane lysates were prepared and fractionated by sucrose density centrifugation. Fractions 1–8 were collected and incubated with buffer only or with hCG (1000 ng/ml final concentration) for 15 min at 37 °C and then analyzed for BRET2. Data shown are the net BRET2 signals (mean ± range of duplicate determinations) taken from one experiment that is representative of at least three independent experiments.
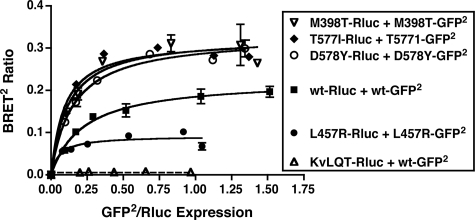
In an independent line of investigation, we also examined if there was a correlation between constitutively active and signaling inactive mutants of the hLHR and their propensity for homodimerization/oligomerization. If an increased or decreased activation state of the hLHR endowed the receptor with an altered propensity for homodimerization, then one would predict that this would be observed regardless of the subcellular localization of the receptor. Therefore, we chose to perform these analyses using BRET2 saturation curves in intact living cells. Within a given experiment comparing the BRET2 saturation curve of the WT hLHR to those of hLHR mutants, care was taken to ensure comparable expression levels for the different Rluc fusion proteins. As shown in Fig. 9, four different strong hLHR CAMs exhibited saturable BRET2 curves, with M398T, T577I, and D578Y displaying greater BRETmax values and L457R a decreased BRETmax compared with the WT hLHR. The BRETmax, however, reflects the distance between donor and acceptor molecules as well as the relative orientations between donor and acceptor (42, 51). Because one cannot assume that the orientations of the donors and acceptors are the same in the different hLHR constructs, it is not possible to infer much from differences between BRETmax values. The BRET50 (the acceptor to donor ratio yielding 50% of the BRETmax as derived from titration curves), however, has been proposed to reflect the relative affinity of donor and acceptor fusion proteins for each (42, 51). Although used initially to compare different GPCR homodimers and heterodimers in a given study (42), the validity of comparing BRET50 values between different constructs has since been questioned. Interpretation of the BRET50 may also be confounded by higher order GPCR oligomerization occurring in addition to dimerization. Therefore, although we report the BRET50 values for hLHR CAMs over several experiments (Table 1), we do so in the context just discussed. As such, the BRET50 values for T577I and D578Y were the same as WT, and those for M398T and L457R were ∼2-fold lower, suggesting a lack of correlation between activation and dimerization.
FIGURE 9.
Constitutively activating mutants of the hLHR do not exhibit an increased propensity to dimerize. For the hLHR(wt) or each of the hLHR activating mutants shown, HEK293T cells were transfected with a fixed amount of Rluc fusion protein (adjusted so that the expression of receptor-Rluc in the absence of receptor-GFP2 was the same for all constructs) and increasing concentrations of the corresponding GFP2 fusion protein. Data shown are the corrected BRET2 signals (mean ± range of duplicate determinations) as a function of GFP2/Rluc expression. The constructs shown were analyzed within the same experiment. The data shown are from one experiment that is representative of at least five independent experiments.
TABLE 1.
BRET50 values for activating and inactivating mutants of the hLHR
HEK293T cells were transiently transfected with Rluc and GFP2 fusion protein pairs of each of the hLHR constructs such that the expression of the Rluc fusion protein was constant while that of the GFP2 fusion protein was increased. Within a given experiment, the expression of the different Rluc fusion proteins was similar among the different donor-acceptor pairs. Data were plotted as the BRET2 ratio as a function of Rluc expression/GFP2 expression, and the BRET50 was taken as the KD value derived from a one-site binding curve. Data shown are the means ± S.E. of the indicated number of experiments. The BRET50 for homodimerization of the WT hLHR was calculated from only those experiments also containing curves examining homodimerization of activating and/or inactivating hLHR mutants.
| hLHR | BRET50 |
|---|---|
| WT | 0.225 ± 0.040 (n = 11) |
| M398T (constitutively active) | 0.104 ± 0.015 (n = 6)a |
| T577I (constitutively active) | 0.230 ± 0.070 (n = 6) |
| D578Y (constitutively active) | 0.271 ± 0.070 (n = 5) |
| L457R (constitutively active) | 0.091 ± 0.031 (n = 7)a |
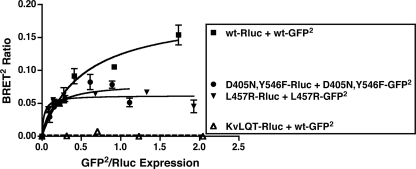
| D405N,Y546F (signaling inactive) | 0.161 ± 0.044 (n = 4) |
BRET50 values of mutant hLHRs that are statistically different from WT receptor (p < 0.05) are noted.
