Abstract
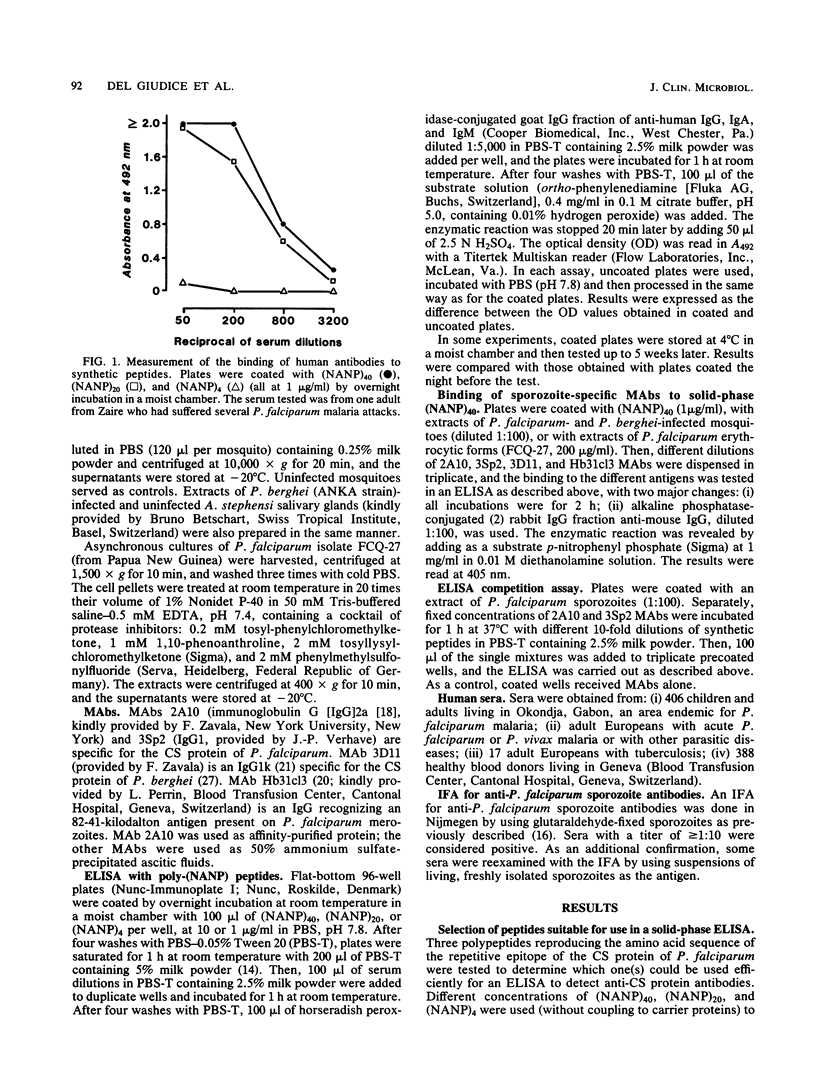
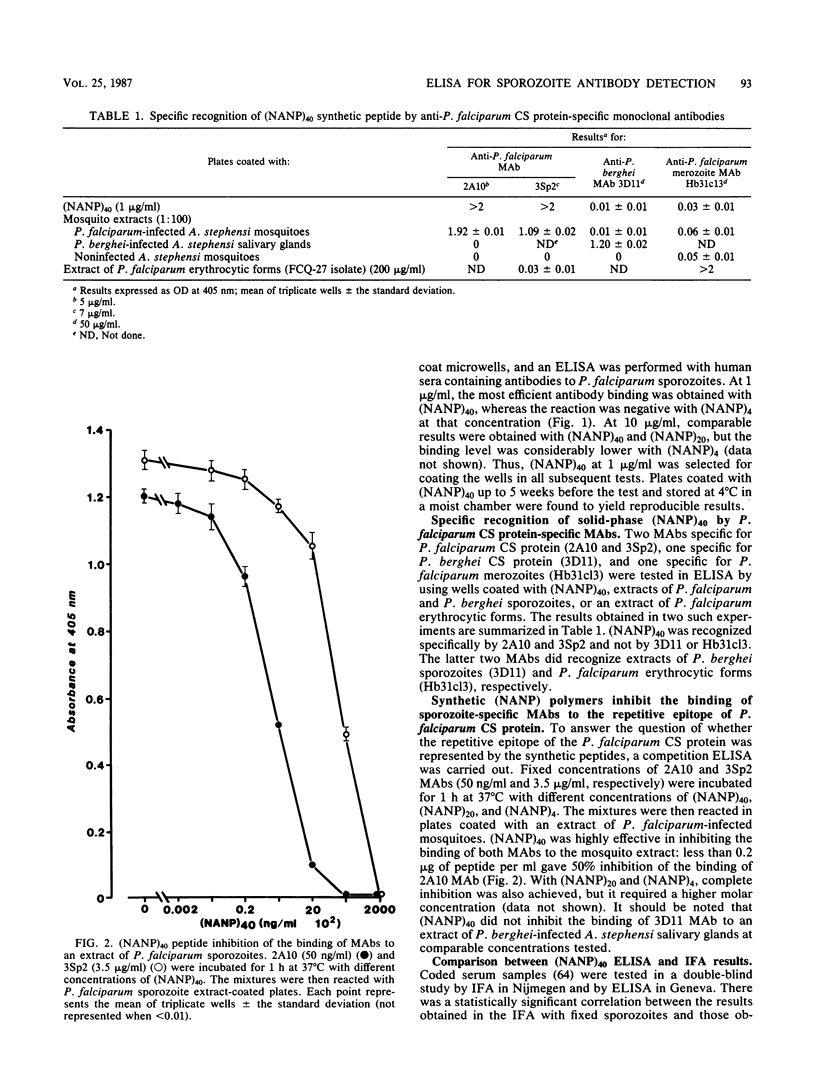
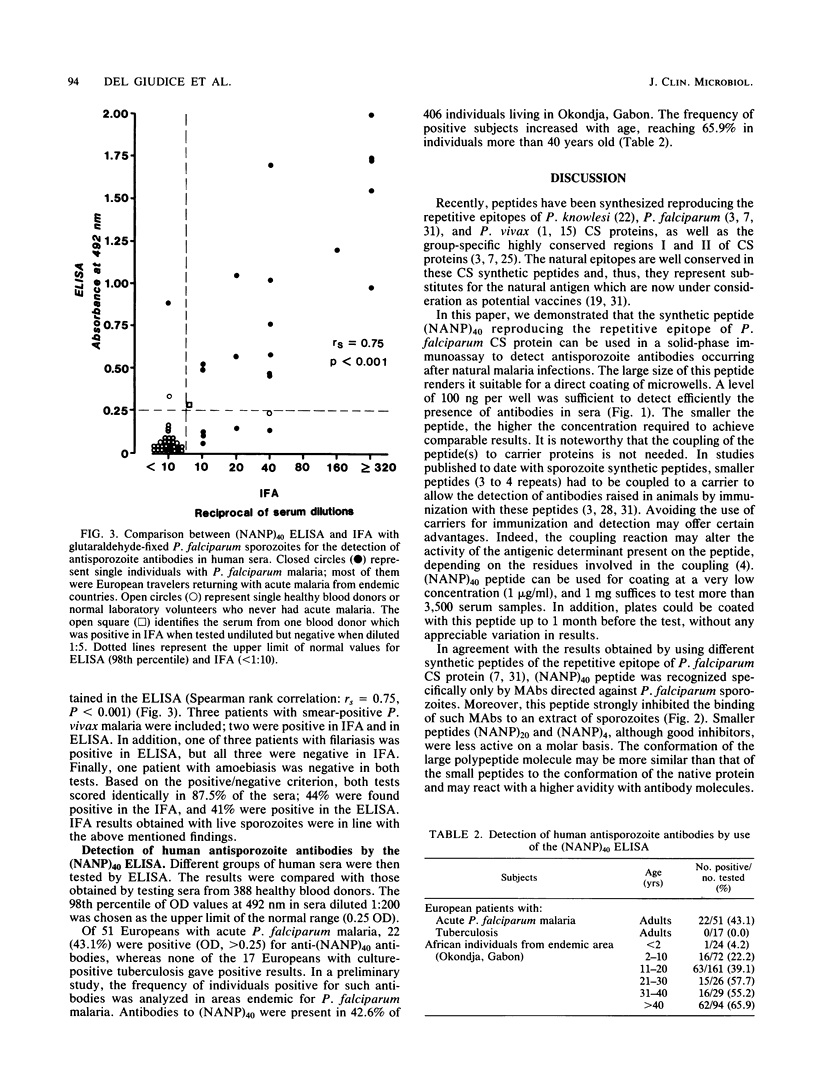
A large peptide consisting of about 40 (Asn-Ala-Asn-Pro) repeats of Plasmodium falciparum circumsporozoite protein, (NANP)40, was synthesized. It was recognized specifically by monoclonal antibodies produced against P. falciparum sporozoites. Moreover, this peptide strongly inhibited the binding of such monoclonal antibodies to antigens present in a sporozoite extract. The (NANP)40 peptide was employed without any carrier to develop an enzyme-linked immunosorbent assay to detect sporozoite-specific serum antibodies arising after natural malaria infections. Antibodies were detected in a high percentage (43.1%) of European patients suffering from acute P. falciparum malaria and in Africans living in an area of Gabon endemic for malaria. In the latter group, the frequency of antisporozoite antibodies increased with age, reaching 65.9% in individuals more than 40 years old. There was a significant correlation between the results obtained with an immunofluorescence assay with glutaraldehyde-fixed sporozoites and those obtained by enzyme-linked immunosorbent assay with (NANP)40. Therefore, such synthetic peptides representing the repetitive epitope of P. falciparum circumsporozoite protein can be used for the detection of antisporozoite antibodies and for the epidemiological studies required to obtain base-line data concerning the immune status of individuals before their participation in a sporozoite vaccine trial.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Tam J. P., Nussenzweig V., Nussenzweig R. S., Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985 Nov 15;230(4727):815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Cochrane A. H., Gwadz R. W., Ojo-Amaize E., Hii J., Nussenzweig V., Nussenzweig R. S. Antigenic diversity of the circumsporozoite proteins in the Plasmodium cynomolgi complex. Mol Biochem Parasitol. 1985 Jan;14(1):111–124. doi: 10.1016/0166-6851(85)90110-0. