Abstract
Activation of G protein–coupled receptor by dopamine and hypoxia-generated reactive oxygen species promote Na+,K+-ATPase endocytosis. This effect is clathrin dependent and involves the activation of protein kinase C (PKC)-ζ and phosphorylation of the Na+,K+-ATPase α-subunit. Because the incorporation of cargo into clathrin vesicles requires association with adaptor proteins, we studied whether phosphorylation of adaptor protein (AP)-2 plays a role in its binding to the Na+,K+-ATPase α-subunit and thereby in its endocytosis. Dopamine induces a time-dependent phosphorylation of the AP-2 μ2 subunit. Using specific inhibitors and dominant-negative mutants, we establish that this effect was mediated by activation of the adaptor associated kinase 1/PKC-ζ isoform. Expression of the AP-2 μ2 bearing a mutation in its phosphorylation site (T156A) prevented Na+,K+-ATPase endocytosis and changes in activity induced by dopamine. Similarly, in lung alveolar epithelial cells, hypoxia-induced endocytosis of Na+,K+-ATPase requires the binding of AP-2 to the tyrosine-based motif (Tyr-537) located in the Na+,K+-ATPase α-subunit, and this effect requires phosphorylation of the AP-2 μ2 subunit. We conclude that phosphorylation of AP-2 μ2 subunit is essential for Na+,K+-ATPase endocytosis in response to a variety of signals, such as dopamine or reactive oxygen species.
Keywords: clathrin, dopamine, hypoxia, kidney tubule cells, lung alveolar cells
The Na+,K+-ATPase migrates to and from the plasma membrane in response to receptor signals. The endocytic traffic of Na+,K+-ATPase molecules could be triggered by the activation of plasma membrane receptors in renal tubule epithelia (1, 2), by mitochondrial reactive oxygen species (ROS) generated by the lung alveolar epithelium in response to hypoxia (3), or by cardiac glycosides in the renal epithelia through their direct binding to the Na+,K+-ATPase molecule (4). Whereas in the renal tubules Na+,K+-ATPase endocytosis in response to dopamine or parathyroid hormone receptor signals represents an important mechanism controlling sodium and phosphate excretion (5), in the alveolar epithelia it may represent a defense mechanism to reduce energy (ATP) consumption in response to hypoxia, albeit resulting in decreased alveolar fluid clearance, which leads to pulmonary edema and impairment of gas exchange.
The intracellular signaling mechanisms involved in Na+,K+-ATPase endocytosis have been partially identified. Phosphorylation of the Na+,K+-ATPase α-subunit constitutes the triggering mechanism that initiates its endocytosis in response to dopamine-parathyroid hormone (2, 6, 7) and hypoxia (3). In these tissues (renal proximal tubules and lung alveolar epithelia), the protein kinase C (PKC)-ζ isoform is responsible for phosphorylating the Na+,K+-ATPase α-subunit. In kidney cells its activation is achieved via a GPCR signal, whereas in alveolar cells during hypoxia it is mediated by mitochondria-generated ROS (3).
Clathrin-dependent traffic is considered to be an important pathway used by vesicles that move along specific compartments within the cell (8). Relevant to this process is its initiation, in which specific proteins located within a particular domain of the cell are selectively incorporated into clathrin-coated vesicles and transported in response to specific stimuli. This process is accomplished by adaptor proteins that recruit the cargo at the plasma membrane and facilitate its integration into clathrin pits (8). Several adaptor isoforms have been described, and they participate in cargo-recruitment processes originated at the plasma membrane or within intracellular organelles.
Binding of the adaptor protein (AP)-2 μ2 subunit to the Na+,K+-ATPase α-subunit has been mapped to the consensus motif of the latter (YLEL) located within the main cytoplasmic loop (9). Little is known, however, about the mechanism that recruits AP-2 μ2 to the Na,K-ATPase. The purpose of this study was to determine whether dopamine and/or ROS induced a further covalent modification in the AP-2 μ2 subunit and whether this is necessary for AP-2 to bind and interact with the Na+,K+-ATPase during its endocytosis.
MATERIALS AND METHODS
Reagents and Antibodies
The following antibodies were used: the Na+,K+-ATPase was immunoprecipitated using an antibody against its α-subunit (α5) developed by Dr. Fambrough and obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biological Sciences. Western blots were performed with an antibody provided by M. J. Caplan (Yale University, New Haven, CT). The antibody against the μ2 subunit of AP-2 was provided by J. Bonifacino (NIH). The AP-2 αC antibody was purchased from Upstate Biotechnology (Buffalo, NY). The antibody against phosphothreonine residues was purchased from Sigma (St. Louis, MO), and the antibody against the hemagglutinin (HA) tag was from Covance (Princeton, NJ). Dopamine (DA) and staurosporine were purchased from Sigma. Rp-cAMP was from Boehringer (Basel, Switzerland). T-butyl-H2O2 was purchased from Sigma. Sulfo-NHS-Biotin was from Pierce (Rockford, IL). All other reagents were of analytical grade.