To examine the potential homodimerization/oligomerization of a signaling inactive hLHR mutant, we initially tested hLHR(K605R), which had previously been reported (using an alternate nomenclature as K583R) to be signaling inactive (33, 34). However, when compared with cells expressing the same cell surface density of WT hLHR, we found K605R responded to hCG with ∼30% of the maximal cAMP response of the WT hLHR. Therefore, our experiments were performed using a novel D405N,Y546F mutant, which we determined to have normal cell surface expression and hCG binding affinity but little or no hCG responsiveness.4 As shown in Fig. 10, the signaling inactive D405N,Y546F mutant can form homodimers, as evidenced by a saturable BRET2 titration curve. In fact, the BRET2 titration curve for the signaling inactive hLHR mutant is similar to that of the hLHR CAM L457R. From several experiments, the BRET50 for homodimerization of the signaling inactive mutant was found to be similar to the WT hLHR (Table 1). If one assumes that the BRET50 measure reflects relative affinities for receptor homodimerization, our results with the signaling inactive and constitutively active hLHR mutants would suggest a lack of correlation between the state of receptor activation and their propensity for homodimerization. Even if one were to take a more skeptical view of a comparison of BRET50 values between different constructs and removed those data from consideration, the results presented still demonstrate that both hLHR CAMs as well as a profoundly signaling impaired hLHR mutant constitutively homodimerize, thus arguing against dimerization/oligomerization of the hLHR being correlated with its activation state.
FIGURE 10.
A signaling impaired mutant of the hLHR does not exhibit a decreased propensity to dimerize. For the hLHR(wt) or signaling impaired hLHR(D405N,Y546F) mutant, HEK293T cells were transfected with a fixed amount of Rluc fusion protein (adjusted so that the expression of receptor-Rluc in the absence of receptor-GFP2 was the same for all constructs) and increasing concentrations of the corresponding GFP2 fusion protein. Data shown are the corrected BRET2 signals (mean ± range of duplicate determinations) as a function of GFP2/Rluc expression. The constructs shown were analyzed within the same experiment. The data shown are from one experiment that is representative of at least four independent experiments.
DISCUSSION
The results presented show that the hLHR can be detected by BRET2 in living cells as constitutive dimers. Cells co-expressing a fixed concentration of energy donor hLHR-Rluc and increasing concentrations of the energy acceptor hLHR-GFP2 gave rise to BRET2 ratios fit by a hyperbolic curve with a saturation plateau, results that are in agreement with those reported by Urizar et al. (47). Previously published studies in which differentially tagged forms of the hLHR were co-immunoprecipitated from unstimulated cells demonstrated self-associated forms of the hLHR that, based upon mobilities on SDS gels, were consistent in size with dimeric and oligomeric forms of the hLHR (39). The BRET2 data presented herein indicate that, at the very least, there is a specific self-association of two hLHR protomers into a dimeric complex under basal conditions in intact cells. Our BRET2 data do not permit the distinction between hLHR dimers and oligomers. That the hLHR homodimerization detected by BRET2 is not because of random protein interactions was also supported by the observation that the BRET2 occurred at many different concentrations of hLHR-Rluc/hLHR-GFP2 held at a fixed ratio and by the fact that no dimers were detected between hLHR-GFP2 and KvLQT1-Rluc.
Additional evidence supporting the constitutive expression of hLHR dimers/oligomers in living cells was obtained from hormone desorption assays. Although it had been previously appreciated that allosteric modulators could affect the binding of ligand to the orthosteric site on a GPCR (52), studies have further demonstrated the modulation of ligand binding between GPCR dimers (48, 53–57). Thus, the binding of ligand to the orthostatic site of one protomer within a GPCR dimer may allosterically affect the binding of ligand to the orthostatic site of the other protomer in the complex. Using an experimental paradigm described by Urizar et al. (47), we show herein that in hLHR-expressing cells the addition of hLH or hCG increases the rate of dissociation of prebound 125I-hLH, indicating an allosteric regulation of hLH binding within an hLHR dimer. In addition to providing additional independent evidence for the constitutive expression of hLHR dimers in living cells, the allosteric modulation of hLH binding demonstrates that hLHR dimers are expressed on the cell surface and that they are functional.
