Abstract
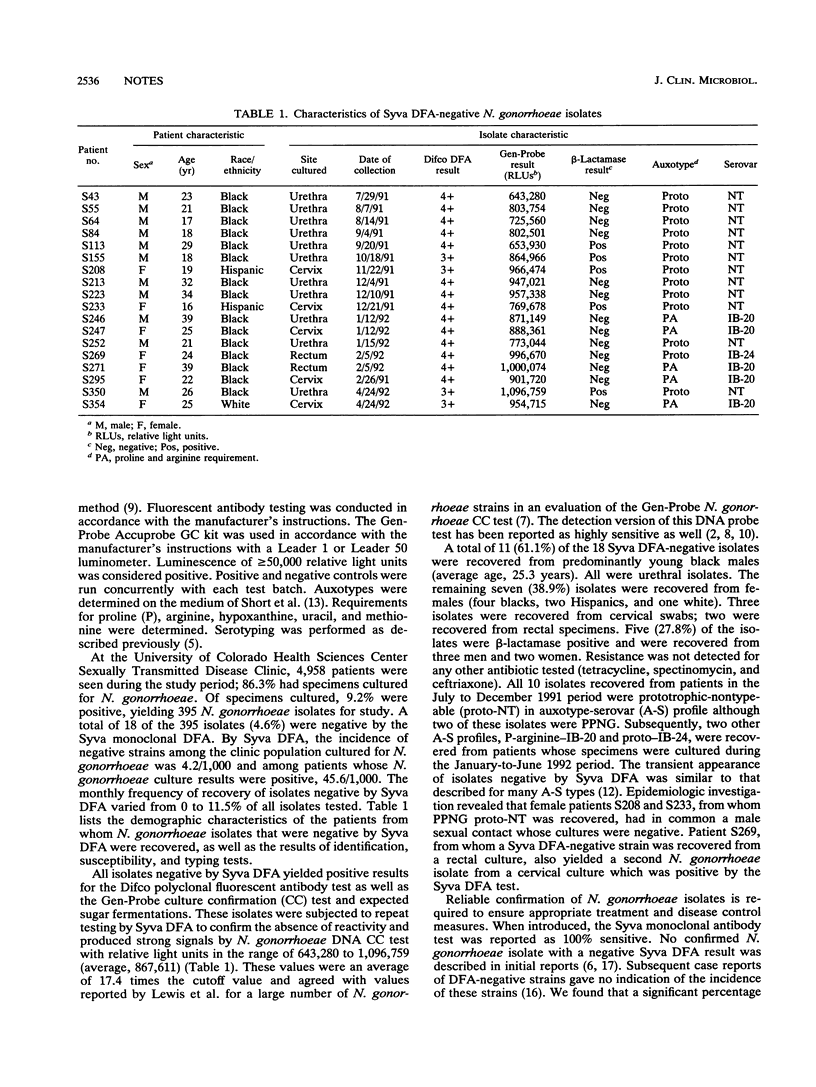
To determine the accuracy of the Syva (Palo Alto, Calif.) direct fluorescent-antibody (DFA) test in comparison with the Gen-Probe (San Diego, Calif.) Accuprobe culture confirmation test, we tested 395 isolates of Neisseria gonorrhoeae from cultures obtained from patients attending a sexually transmitted disease clinic from 1 July 1991 through 30 June 1992. All isolates were tested for DFA reactivity with a polyclonal reagent (Difco Laboratories, Detroit, Mich.) and a monoclonal reagent (Syva, Inc., direct specimen test) and for specific molecular probe reactivity by the Gen-Probe Accuprobe culture confirmation test for N. gonorrhoeae. The 395 isolates gave positive results for the Gen-Probe culture confirmation test and the Difco polyclonal direct specimen test. However, 18 (4.6%) of the isolates were negative for N. gonorrhoeae by the Syva DFA test. With the exception of six beta-lactamase-positive isolates, all isolates that were negative by Syva DFA were sensitive to penicillin, tetracycline, spectinomycin, and ceftriaxone by disk-diffusion susceptibility testing. Auxotyping and serotyping studies indicated that strains negative by Syva DFA consisted of several variants. The frequency of N. gonorrhoeae isolates showing negative results by Syva DFA in this patient population ranged from 0 to 11.5%/month. Laboratories using only the Syva DFA test for confirmation of N. gonorrhoeae may incur a significant risk of misidentification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hare M. J. Comparative assessment of microbiological methods for the diagnosis of gonorrhea in women. Br J Vener Dis. 1974 Dec;50(6):437–441. doi: 10.1136/sti.50.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosein I. K., Kaunitz A. M., Craft S. J. Detection of cervical Chlamydia trachomatis and Neisseria gonorrhoeae with deoxyribonucleic acid probe assays in obstetric patients. Am J Obstet Gynecol. 1992 Sep;167(3):588–591. doi: 10.1016/s0002-9378(11)91554-3. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Gavan T. L., Thornsberry C., Fuchs P. C., Gerlach E. H., Knapp J. S., Murray P., Washington J. A., 2nd Standardization of disk diffusion and agar dilution susceptibility tests for Neisseria gonorrhoeae: interpretive criteria and quality control guidelines for ceftriaxone, penicillin, spectinomycin, and tetracycline. J Clin Microbiol. 1989 Dec;27(12):2758–2766. doi: 10.1128/jcm.27.12.2758-2766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S. Historical perspectives and identification of Neisseria and related species. Clin Microbiol Rev. 1988 Oct;1(4):415–431. doi: 10.1128/cmr.