Plasmids
Site-directed mutagenesis of the AP-2 μ2 subunit was performed directly on pcDNA3.HA-μ2 by using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The T165 mutant was generated by exchanging nucleotides as follows: ACC versus GCC. The respective oligonucleotides were purchased from Poligo (Paris, France). All mutations were verified by DNA sequence analysis. Transfection of the PKC-ζ wild-type and dominant-negative mutant was performed as previously described (10).
Cell Culture and Transfection
Opossum kidney (OK) cells were cultured in 100-mm tissue culture dishes (∼ 80% confluent) containing 5 ml Dulbecco's modified Eagle's medium/10% bovine serum. Transient transfection with the different AP-2 μ2 was performed using lipofection (11). The cDNA carried a tag (HA), and an antibody against this tag was used to monitor expression levels during transient transfection.
To generate stable clones, A549 cells (ATCC CCL 185) were plated in 6-cm plates at 2–3 × 105 cells/plate and transfected with 2 μg of plasmid DNA (pcDNA3.HA-μ2 or pcDNA3.HA-μ2 T156 A) by using lipofection (LipofectAmine 2000; Invitrogen, Carlsbad, CA) as indicated by the manufacturer. Transfectants were selected in the presence of 400 μg/ml geneticin (G418; Mediatech, Herndon, VA). Individual colonies were isolated using cloning cylinders (PGC Scientifics, Frederick, MO), and the resulting cell lines were propagated in complete Dulbecco's modified Eagle's medium supplemented with G418.
Generation of the Hypoxic Condition
A549 cells and permanent cell lines of A549 cells expressing a GFP-tagged rat α1 subunit (GFP-A549) (9) were incubated under normoxic (16% O2, 5% CO2, 79% N2) and hypoxic (1.5% O2, 93.5% N2, and 5% CO2) conditions in a humidified workstation (in vivo O2; Ruskinn Technologies, Kansas City, MO) for continuous monitoring of the chamber oxygen tension.
Determination of Na+,K+-ATPase Activity
OK cells were incubated with DA 1 μM or vehicle (Hanks' medium), and Na+,K+-ATPase transport activity was determined as previously described (12) in intact cells as the rate of rubidium transport (nmol Rb/mg protein/min) in the presence or absence of 5 mM ouabain.
Cell Surface Biotinylation
After treatment with agonists or vehicle, the incubation was stopped by placing the samples on ice. The medium changed to ice-cold biotinylation buffer (10 mM Tris-HCl [pH 7.5], 2 mM CaCl2, 150 mM NaCl, 1.5 mg/ml Sulfo-NHS-Biotin), and the cells were incubated for 1 h at 4°C. The cells were scraped in immunoprecipitation (IP) buffer (20 mM Tris, 2 mM EDTA, 2 mM EGTA, 30 mM sodium pyrophosphate [pH 7.3]) containing a protease inhibitor cocktail frozen in liquid nitrogen, thawed rapidly, probe sonicated twice by immersion for 10 s in an ice-water bath, and frozen and thawed again. The cell suspension was centrifuged at 14,000 × g at 4°C for 5 min. After the supernatants were transferred to clean tubes, 1% Triton X-100 and 0.2% SDS were added. The Na+,K+-ATPase antibody (α5) was added and incubated for 1 h at 4°C with end-over-end shaking. Protein A/G agarose, prewashed three times with PBS and once with IP buffer containing 1% Triton X-100, was added and incubated for 4 h. The pellet was washed four times with IP buffer containing 1% Triton X-100 and 0.1% SDS and once with 50 mM Tris-HCl (pH 7.4) and was resuspended in Laemmli sample buffer (13). Electrophoresis, Western blot analysis with extravidin (Sigma), and densitometric analysis were performed.