Using sucrose gradient centrifugation to fractionate membranes isolated from cells co-expressing hLHR energy donor and acceptor fusion proteins, we have also been able to show by both BRET2 analyses and by Western blotting that constitutively expressed hLHR dimers/oligomers are localized to both the plasma membrane and ER. These results indicate that hLHR dimers/oligomers form early in the biosynthetic process and are most likely transported to the plasma membrane as constitutive dimers. The hLHR, therefore, can be added to the growing list of GPCRs that have been shown to homo- or heterodimerize within the ER (28, 59–63). Our studies lend further support to the hypothesis that GPCR dimerization may be an obligate process necessary for the transport of the GPCR to the plasma membrane (64, 65).
Although the majority of the hLHR detected on Western blots of unfractionated and fractionated membranes is in the dimeric/oligomeric form, some hLHR is also detected in a monomeric form as well. Interestingly, less monomeric hLHR is detected in fractions enriched for the ER compared with plasma membrane-enriched fractions (compare fractions 2 and 8 in the Western blots of Fig. 4). These data may indicate that although all or most of the hLHR is transported from the ER to the cell surface as dimers/oligomers, a fraction of the hLHR dimers/oligomers dissociate into monomers once in the plasma membrane. Alternatively, however, there may be a differential sensitivity of hLHR monomers/dimers in the plasma membrane versus the ER to dissociation by SDS. Although the ability to detect hLHR dimers/oligomers on Western blots suggests that they are in large part resistant to dissociation by SDS (at the room temperature conditions we typically use because boiling the hLHR in Laemmli buffer causes nonspecific aggregation), this does not rule out the possibility that a small fraction of dimers/oligomers may dissociate as a result of SDS treatment. Nor is it unreasonable to postulate that differing lipid compositions of cellular membranes may affect the ability of SDS to dissociate preformed hLHR dimers/oligomers or that different populations of other interacting proteins or chaperones might affect dimer stability in the different compartments. Clearly, further studies are required to examine these possibilities in more depth before a meaningful interpretation of the data can be made.
Additional support for the dimerization of the hLHR initiating in the ER is derived from our data showing specific homodimerization, as determined by BRET2 saturation curves in living cells, of the hLHR mutants S616Y and A593P. These are the gene products of naturally occurring loss-of-function mutations of the hLHR gene (66–68). These two mutants have been shown to be retained by the cell's quality control system, presumably due to misfolding of the mutants, which in turn results in decreased cell surface expression of the mutant receptors (39, 50). Despite the misfolding of the S616Y and A593P mutants, at least some of the mutant receptor proteins undergo specific homodimerization within the ER, as indicated by the BRET2 data presented herein. These results are in agreement with previous data from our laboratory showing that high molecular weight complexes consistent with dimers and oligomers of immature hLHR are detected on Western blots prepared from cells expressing each of these misfolded mutants (39). We had previously reported that the S616Y and A593P mutants, when co-expressed with the WT hLHR, have a dominant-negative effect on the cell surface expression of the WT receptor and that this correlated with the physical association of each of the mutants with the WT hLHR as determined by co-immunoprecipitation (39). The current BRET2 studies examining this phenomenon indicate a specific and saturable association between each of the misfolded receptors and the WT hLHR. The current BRET2 data are in agreement with the previous co-immunoprecipitation data, providing further support for the premise that the heterodimerization of the misfolded hLHR mutant with the WT receptor in the ER underlies the subsequent retention of the WT hLHR within the ER by cellular quality control mechanisms. These findings further suggest that, once associated, only homo- or heterodimers composed of properly folded receptor protomers would be permitted to exit the ER for further processing and eventual transport to the plasma membrane.