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Enea V., Arnot D., Schmidt E. C., Cochrane A., Gwadz R., Nussenzweig R. S. Circumsporozoite gene of plasmodium cynomolgi (Gombak):cDNA cloning and expression of the repetitive circumsporozoite epitope. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7520–7524. doi: 10.1073/pnas.81.23.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Nardin E. H., Tharavanij S., Schwartz A. L., Nussenzweig R. S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984 Feb;132(2):909–913. [PubMed] [Google Scholar]
- Hollingdale M. R., Zavala F., Nussenzweig R. S., Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol. 1982 Apr;128(4):1929–1930. [PubMed] [Google Scholar]
- Hope I. A., Hall R., Simmons D. L., Hyde J. E., Scaife J. G. Evidence for immunological cross-reaction between sporozoites and blood stages of a human malaria parasite. Nature. 1984 Mar 8;308(5955):191–194. doi: 10.1038/308191a0. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Mackay M., Hyde J. E., Goman M., Scaife J. The gene for an exported antigen of the malaria parasite Plasmodium falciparum cloned and expressed in Escherichia coli. Nucleic Acids Res. 1985 Jan 25;13(2):369–379. doi: 10.1093/nar/13.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Lal A. A., de la Cruz V. F., Miller L. H., Maloy W. L., Charoenvit Y., Beaudoin R. L., Guerry P., Wistar R., Jr, Hoffman S. L. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science. 1985 Dec 20;230(4732):1381–1383. doi: 10.1126/science.2416057. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., McGregor I. A., Bryan J. H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979 Nov 2;206(4418):597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig V., Nussenzweig R. S., Collins W. E., Harinasuta K. T., Tapchaisri P., Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982 Jul 1;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin E., Gwadz R. W., Nussenzweig R. S. Characterization of sporozoite surface antigens by indirect immunofluorescence: detection of stage- and species-specific antimalarial antibodies. Bull World Health Organ. 