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Ehret J. M., Tanino T. T., Van der Pol B., Handsfield H. H., Jones R. B., Judson F. N., Hook E. W., 3rd Fluorescent monoclonal antibody for confirmation of Neisseria gonorrhoeae cultures. J Clin Microbiol. 1987 Dec;25(12):2388–2390. doi: 10.1128/jcm.25.12.2388-2390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. S., Kranig-Brown D., Trainor D. A. DNA probe confirmatory test for Neisseria gonorrhoeae. J Clin Microbiol. 1990 Oct;28(10):2349–2350. doi: 10.1128/jcm.28.10.2349-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Biega R., Evancoe A., McCarthy L., Slivienski L., Kirkwood M. Evaluation of culture and the Gen-Probe PACE 2 assay for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in endocervical specimens transported to a state health laboratory. J Clin Microbiol. 1992 May;30(5):1162–1166. doi: 10.1128/jcm.30.5.1162-1166.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panke E. S., Yang L. I., Leist P. A., Magevney P., Fry R. J., Lee R. F. Comparison of Gen-Probe DNA probe test and culture for the detection of Neisseria gonorrhoeae in endocervical specimens. J Clin Microbiol. 1991 May;29(5):883–888. doi: 10.1128/jcm.29.5.883-888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock H. M. Evaluation of methods for the rapid identification of Neisseria gonorrhoeae in a routine clinical laboratory. J Clin Microbiol. 1976 Jul;4(1):19–21. doi: 10.1128/jcm.4.1.19-21.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian S. K., Knapp J. S. Molecular epidemiology of gonorrhea. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S49–S55. doi: 10.1128/cmr.2.suppl.s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short H. B., Ploscowe V. B., Weiss J. A., Young F. E. Rapid method for auxotyping multiple strains of Neisseria gonorrhoeae. J Clin Microbiol. 1977 Sep;6(3):244–248. doi: 10.1128/jcm.6.3.244-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thin R. N. Immunofluorescent method for diagnosis of gonorrhoea in women. Br J Vener Dis. 1970 Feb;46(1):27–30. doi: 10.1136/sti.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitkins S. A., Anderson R. D. Failure of the fluorescent antibody reaction to identify penicillinase-producing gonococci. J Clin Pathol. 1982 Feb;35(2):215–218. doi: 10.1136/jcp.35.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. T. Fluorescent-antibody-negative penicillinase-producing Neisseria gonorrhoeae. J Clin Microbiol. 1989 Aug;27(8):1885–1886. doi: 10.1128/jcm.27.8.1885-1886.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. D., Cartwright G. Fluorescent monoclonal antibody compared with carbohydrate utilization for rapid identification of Neisseria gonorrhoeae. J Clin Microbiol. 1988 Feb;26(2):293–296. doi: 10.1128/jcm.26.2.293-296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