There has been a great deal of attention given to the question of whether or not the dimerization status of GPCR homodimers is affected by activation of the receptor. Despite this, there appears to be little consensus in the literature (10). Some of the apparent discrepancies may have arisen because the data derived from many of the experimental approaches used to address this question are open to different interpretations, and these were not necessarily taken into account (10, 69). In light of this, it has been argued that complementary experimental approaches may be required to more reliably characterize agonist effects on GPCR dimerization. Our previous studies had indicated an increased ratio of hLHR dimer/oligomer relative to the monomer observed on Western blots of detergent-solubilized extracts of hCG-treated cells as compared with untreated cells (39). As had been discussed, these data could be interpreted to suggest that hCG increases dimerization/oligomerization of the hLHR or that hCG stabilizes preformed dimers/oligomers to detergent solubilization. Therefore, in this study alternative methods were used to address whether the activation of the hLHR affected its dimerization. In one approach we asked whether the addition of hCG resulted in a change in the net BRET2 ratio for hLHR dimerization/oligomerization. Because a change in BRET2 ratio obtained from a single set of donor and acceptor concentrations could reflect a change in the amount of dimers formed and/or from conformational changes altering the orientations of the donor and acceptor relative to each other (10, 40, 41), we performed saturation curves on cells that had been treated with or without hCG. Bouvier and co-workers (42) have proposed that under these conditions the BRET50 reflects the relative affinity of two GPCR protomers to dimerize with each other. Because the saturation curves and BRET50 values were nearly identical, these data suggest that hCG does not affect the propensity of the hLHR to dimerize. Using a similar experimental approach, Urizar et al. (47) had also concluded that agonist binding had no effect on dimerization of the structurally related hTSHR. The caveat to this experimental design, however, is that only the cell surface receptor would be accessible to hormone. Therefore, if there were an agonist-induced change in cell surface receptor dimerization, it may be difficult to discern above the high background of BRET2 derived from ER-localized receptor dimers/oligomers, which would remain unchanged with agonist treatment of intact cells. Therefore, we also examined the effects of hCG on BRET2 ratios measured in membranes isolated from cells expressing hLHR energy donor and acceptor fusion proteins. Even in highly purified plasma membranes, no effects of hCG on BRET2 were observed. In addition, we examined if constitutively active and signaling inactive mutants of the hLHR had altered propensities for homodimerization as determined by BRET2 saturation curves in living cells. Even though our results reflect the total pool of cellular receptor rather than those localized to the plasma membrane, it should not matter as any changes in the dimerization/oligomerization of the hLHR mutants because of their activation state would be expected to be present in the ER as well, where dimerization is initiated. The data presented showed homodimerization of four different strong hLHR CAMs (M398T, T577I, L457R, and D578Y) as well as a signaling inactive mutant, where the BRET2 saturation curve of the signaling inactive mutant was quite similar to one of the activating mutants. These data further suggest a lack of correlation between hLHR activation and homodimerization/oligomerization.
Based on our data on the effects of hCG on hLHR dimerization/oligomerization from our previous studies (39) and the experiments presented herein, we conclude the following points. (i) The hLHR is expressed constitutively (on the cell surface as well as in the ER) as dimers/oligomers. (ii) hCG binding to cells stabilizes preformed hLHR dimers/oligomers to detergent solubilization. (iii) Neither agonist stimulation nor mutation-induced activation of the hLHR results in an altered propensity of the hLHR to homodimerize. Previous studies have suggested that upon agonist treatment, the LHR on the plasma membrane becomes aggregated (37). Our findings that hCG does not affect the dimerization/oligomerization of the hLHR do not rule out possible effects of agonist on large scale clustering of the receptor. Rather, they suggest that dimerization per se of the hLHR is not enhanced by agonist treatment. In this respect, our findings would be in accord with those reported of Jastrzebska et al. (70), who showed that the degree of oligomerization of rhodopsin does not correlate with the magnitude of Gt activation. Rather, there was a correlation between the extent of rhodopsin oligomerization and the kinetics of Gt activation.
Our observations on the constitutive expression of hLHR dimers/oligomers are not in agreement with reports by another group who, using FRET analyses, detected little or no energy transfer LHR molecules under basal conditions (38, 71). Because RET is dependent on the relative concentrations of energy donor and acceptor and their experiments were performed with a single donor/acceptor ratio, it is possible that they may not have had the appropriate conditions to detect self-association of the LHR under basal conditions (41). Also, given that RET depends on the relative orientations between the energy donor and acceptor, false negative results may arise even when two proteins are in a physical complex if the conformations are not compatible for detecting energy transfer (10). Using BRET2 saturation experiments, which entail a wide range of donor/acceptor ratios, it has been independently reported by both our group in this study and that of Urizar et al. (47) that specific hLHR dimerization/oligomerization under basal conditions can be observed. As shown herein, constitutive hLHR dimerization/oligomerization was detected by BRET2 in plasma membrane as well as ER cellular compartments. Constitutive hLHR dimerization/oligomerization has also been observed under basal conditions examining co-immunoprecipitation of differentially tagged hLHRs (39). Furthermore, the high molecular weight complexes observed on Western blots of membrane lysates (present study) or detergent-solubilized extracts (39) are of the same mobilities as those brought down by co-immunoprecipitation of differentially tagged hLHRs, strongly suggesting that the high molecular weight complexes seen on Western blots represent constitutively expressed hLHR dimers/oligomers. Therefore, there are several lines of evidence that support the presence of constitutive hLHR dimers/oligomers. That the hLHR is expressed as dimers/oligomers under basal conditions is consistent with reports on the structurally related follitropin receptor (72) and thyrotropin receptor (47) and several other GPCRs (27–30).