Phosphorylation of the AP-2 μ2 Subunit
Nontransfected OK cells and cells transfected transiently with the wild-type or the T156A AP-2 μ2 subunit cDNA was studied 48 h after transfection. The culture medium was replaced by Hanks' medium 30 min before incubation with DA or vehicle. The incubation period was terminated by placing the samples on ice and adding homogenization medium. The AP-2 μ2 subunit was immunoprecipitated as a complex with an AP-2 αC antibody (500 μg protein/5 μg antibody) as described previously (14). The presence of the μ2 subunit in the immunoprecipitated material was corroborated by Western blot with a polyclonal antibody (1:100). Phosphorylation of the immunoprecipitated AP-2 μ2 subunit was examined by Western blot using an antibody against a phosphothreonine residue (1:500).
Coimmunoprecipitation
OK cells grown in Petri dishes (10 cm) were incubated in Hanks' medium (pH 7.4) for 30 min at room temperature before incubation in the presence or absence of 1 μM DA for 5 min at room temperature. After the media were removed, the cells were homogenized in 500 μl immunoprecipitation buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 30 mM NaF, 2 mM EDTA, 2 mM EGTA, 1% Triton X-100, 10 μg/ml aprotinin, 1 mM PMSF, 5 μg/ml pepstatin A, 5 μg/ml leupeptin, 5 μg/ml antipain) by passing through a 27.5-G needle for 20 times and ground in a motor pestle for 30 s. The homogenates were precleared by centrifugation at 800 × g for 10 min, and the supernatants were incubated in 40 μl A/G agarose (Amersham Biosciences AB, Uppsala, Sweden) with rotation for 1 h at 4°C. The A/G agarose beads were removed by centrifugation at 2,000 × g for 1 min. Aliquots (600 μg of protein) were incubated overnight at 4°C with 70 μl of a Na+,K+-ATPase antibody (α5) and the simultaneous addition of excess protein A/G-agarose beads (40 μl) for 2 h. We have efficiently immunoprecipitated the Na+,K+-ATPase α-subunit with the α5 antibody despite this technique being described as a more efficient tool under denaturing conditions (15). The beads were washed with immunoprecipitation buffer four times and analyzed by SDS-PAGE using the Laemmli buffer system (13). Proteins were transferred to polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences AB), and Western blots were performed using antibodies against the Na+,K+-ATPase α-subunit and the hemagglutinin (HA).
Miscellaneous
All incubations with 1 μM DA (diluted in Hanks' vehicle) were performed at room temperature. Protein content was determined according to Bradford (16) using a commercial reagent (BioRad, Richmond, CA). Western blots were developed with an ECL Plus kit (Amersham Pharmacia Biotech, Uppsala, Sweden). Quantitation of the Western blots was performed as described (1).
Statistical Analysis
Statistical analysis of the data was performed with the unpaired Student's t test. P values < 0.05 were considered significant.
RESULTS
Phosphorylation of AP-2 by Dopamine
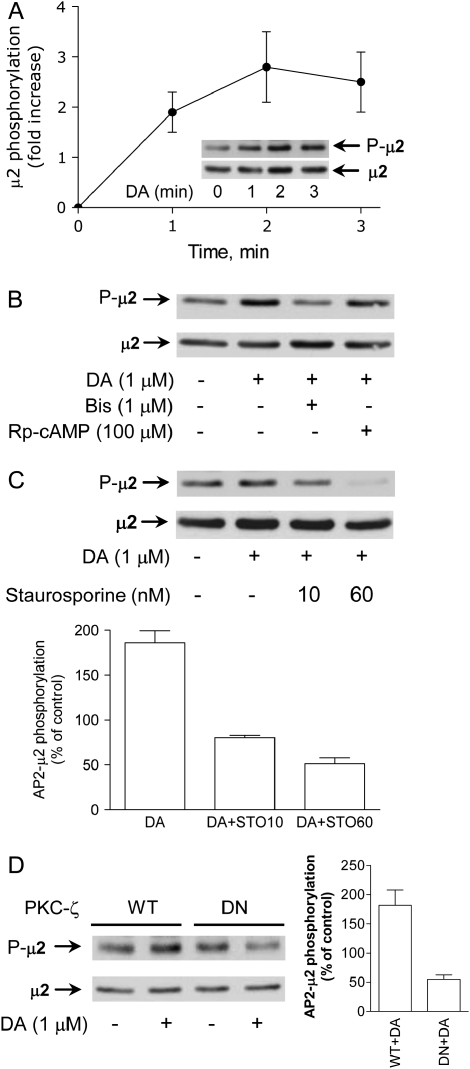
Clathrin-dependent endocytosis of Na+,K+-ATPase molecules requires its binding to AP-2 (8). It has been demonstrated in cell-free systems and in intact cells that AP-2 undergoes phosphorylation in its μ2 subunit (17, 18) and that this effect is required for cargo recognition during transferrin receptor endocytosis. In our studies AP-2 (μ2 subunit) phosphorylation in OK cells increased in a time-dependent manner in response to DA (Figure 1A). Several protein kinase inhibitors were used to search for the identity of the kinase responsible for AP-2 μ2 phosphorylation. In the presence of a cAMP-dependent kinase inhibitor (Rp-cAMP), DA