1979;57 (Suppl 1):211–217. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Malaria vaccine against sporozoites? Ann Inst Pasteur Immunol. 1985 Nov-Dec;136D(3):301–312. doi: 10.1016/s0769-2625(85)80115-x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger D. H., Cochrane A. H., Gwadz R. W., Godson G. N., Melton R., Nussenzweig R. S., Nussenzweig V. Structure of an immunodominant epitope of the circumsporozoite surface protein of Plasmodium knowlesi. Biochemistry. 1984 Nov 6;23(23):5665–5670. doi: 10.1021/bi00318a043. [DOI] [PubMed] [Google Scholar]
- Sharma S., Svec P., Mitchell G. H., Godson G. N. Diversity of circumsporozoite antigen genes from two strains of the malarial parasite Plasmodium knowlesi. Science. 1985 Aug 23;229(4715):779–782. doi: 10.1126/science.4023712. [DOI] [PubMed] [Google Scholar]
- Tapchaisri P., Chomcharn Y., Poonthong C., Asavanich A., Limsuwan S., Maleevan O., Tharavanij S., Harinasuta T. Anti-sporozoite antibodies induced by natural infection. Am J Trop Med Hyg. 1983 Nov;32(6):1203–1208. doi: 10.4269/ajtmh.1983.32.1203. [DOI] [PubMed] [Google Scholar]
- Vergara U., Ruiz A., Ferreira A., Nussenzweig R. S., Nussenzweig V. Conserved group-specific epitopes of the circumsporozoite proteins revealed by antibodies to synthetic peptides. J Immunol. 1985 May;134(5):3445–3448. [PubMed] [Google Scholar]
- Weber J. L., Hockmeyer W. T. Structure of the circumsporozoite protein gene in 18 strains of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;15(3):305–316. doi: 10.1016/0166-6851(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Nussenzweig R. S., Potocnjak P., Nussenzweig V., Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Masuda A., Graves P. M., Nussenzweig V., Nussenzweig R. S. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol. 1985 Oct;135(4):2790–2793. [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Masuda A. Synthetic peptides as antigens for the detection of humoral immunity to Plasmodium falciparum sporozoites. J Immunol Methods. 1986 Oct 23;93(1):55–61. doi: 10.1016/0022-1759(86)90432-1. [DOI] [PubMed] [Google Scholar]