Our conclusion that dimerization/oligomerization of the hLHR is not affected by the activation state of the hLHR is also in conflict with studies published by others (38, 71). As noted above, these other investigators detected very low FRET between LHR molecules under basal conditions. However, their studies reported an increased efficiency of energy transfer after hCG treatment (38, 71). Based upon the change in FRET (using one ratio of energy donor and acceptor), they concluded that hCG increased the dimerization of the hLHR. Using photobleaching FRET analyses, it was also reported that the energy transfers in the hLHR CAMs D578Y, D578H, and D578Y were greater than that of the hLHR(wt), and that the energy transfer of a signaling impaired rLHR mutant was less than that of the rLHR(wt) (35, 38). Therefore, they concluded that these data further showed a correlation between the activation of the hLHR and its increased homodimerization. With respect to hCG treatment, changes in a FRET or BRET signal arising from a single ratio of donor/acceptor may be due not only to changes in the dimerization of the GPCR but also to conformational changes of the receptor (10, 40, 41). However, this alternative interpretation of their data was not considered. Also, in the earlier studies, cells were incubated with hCG for 1 h at 37 °C, conditions that promote internalization of the agonist-occupied receptor and its subsequent degradation and/or recycling (73). Therefore, it is possible that changes in receptor localization and receptor numbers may have affected the resulting FRET signals. With respect to the apparent discrepancies regarding our results on activating and inactivating LHR mutants and those previously reported, there are several points to consider. First, because in vitro (74) and in silico (45, 58, 75–79) data suggest that the conformations of hLHR CAMs are different from the WT hLHR, one cannot assume that, within a dimeric complex, the distances between and/or orientations of an energy donor and acceptor on constitutively active or signaling inactive mutants are identical to those on the WT hLHR. Furthermore, energy transfer is also dependent on the ratio of donor to acceptor. There are no data in the FRET studies on the hLHR, however, to indicate whether the ratios between the expression levels of energy donors and acceptors for the different constructs were the same or not. Therefore, the greater FRET signals derived from cells expressing activating hLHR mutants and decreased FRET signals of the signaling inactive mutant as compared with the WT may have arisen from differences in the expression ratios of donor and acceptors, the relative orientations of the donor and acceptor to each other, differences in the distances between energy donor and acceptor within a mutant LHR dimer and the WT LHR dimer, and/or the relative propensity of the mutants or the WT receptor to dimerize (10, 40–42).
In summary, this study demonstrates the obligate and constitutive expression of hLHR dimers/oligomers. Dimerization/oligomerization occurs early in the biosynthetic pathway, with hLHR dimers/oligomers detected in the ER as well as the plasma membrane under basal conditions. Misfolded mutants that are retained in the ER similarly undergo specific homodimerization and, when co-expressed with the WT hLHR, heterodimerize with the WT receptor, thus accounting for the dominant-negative effect of misfolded mutants on the cell surface expression of the WT hLHR (39). Using a variety of experimental approaches, our studies further suggest that the dimerization/oligomerization of the hLHR is not affected by the activation state of the receptor. Although the functional basis for hLHR homodimerization/oligomerization remains elusive, the ability of the hLHR to dimerize/oligomerize may have consequences in the context of hLHR interactions with other GPCRs, a question that we are currently examining.
Acknowledgments
We thank Nathan Johnson and Nathalie Ethier for contributions in obtaining preliminary data for the study. We also thank Dr. Mark Stamnes (University of Iowa) for helpful discussions and designing the conditions described for subcellular fractionation by sucrose gradient centrifugation.
This work was supported, in whole or in part, by National Institutes of Health Grants DK068614 and HD22196 (to D. L. S.). This work was also supported by grants from the Canadian Institutes of Health Research (to T. E. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: hLHR, human lutropin receptor; LHR, lutropin receptor; LH, lutropin; hCG, human chorionic gonadotropin; GFP, green fluorescent protein; D-PBS, Dulbecco's phosphate-buffered saline; GPCR, protein-coupled receptor; BRET, bioluminescence resonance energy transfer; ER, endoplasmic reticulum; CAM, constitutively active mutant; FRET, fluorescent resonance energy transfer; RET, resonance energy transfer.