efficiently phosphorylated the AP-2 μ2 subunit; however, this effect was blocked by 1 μM bisindolylmaleimide (Figure 1B). Although the concentration of bisindolylmaleimide is likely to be specific for PKC-ζ (19), we tested the effect of another PKC inhibitor. Staurosporine, at concentrations that would likely affect all PKC isoforms except the PKC-ζ (20), also blocked the increase of AP-2 μ2 subunit phosphorylation induced by DA (Figure 1C). It has recently been reported that in vitro phosphorylation of AP-2 could be under the control of an adaptor kinase (AAK1) (21, 22), and in vitro experiments have demonstrated that staurosporine at concentrations within the nM range inhibits AAK1. The lack of AAK1-specific inhibitors and specific dominant-negative modulators prevented a more conclusive inclusion of AAK1 in this process. Further proof that a simultaneous activation of PKC-ζ (the kinase isoform activated by DA during inhibition of Na+,K+-ATPase activity) (12) was involved in μ2 phosphorylation was provided by the ability of a PKC-ζ dominant-negative mutant (10) to block the DA-dependent phosphorylation of μ2 (Figure 1D).
Figure 1.
Effect of DA on AP-2 μ2 phosphorylation. (A) OK cells incubated with DA for different periods of time. The AP-2 μ2 subunit phosphorylation was examined by Western blot using a phosphothreonine antibody in the immunoprecipitated material with an AP-2 αC antibody. The amount of immunoprecipitated μ2 subunit was established in the same blot using a μ2 antibody (dilution 1:50). A representative Western blot (inset) and the quantitative analysis are shown (n = 3). Closed circles represent the mean ± SE. (B) Phosphorylation of AP-2 μ2 in response to DA (1 μM for 2 min at 23°C) was evaluated as described previously in the presence (30 min before DA) or absence of bisindolylmaleimide (Bis) (1 μM) or Rp-cAMP (100 μM). Representative Western blots are shown (n = 2). (C) PKC-dependent phosphorylation of AP-2 μ2 in response to DA (1 μM for 2 min at 23°C) was evaluated as described previously in the presence (30 min before DA) or absence of staurosporine (STO) (10 and 60 nM). Representative Western blot (upper panel) and quantitative analysis of three experiments (lower panel). Bars represent the mean ± SE. (D) The effect of DA (1 μM for 2 min at 23°C) on μ2 phosphorylation in response to DA was examined in OK cells transiently expressing the wild-type PKC-ζ isoform or a dominant-negative (DN) mutant. A representative Western blot (left panel) and quantitative analysis of three experiments (right panel) are shown. Bars represent the mean ± SE.
Phosphorylation of AP-2 Is Essential for Na+,K+-ATPase Endocytosis
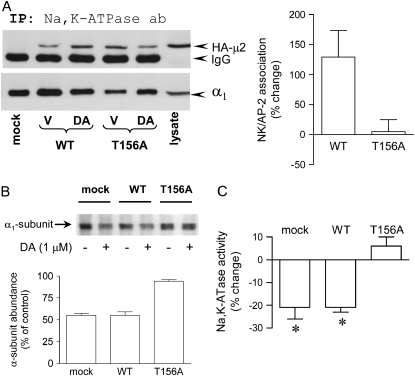
The functional relevance of μ2 phosphorylation for Na+,K+-ATPase endocytosis in response to DA was examined in cells transiently expressing the μ2 subunit in which Thr-156 (the putative phosphorylation site [18]) has been replaced by Ala (T156A). Expression of this mutant does not affect basal-stimulated transferrin receptor endocytosis, but it does affect temperature-stimulated transferrin receptor endocytosis. Similarly, in our studies it did not affect basal Na+,K+-ATPase activity (a reflection of copies at the plasma membrane). Control experiments indicated that the μ2 constructs carrying the HA tag were efficiently expressed in OK cells (Figure 2A) and that it incorporates into the AP-2 complex because the HA-μ2 could be immunoprecipitated with an AP-2 antibody (data not shown). DA increased the association of wild-type but not the T156A AP-2 μ2 subunit with the Na+,K+-ATPase α-subunit (Figure 2A).
Figure 2.
