Abstract
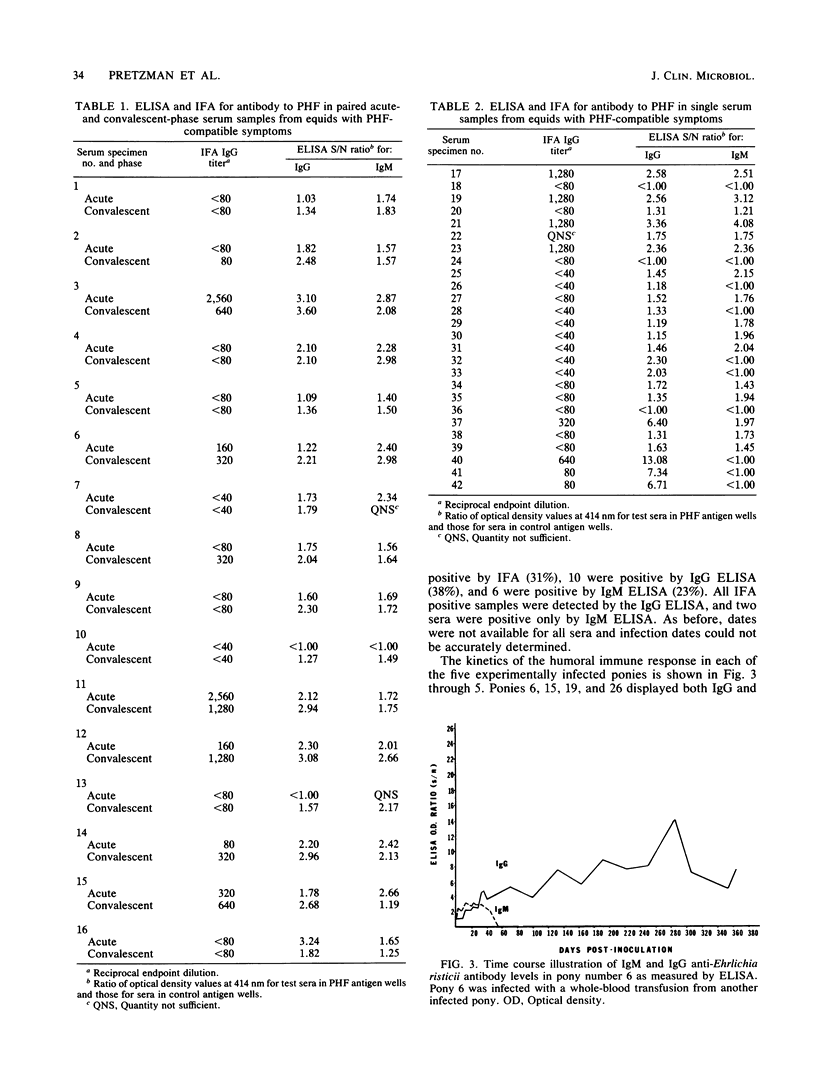
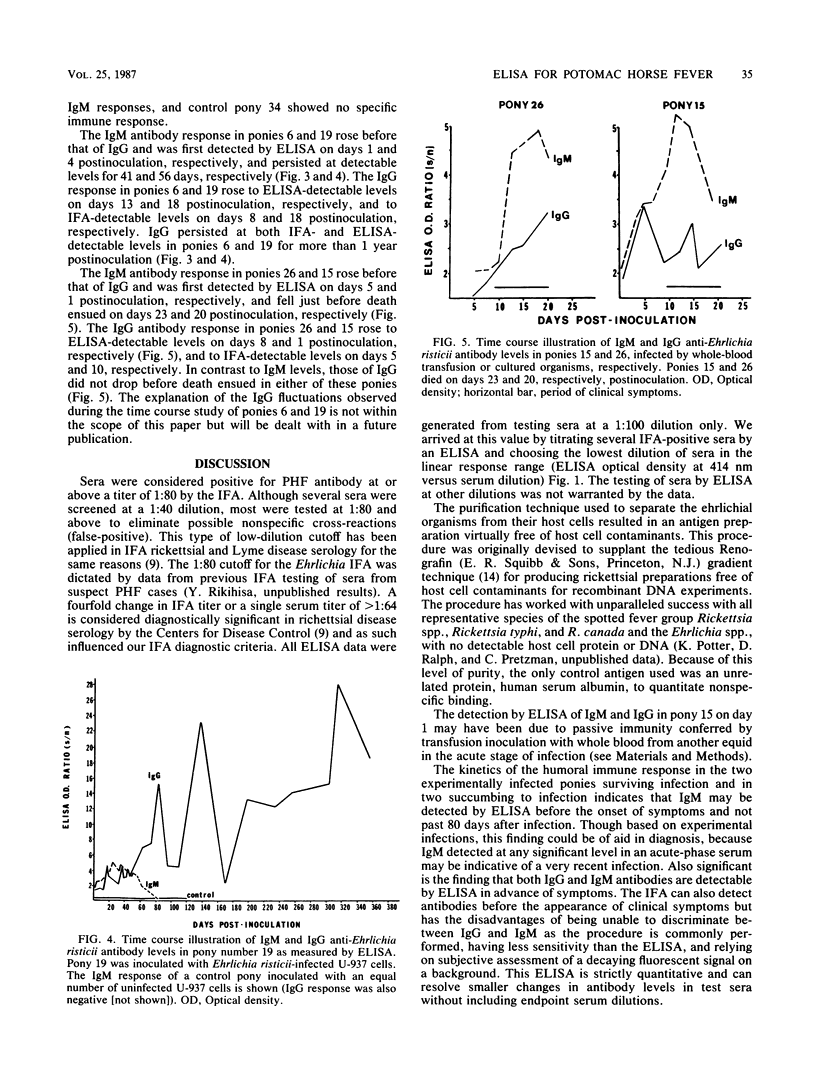
An enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G (IgG) and IgM in natural and experimental infections of equids with Ehrlichia risticii was developed. Ehrlichial organisms purified from an infected mouse macrophage cell line were used as the antigen. IgM was separated from serum IgG by the expedient of spun-column chromatography, allowing the use of an indirect ELISA for quantitation of both IgG and IgM in the test sera. Among 16 paired sera from horses exhibiting clinical signs of Potomac horse fever, 8 were positive by the indirect fluorescent-antibody test (IFA), 11 were positive by the IgG ELISA, and 8 were positive by the IgM ELISA. All IFA-positive specimens were positive by the IgG ELISA, which appeared to be more sensitive than the IFA. In all cases, the IgG ELISA alone would have sufficed for diagnosis when acute- and convalescent-phase sera were available. When 26 single acute- or convalescent-phase serum samples were tested, the IFA detected 8, the IgG ELISA detected 10, and the IgM ELISA detected 6 positive serum specimens. The kinetics of IgG and IgM responses as determined by ELISA in two experimentally infected ponies which survived infection and challenges revealed that specific IgM was short-lived, falling to undetectable levels by day 60 postinoculation, whereas specific IgG persisted for more than 1 year. IgM and IgG were detected as early as days 1 and 10, respectively, postinoculation. The