M. Zhang, R. Guan, T. Hébert, and D. L. Segaloff, submitted for publication.
References
- 1.Latronico, A., and Segaloff, D. (1999) Am. J. Hum. Genet. 65 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Themmen, A. P. N., and Huhtaniemi, I. T. (2000) Endocr. Rev. 21 551–583 [DOI] [PubMed] [Google Scholar]
- 3.Ascoli, M., Fanelli, F., and Segaloff, D. L. (2002) Endocr. Rev. 23 141–174 [DOI] [PubMed] [Google Scholar]
- 4.Vassart, G., Pardo, L., and Costagliola, S. (2004) Trends Biochem. Sci. 29 119–126 [DOI] [PubMed] [Google Scholar]
- 5.Hirakawa, T., and Ascoli, M. (2003) Endocrinology 144 3872–3878 [DOI] [PubMed] [Google Scholar]
- 6.Ascoli, M. (2007) Mol. Cell. Endocrinol. 260–262 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulenger, S., Marullo, S., and Bouvier, M. (2005) Trends Pharmacol. Sci. 26 131–137 [DOI] [PubMed] [Google Scholar]
- 8.Milligan, G. (2007) Biochim. Biophys. Acta 1768 825–835 [DOI] [PubMed] [Google Scholar]
- 9.Dalrymple, M. B., Pfleger, K. D., and Eidne, K. A. (2008) Pharmacol. Ther. 118 359–371 [DOI] [PubMed] [Google Scholar]
- 10.Hebert, T. E., Gales, C., and Rebois, R. V. (2006) Cell Biochem. Biophys. 45 85–109 [DOI] [PubMed] [Google Scholar]
- 11.Mellado, M., Rodriguez-Frade, J. M., Vila-Coro, A. J., Fernandez, S., Martin de Ana, A., Jones, D. R., Toran, J. L., and Martinez, A. C. (2001) EMBO J. 20 2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocheville, M., Lange, D. C., Kumar, U., Patel, S. C., Patel, R. C., and Patel, Y. C. (2000) Science 288 154–157 [DOI] [PubMed] [Google Scholar]
- 13.AbdAlla, S., Lother, H., and Quitterer, U. (2000) Nature 407 94–98 [DOI] [PubMed] [Google Scholar]
- 14.Breit, A., Gagnidze, K., Devi, L. A., Lagace, M., and Bouvier, M. (2006) Mol. Pharmacol. 70 686–696 [DOI] [PubMed] [Google Scholar]
- 15.El-Asmar, L., Springael, J. Y., Ballet, S., Andrieu, E. U., Vassart, G., and Parmentier, M. (2005) Mol. Pharmacol. 67 460–469 [DOI] [PubMed] [Google Scholar]
- 16.Ellis, J., Pediani, J. D., Canals, M., Milasta, S., and Milligan, G. (2006) J. Biol. Chem. 281 38812–38824 [DOI] [PubMed] [Google Scholar]
- 17.Gomes, I., Gupta, A., Filipovska, J., Szeto, H. H., Pintar, J. E., and Devi, L. A. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 5135–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes, I., Jordan, B. A., Gupta, A., Trapaidze, N., Nagy, V., and Devi, L. A. (2000) J. Neurosci. 20 RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilairet, S., Bouaboula, M., Carriere, D., Le Fur, G., and Casellas, P. (2003) J. Biol. Chem. 278 23731–23737 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Frade, J. M., Vila-Coro, A. J., de Ana, A. M., Albar, J. P., Martinez, A. C., and Mellado, M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3628–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vila-Coro, A. J., Mellado, M., de Ana, A. M., Lucas, P., del Real, G., Martinez, A. C., and Rodriguez-Frade, J. M. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 3388–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vila-Coro, A. J., Rodriguez-Frade, J. M., De Ana, A. M., Moreno-Ortiz, M. C., Martinez, A. C., and Mellado, M. (1999) FASEB J. 13 1699–1710 [PubMed] [Google Scholar]
- 23.AbdAlla, S., Zaki, E., Lother, H., and Quitterer, U. (1999) J. Biol. Chem. 274 26079–26084 [DOI] [PubMed] [Google Scholar]
- 24.Kroeger, K. M., Hanyaloglu, A. C., Seeber, R. M., Miles, L. E., and Eidne, K. A. (2001) J. Biol. Chem. 276 12736–12743 [DOI] [PubMed] [Google Scholar]
- 25.Horvat, R. D., Roess, D. A., Nelson, S. E., Barisas, B. G., and Clay, C. M. (2001) Mol. Endocrinol. 15 695–703 [DOI] [PubMed] [Google Scholar]