Functional relevance of AP-2 μ2 subunit phosphorylation on Na+,K+-ATPase endocytosis. (A) The AP-2 μ2 constructs bearing an HA tag (wild type [WT] or mutant [T156A]) were coimmunoprecipitated with a Na+,K+-ATPase α-subunit antibody (α5), and the HA-μ2 subunit was identified by Western blot with an HA antibody from cells treated with or without DA (1 μM for 2.5 min at room temperature). Representative blot (left panel) and quantitation (right panel) of four experiments (mean ± SE). For quantitation, the amount of coimmunoprecipitated HA-μ2 was corrected for the amount of Na+,K+-ATPase α-subunit immunoprecipitated, and the percent change was established from those ratios. (B) Na+,K+-ATPase α-subunit abundance in the plasma membrane (cell surface biotinylation) in response to DA (5 min) was examined in OK cells mock transfected (mock) or transiently expressing the μ2 cDNA wild type (WT) or carrying a mutation in its phosphorylation site (T156A). A representative Western blot (upper panel) and the quantitative analysis (lower panel) are shown. Bars represent the mean ± SD (n = 2). (C) Na+,K+-ATPase activity in response to DA (5 min) was examined in OK cells mock transfected (mock) or transiently expressing the μ2 cDNA WT or carrying a mutation in its phosphorylation site (T156A). Na+,K+-ATPase activity was measured as ouabain-sensitive rubidium transport and expressed as percent change. Each bar represents the mean ± SD (n = 3). *P < 0.05.
DA promoted the endocytosis of Na+,K+-ATPase (i.e., it reduced abundance at the plasma membrane) in nontransfected OK cells and in cells transfected with the wild-type μ2 cDNA, but it failed to elicit such an effect in OK cells expressing the μ2 cDNA carrying a mutation within the phosphorylation site (Figure 2B). Additionally, dopamine failed to trigger Na+,K+-ATPase endocytosis in OK cells in which Thr-156 was replaced by negatively charged residues (T156D and T156E) (data not shown). If one considers that a negatively charged residue would resemble in part a phosphorylated amino acid, these results further suggest that a dynamic phosphorylation/dephosphorylation of adaptor protein μ-subunits is needed for the efficient recognition of cargo proteins, as proposed previously (23). The functional role of AP-2 μ2 phosphorylation was also evaluated during the regulation of Na+,K+-ATPase activity (Figure 2C). DA inhibits Na+,K+-ATPase activity in cells transiently transfected with the AP-2 μ2 wild type but not in cells transfected with the T156A mutant.
AP-2 Phosphorylation and Binding to Na+,K+-ATPase during Hypoxia
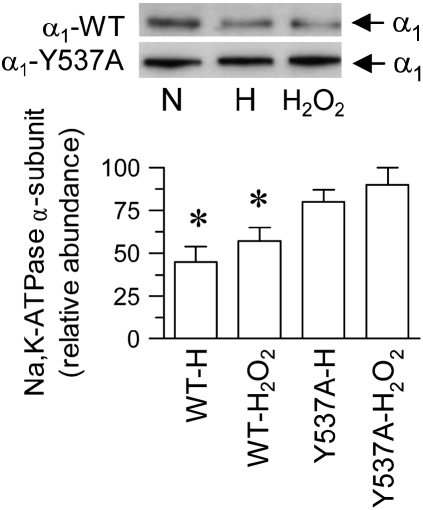
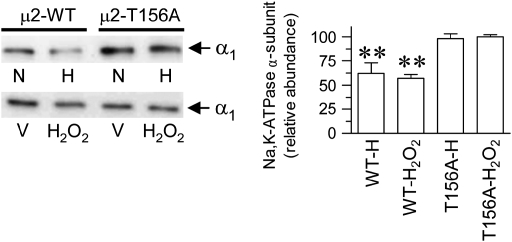
To examine whether the requirement of AP-2 μ2 phosphorylation for Na+,K+-ATPase endocytosis has a wider biological significance beyond the renal epithelia and GPCR signals, we studied this phenomenon in alveolar epithelial cells in response to hypoxia. Decreased Na+,K+-ATPase activity in alveolar epithelial cells in response to hypoxia is also a result of a reduced number of Na+,K+-ATPase molecules at the plasma membrane (3) via endocytosis mediated by clathrin. Thus, we evaluated whether in this epithelium it requires the binding of AP-2 to the Na+,K+-ATPase α-subunit. A549 cells (an established model of lung alveolar epithelium) were transfected stably with the wild-type Na+,K+-ATPase α-subunit carrying a GFP tag and an isoform carrying a mutation in the AP-2 binding site (Y537A), as previously established (9). When these cells were transferred from normal O2 tension to a controlled hypoxic condition of 1.5% O2 (3), there was a reduction in the number of Na+,K+-ATPase molecules at the plasma membrane (Figure 3), as previously described (3). However, if cells were carrying a mutation in the AP-2 binding site (within the α-subunit), the response to hypoxia was absent. It was previously established that the effect of hypoxia on Na+,K+-ATPase endocytosis was mediated by ROS (3). Similarly, although H2O2 induced a reduction in Na+,K+-ATPase at the plasma membrane from cells expressing the wild-type isoform, it failed to elicit the same effect in cells transfected with the α-subunit carrying a mutation in the AP-2 binding site (Figure 3). We further examined whether phosphorylation of AP-2 μ2 subunit was relevant for reduction in Na+,K+-ATPase activity by using cells expressing the AP-2 μ2 bearing a mutation in the phosphorylation site (T156A). Hypoxia as well as H2O2 reduced Na+,K+-ATPase activity in AP-2 μ2 wild-type–transfected cells, whereas it failed to elicit such an effect in cells expressing the AP-2 μ2 bearing the T156A mutation (Figure 4).