results suggest that the ELISA is more sensitive than the IFA and that the IgM ELISA may provide a means for early diagnosis of Potomac horse fever at or before the onset of clinical signs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carroll J. F., Schmidtmann E. T. American dog tick (Acari: Ixodidae), summer activity on equine premises enzootic for Potomac horse fever in south-central Maryland. J Econ Entomol. 1986 Feb;79(1):62–66. doi: 10.1093/jee/79.1.62. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Newhouse V. F., Shepard C. C., Redus M. D., Tzianabos T., McDade J. E. A comparison of the complement fixation, indirect fluorescent antibody, and microagglutination tests for the serological diagnosis of rickettsial diseases. Am J Trop Med Hyg. 1979 Mar;28(2):387–395. doi: 10.4269/ajtmh.1979.28.387. [DOI] [PubMed] [Google Scholar]
- Pretzman C. I., Jr, Ralph D., Mishler L., Bodine J. Rapid separation of IgM from whole serum using spun column chromatography. J Immunol Methods. 1985 Nov 7;83(2):301–307. doi: 10.1016/0022-1759(85)90251-0. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B., Cordes D. Rickettsial link with acute equine diarrhoea. Vet Rec. 1984 Oct 13;115(15):390–390. doi: 10.1136/vr.115.15.390-a. [DOI] [PubMed] [Google Scholar]
- Ristic M., Holland C. J., Dawson J. E., Sessions J., Palmer J. Diagnosis of equine monocytic ehrlichiosis (Potomac horse fever) by indirect immunofluorescence. J Am Vet Med Assoc. 1986 Jul 1;189(1):39–46. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