- 26.Latif, R., Graves, P., and Davies, T. F. (2002) J. Biol. Chem. 277 45059–45067 [DOI] [PubMed] [Google Scholar]
- 27.Issafras, H., Angers, S., Bulenger, S., Blanpain, C., Parmentier, M., Labbe-Jullie, C., Bouvier, M., and Marullo, S. (2002) J. Biol. Chem. 277 34666–34673 [DOI] [PubMed] [Google Scholar]
- 28.Terrillon, S., Durroux, T., Mouillac, B., Breit, A., Ayoub, M. A., Taulan, M., Jockers, R., Barberis, C., and Bouvier, M. (2003) Mol. Endocrinol. 17 677–691 [DOI] [PubMed] [Google Scholar]
- 29.Romano, C., Yang, W. L., and O'Malley, K. L. (1996) J. Biol. Chem. 271 28612–28616 [DOI] [PubMed] [Google Scholar]
- 30.Bai, M., Trivedi, S., and Brown, E. M. (1998) J. Biol. Chem. 273 23605–23610 [DOI] [PubMed] [Google Scholar]
- 31.Kusuda, S., and Dufau, M. L. (1988) J. Biol. Chem. 263 3046–3049 [PubMed] [Google Scholar]
- 32.Crine, P., Aubry, M., and Potier, M. (1984) Ann. N. Y. Acad. Sci. 438 224–237 [DOI] [PubMed] [Google Scholar]
- 33.Lee, C.-W., Ji, I., Ryu, K.-S., Song, Y.-S., Conn, P. M., and Ji, H. (2002) J. Biol. Chem. 277 15795–15800 [DOI] [PubMed] [Google Scholar]
- 34.Ji, I., Lee, C. W., Song, Y. S., Conn, P. M., and Ji, T. H. (2002) Mol. Endocrinol. 16 1299–1308 [DOI] [PubMed] [Google Scholar]
- 35.Roess, D. A., Horvat, R. D., Munnelly, H., and Barisas, B. G. (2000) Endocrinology 141 4518–4523 [DOI] [PubMed] [Google Scholar]
- 36.Roess, D. A., and Smith, S. M. (2003) Biol. Reprod. 69 1765–1770 [DOI] [PubMed] [Google Scholar]
- 37.Hunzicker-Dunn, M., Barisas, G., Song, J., and Roess, D. A. (2003) J. Biol. Chem. 278 42744–42749 [DOI] [PubMed] [Google Scholar]
- 38.Lei, Y., Hagen, G. M., Smith, S. M., Liu, J., Barisas, G., and Roess, D. A. (2007) Mol. Cell. Endocrinol. 260 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao, Y. X., Johnson, N. B., and Segaloff, D. L. (2004) J. Biol. Chem. 279 5904–5914 [DOI] [PubMed] [Google Scholar]
- 40.Milligan, G., and Bouvier, M. (2005) FEBS J. 272 2914–2925 [DOI] [PubMed] [Google Scholar]
- 41.Pfleger, K. D., and Eidne, K. A. (2006) Nat. Meth. 3 165–174 [DOI] [PubMed] [Google Scholar]
- 42.Mercier, J.-F., Salahpour, A., Angers, S., Breit, A., and Bouvier, M. (2002) J. Biol. Chem. 277 44925–44931 [DOI] [PubMed] [Google Scholar]
- 43.Rebois, R. V., Robitaille, M., Gales, C., Dupre, D. J., Baragli, A., Trieu, P., Ethier, N., Bouvier, M., and Hebert, T. E. (2006) J. Cell Sci. 119 2807–2818 [DOI] [PubMed] [Google Scholar]
- 44.Ascoli, M., and Puett, D. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, M., Mizrachi, D., Fanelli, F., and Segaloff, D. L. (2005) J. Biol. Chem. 280 26169–26176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenworthy, A. K., and Edidin, M. (1998) J. Cell Biol. 142 69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urizar, E., Montanelli, L., Loy, T., Bonomi, M., Swillens, S., Gales, C., Bouvier, M., Smits, G., Vassart, G., and Costagliola, S. (2005) EMBO J. 24 1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Springael, J. Y., Urizar, E., Costagliola, S., Vassart, G., and Parmentier, M. (2007) Pharmacol. Ther. 115 410–418 [DOI] [PubMed] [Google Scholar]
- 49.Fabritz, J., Ryan, S., and Ascoli, M. (1998) Biochemistry (Mosc.) 37 664–672 [DOI] [PubMed] [Google Scholar]
- 50.Mizrachi, D., and Segaloff, D. L. (2004) Mol. Endocrinol. 18 1768–1777 [DOI] [PubMed] [Google Scholar]
- 51.Bouvier, M., Heveker, N., Jockers, R., Marullo, S., and Milligan, G. (2007) Nat. Meth. 4 3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christopoulos, A., and Kenakin, T. (2002) Pharmacol. Rev. 54 323–374 [DOI] [PubMed] [Google Scholar]
- 53.Wong, H. M., Sole, M. J., and Wells, J. W. (1986) Biochemistry (Mosc.) 25 6995–7008 [DOI] [PubMed] [Google Scholar]