Figure 3.
Relevance of a tyrosine-base motif interaction with AP-2 μ2 during hypoxia/ROS-mediated Na+,K+-ATPase α-subunit endocytosis in alveolar epithelial cells. A549 cells were stably expressing the wild-type or the Na+,K+-ATPase α-subunit bearing the Y537A mutation tagged with GFP as reporter. Cells maintained on normal O2 tension (N) were submitted to a hypoxic condition (H) or incubation with 100 μM (H2O2) for 40 min at 37°C. Na+,K+-ATPase α-subunit abundance in the plasma membrane was examined by cell surface biotinylation and Western blot (upper panel), and quantitation (mean ± SE) (lower panel) of three experiments was performed independently. *P < 0.05.
Figure 4.
A549 cells stably expressing the AP-2 μ2 wild type (μ2-WT) or T156A mutant (μ2-T156A) were used. Na+,K+-ATPase α-subunit abundance in the plasma membrane (cell surface biotinylation) in response to normal O2 tension (N) or hypoxia (H) was examined. In another set of experiments, A549 cells were incubated with 100 μM H2O2 or vehicle (V) for 40 min at 37°C. Left panel: Representative Western blot. Right panel: Quantitative analysis (bars represent mean ± SE of three experiments performed independently). **P < 0.01.
DISCUSSION
In this study, we provide evidence that phosphorylation of the μ2 isoform of AP-2 is necessary for GPCR-induced endocytosis of Na+,K+-ATPase molecules. Our results demonstrate that other intracellular signals, such as hypoxia-induced ROS, also required μ2 phosphorylation to promote Na+,K+-ATPase endocytosis in lung alveolar epithelial cells.
Clathrin-dependent endocytosis of membrane proteins is initiated by adaptor proteins (adaptins) that simultaneously bind to cargo proteins, recruit clathrin, and promote the formation of clathrin-coated pits (8). AP-2 phosphorylation is a key event that determines the binding affinity of the μ2 subunit to cargo proteins (18, 21). In the present study, DA increased the state of AP-2 μ2 subunit phosphorylation in a time-dependent manner that coincided with maximal association of AP-2 with the Na+,K+-ATPase and preceded its appearance in clathrin vesicles (1). The transition between phospho- and dephospoAP-2 μ2 facilitates its association with the cargo molecules, as demonstrated by several in vitro studies (23). In our studies, the presence of negatively charged residues (T156D and T156E) also prevented AP-2 μ2 binding to the α-subunit and Na+,K+-ATPase endocytosis (not shown).
In intact cells, the AP-2 μ2 subunit could be targeted by myriad protein kinases. A new kinase (AAK1) responsible for phosphorylating the AP-2 μ2 subunit has been described (21, 22). A detailed in vitro study revealed that AAK1 (21) could be inhibited by several chemical inhibitors at doses that overlap their actions on other kinases. In this study, when we used several inhibitors of PKC and dominant-negative mutants of the PKC-ζ isoform, it was possible to block AP-2 μ2 phosphorylation. Coincidentally, this isoform of PKC is also responsible for phosphorylating the Na+,K+-ATPase α-subunit at the ser-18 residue (a necessary step for Na+,K+-ATPase subunit endocytosis). Staurosporine, at a concentration that would not affect the activation of PKC-ζ, does inhibit the activation of AAK1 (21). Therefore, it is possible that the PKC-ζ isoform would form a complex within the endocytic network and thereby express a dual effect (i.e., phosphorylating the Na+,K+-ATPase and facilitating AP2 phosphorylation), which together would be responsible for Na+,K+-ATPase internalization. Proven specific inhibitors of AAK1 in intact cells are not yet available.
The generation of ROS by lung alveolar epithelial cells during hypoxia results in a series of events leading to Na+,K+-ATPase internalization via a clathrin-coated–dependent mechanism (3). Endocytosis is initiated by phosphorylation of the Na+,K+-ATPase α-subunit via a PKC-ζ–dependent mechanism, a process that leads to activation of phosphatidylinositol 3-kinase. The lipids generated by this kinase at the plasma membrane interface favor the association of AP-2 with the cargo. Similarly to renal epithelial cells (9), association of AP-2 to the Na+,K+-ATPase requires the binding of the μ2 subunit (within the AP-2 complex) to a tyrosine-based motif (Y537) located in the α-subunit. Moreover, internalization of Na+,K+-ATPase molecules in response to hypoxia or exogenously administrated H2O2 was abolished in cells expressing the AP-2 μ2 lacking the phosphorylation site (T156).
The effects of AP-2 μ2 phosphorylation have been extensively studied mostly in reconstituted systems and during basal endocytosis in intact cells exposed to changes in temperature, such as transferrin receptor trafficking (18, 22). In this report, we provide the first evidence that endocytosis of an integral membrane protein (the Na+,K+-ATPase) in response to a physiological condition (activation of GPCR by dopamine in renal epithelial cells) or a pathophysiologic condition (e.g., hypoxia in lung alveolar epithelial cells) requires phosphorylation of AP-2 μ2 subunit (Figure 5). Additionally, the results from this study suggest that during endocytosis the Na+,K+-ATPase may represent its own scaffold system, organizing the signaling network independently of whether it is a GPCR or a ROS signal that triggers the process, thus making the activation of PKC-ζ a possible converging point during endocytosis in kidney or lung epithelial cells.
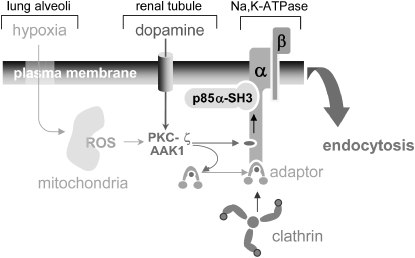
Figure 5.
Model of Na+,K+-ATPase endocytosis in response to GPCR signals and those generated by hypoxia. PKC-ζ and possibly AAK1 are the cellular targets where the GPCR or hypoxia signals converge. In the case of GPCR (dopamine), activation of PKC-ζ is mediated by the arachidonic acid metabolite, 12-HETE (24), whereas during hypoxia activation of PKC-ζ is mediated by ROS. Activation of PKC-ζ may have a dual effect: (1) phosphorylation of the Na+,K+-ATPase α-subunit (serine-18), which leads to the activation of phosphoinositide 3-kinase by its binding to the α-subunit (25) and production of phosphatidylinositol 3-phosphate necessary for AP-2 binding to the cargo (26, 27), and (2) phosphorylation of AP-2 μ2 to facilitate its binding to the Tyrosine-537 residue within the Na+,K+-ATPase α-subunit and thereby permitting the recruitment of clathrin to the membrane interface where the endocytic process occurs.
Acknowledgments
The authors thank Lynn Welsh for technical assistance, J. Bonifacino for providing the antibody against the μ2 subunit of AP-2, A. Sorkin for providing the AP-2 μ2 cDNA, and H. Takeda for providing the PKC-ζ cDNA.