- 54.Wreggett, K. A., and Wells, J. W. (1995) J. Biol. Chem. 270 22488–22499 [DOI] [PubMed] [Google Scholar]
- 55.Chidiac, P., Green, M. A., Pawagi, A. B., and Wells, J. W. (1997) Biochemistry (Mosc.) 36 7361–7379 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, Y., Moriyoshi, E., Tsuchiya, D., and Jingami, H. (2004) J. Biol. Chem. 279 35526–35534 [DOI] [PubMed] [Google Scholar]
- 57.Milligan, G., and Smith, N. J. (2007) Trends Pharmacol. Sci. 28 615–620 [DOI] [PubMed] [Google Scholar]
- 58.Feng, X., Muller, T., Mizrachi, D., Fanelli, F., and Segaloff, D. L. (2008) Endocrinology 149 1705–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salahpour, A., Angers, S., Mercier, J. F., Lagace, M., Marullo, S., and Bouvier, M. (2004) J. Biol. Chem. 279 33390–33397 [DOI] [PubMed] [Google Scholar]
- 60.Wilson, S., Wilkinson, G., and Milligan, G. (2005) J. Biol. Chem. 280 28663–28674 [DOI] [PubMed] [Google Scholar]
- 61.Zhou, F., Filipeanu, C. M., Duvernay, M. T., and Wu, G. (2006) Cell. Signal. 18 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pidasheva, S., Grant, M., Canaff, L., Ercan, O., Kumar, U., and Hendy, G. N. (2006) Hum. Mol. Genet. 15 2200–2209 [DOI] [PubMed] [Google Scholar]
- 63.Herrick-Davis, K., Weaver, B. A., Grinde, E., and Mazurkiewicz, J. E. (2006) J. Biol. Chem. 281 27109–27116 [DOI] [PubMed] [Google Scholar]
- 64.Terrillon, S., and Bouvier, M. (2004) EMBO Rep. 5 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milligan, G. (2008) Br. J. Pharmacol. 153 S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kremer, H., Kraaij, R., Toledo, S. P. A., Post, M., Fridman, J. B., Hayashida, C. Y., van Reen, M., Milgrom, E., Ropers, H.-H., Mariman, E., Themmen, A. P. N., and Brunner, H. G. (1995) Nat. Genet. 9 160–164 [DOI] [PubMed] [Google Scholar]
- 67.Toledo, S. P. A., Brunner, H. G., Kraaij, R., Post, M., Dahia, P. L. M., Hayashida, C. Y., Kremer, H., and Themmen, A. P. N. (1996) J. Clin. Endocrinol. Metab. 81 3850–3854 [DOI] [PubMed] [Google Scholar]
- 68.Latronico, A. C., Anasti, J., Arnhold, I. J. P., Rapaport, R., Mendonca, B. B., Bloise, W., Castro, M., Tsigos, C., and Chrousos, G. P. (1996) New Engl. J. Med. 334 507–512 [DOI] [PubMed] [Google Scholar]
- 69.Bai, M. (2004) Cell. Signal. 16 175–186 [DOI] [PubMed] [Google Scholar]
- 70.Jastrzebska, B., Fotiadis, D., Jang, G. F., Stenkamp, R. E., Engel, A., and Palczewski, K. (2006) J. Biol. Chem. 281 11917–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horvat, R. D., Barisas, B. G., and Roess, D. A. (2001) Mol. Endocrinol. 15 534–542 [DOI] [PubMed] [Google Scholar]
- 72.Thomas, R. M., Nechamen, C. A., Mazurkiewicz, J. E., Muda, M., Palmer, S., and Dias, J. A. (2007) Endocrinology 148 1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kishi, M., Liu, X., Hirakawa, T., Reczek, D., Bretscher, A., and Ascoli, M. (2001) Mol. Endocrinol. 15 1624–1635 [DOI] [PubMed] [Google Scholar]
- 74.Jaquette, J., and Segaloff, D. L. (2002) Mol. Cell. Endocrinol. 194 211–215 [DOI] [PubMed] [Google Scholar]
- 75.Fanelli, F. (2000) J. Mol. Biol. 296 1333–1351 [DOI] [PubMed] [Google Scholar]
- 76.Fanelli, F., Themmen, A. P., and Puett, D. (2001) IUBMB Life 51 149–155 [DOI] [PubMed] [Google Scholar]
- 77.Angelova, K., Fanelli, F., and Puett, D. (2002) J. Biol. Chem. 277 32202–32213 [DOI] [PubMed] [Google Scholar]
- 78.Fanelli, F., Verhoef-Post, M., Timmerman, M., Zeilemaker, A., Martens, J. W., and Themmen, A. P. (2004) Mol. Endocrinol. 18 1499–1508 [DOI] [PubMed] [Google Scholar]
- 79.Zhang, M., Tao, Y. X., Ryan, G. L., Feng, X., Fanelli, F., and Segaloff, D. L. (2007) J. Biol. Chem. 282 25527–25539 [DOI] [PubMed] [Google Scholar]