This work was supported by the Swedish Research Council (32X-10860 and 32P-14879), the Swedish Heart and Lung Foundation, the National Institutes of Health (PO1HL-071643, HL-48129, and DK62195), and the American Heart Association (0455110Y).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0044OC on February 23, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Chibalin AV, Katz AI, Berggren P-O, Bertorello AM. Receptor-mediated inhibition of renal Na+, K+-ATPase is associated with endocytosis of its α- and β-subunits. Am J Physiol 1997;273:C1458–C1465. [DOI] [PubMed] [Google Scholar]
- 2.Khundmiri SJ, Bertorello AM, Delamere N, Lederer ED. Clathrin-mediated endocytosis of Na+, K+-ATPase in response to PTH requires ERK-dependent phosphorylation of Ser-11 within the α1-subunit. J Biol Chem 2004;279:17418–17427. [DOI] [PubMed] [Google Scholar]
- 3.Dada LA, Chandel NS, Ridge KM, Pedemonte CH, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J Clin Invest 2003;111:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Kesiry R, Periyasami SM, Malhorta D, Xie Z, Shapiro JL. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int 2004;66:227–241. [DOI] [PubMed] [Google Scholar]
- 5.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 2001;81:345–418. [DOI] [PubMed] [Google Scholar]
- 6.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren P-O, Bertorello AM. Phosphorylation of the catalytic α-subunit constitutes a triggering signal for Na+, K+-ATPase endocytosis. J Biol Chem 1998;273:8814–8819. [DOI] [PubMed] [Google Scholar]
- 7.Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren P-O, Bertorello AM. Dopamine-induced endocytosis of Na+, K+-ATPase is initiated by phosphorylation of Ser-18 in the rat α subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem 1999;274:1920–1927. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol 2000;3:187–198. [DOI] [PubMed] [Google Scholar]
- 9.Doné SC, Leibiger IB, Efendiev R, Katz AI, Leibiger B, Berggren P-O, Pedemonte CH, Bertorello AM. Tyrosine 537 within the Na+, K+-ATPase α subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J Biol Chem 2002;277:17108–17111. [DOI] [PubMed] [Google Scholar]
- 10.Takeda H, Takeda H, Matozaki T, Takada T, Noguchi T, Yamao T, Tsuda M, Ochi F, Fukunaga K, Inagaki K, et al. PI 3-kinase γ and protein kinase C-ζ mediate RAS-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J 1999;18:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efendiev R, Yudowski GA, Zwiller J, Leibiger B, Katz AI, Berggren PO, Pedemonte CH, Leibiger IB, Bertorello AM. Relevance of dopamine signals anchoring dynamin-2 to the plasma membrane during Na+,K+-ATPase endocytosis. J Biol Chem 2002;277:44108–44114. [DOI] [PubMed] [Google Scholar]
- 12.Efendiev R, Bertorello AM, Pedemonte CH. PKC-β and PKC-ξ mediate opposing effects on proximal tubule Na+, K+-ATPase activity. FEBS Lett 1999;456:45–48. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 14.Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, Bianchi G, Pedemonte CH, Bertorello AM. Hypertension-linked mutation in the adducin α-subunit leads to higher AP2-μ2 phosphorylation and impaired Na+,K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res 2004;95:1100–1108. [DOI] [PubMed] [Google Scholar]
- 15.Takeyasu K, Tamkun MM, Renaud KJ, Fambrought DM. Ouabain-sensitive (Na+,K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem 1988;263:4347–4354. [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 17.Fingerhut A, von Figura K, Höning S. Binding of AP2 to storing signals is modulated by AP2 phosphorylation. J Biol Chem 2001;276:5476–5482. [DOI] [PubMed] [Google Scholar]
- 18.Olusanya O, Andrews PD, Swedlow JR, Smythe E. Phosphorylation of threonine 156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr Biol 2001;11:896–900. [DOI] [PubMed] [Google Scholar]
- 19.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 1991;266:15771–15781. [PubMed] [Google Scholar]
- 20.Marte BM, Meyer T, Stabel S, Standke GJ, Jaken S, Fabbro D, Hynes NE. Protein kinase C and mammary cell differentiation: involvement of protein kinase C alpha in the induction of beta-casein expression. Cell Growth Differ 1994;5:239–247. [PubMed] [Google Scholar]
- 21.Ricotta D, Conner SD, Schmid SL, von Figura K, Höning S. Phosphorylation of the AP2 μ2 subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol 2002;156:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathin-mediated endocytosis. J Cell Biol 2002;156:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh P, Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J Cell Biol 2003;160:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowicki S, Chen SL, Aizman O, Cheng XJ, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C: role in regulation of rat renal Na+,K+-ATPase. J Clin Invest 1997;99:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren P-O, Bertorello AM. Phosphoinositide-3kinase binds to a proline-rich motif in the Na+, K+-ATPase α subunit and regulates its trafficking. Proc Natl Acad Sci USA 2000;97:6556–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogimoto G, Yudowski GA, Barker CJ, Köhler M, Katz AI, Féraille E, Pedemonte CH, Berggren P-O, Bertorello AM. G protein-coupled receptors regulate Na+, K+-ATPase activity and endocytosis by modulating the recruitment of adaptor protein 2 and clathrin. Proc Natl Acad Sci USA 2000;97:3242–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell 2002;109:523–535. [DOI] [PubMed] [Google Scholar]