Abstract
The injection of antigen into the anterior chamber (AC, intracameral injection)2 of a murine eye induces the generation of splenic CD8+ regulatory T cells (AC-SPL cells) that effect the antigen-specific suppression of a Delayed-Type Hypersensitivity (DTH) reaction. Here we show (i) for the first time that the local antigen-specific suppression of DTH-induced swelling in immunized mice by either an intracameral injection of antigen or by the direct injection of CD8+ AC-SPL cells into an antigen-challenged site is associated with an absence of infiltrated mononuclear cells, (ii) the local antigen-specific suppression of the DTH reaction by CD8+ AC-SPL cells requires compatibility between the Qa-1 but not H2 antigen haplotype of the immunized recipient and the injected AC-SPL regulatory T cells, (iii) The suppression of the DTH reaction by CD8+ AC-SPL cells requires the expression of Qa-1 but not H2 antigens and is not due to bystander suppression.
Keywords: CD8+ regulatory cells, anterior chamber, Qa-1, suppression, hypersensitivity
INTRODUCTION
In vivo and in vitro investigations have shown that CD8+ regulatory T cells effect the antigen-specific suppression of activated T cells but CD4/CD25/FoxP3+ regulatory T cells inhibit the activation of naïve T cells1–4. Because CD8+ regulatory T cells are induced by immunization, it has been suggested that the number of these cells, specific for the immunizing antigen, may increase during a primary immune response 5–7. Other reports have suggested that the antigen specificity of CD8+ regulatory T cells may be determined by T cell Receptor (TCR) variable region peptides that are presented by major histocompatibility complex (MHC) -related Qa-1 molecules5.8.9. However, in addition to the generation of CD8+ hregulatory T cells responding to T cell receptor (TCR) V-region peptides, MHC class I-specific CD8+ effector T cells have also been converted in vitro to a suppressive phenotype by exposure to TGF-β–treated F4/80+ monocytic cells or TGF-β only10,11.
In addition to an environment that prevents or mitigates immune/inflammatory reactions, the eye responds to an antigenic insult or injury by the induction of antigen-specific splenic CD4+ and CD8+ regulatory T cells when antigen is introduced into the anterior chamber (AC) of an eye12. As such it is useful to investigate the nature and mechanisms of systemic regulatory T cells induced by the injection of antigen into the anterior chamber. Although CD8+ regulatory T cells induced by the intracameral injection of antigen (AC-SPL cells) suppress cell-mediated immunity effected by immunized T cells12–15, several cell types participate in the induction of the CD8+ regulatory splenic T cells after the injection of antigen into the AC 7,12–14, 16–20. Moreover, the activation of splenic CD8+ regulatory T cells by intracameral antigen is restricted by Qa-1 antigens expressed by B cells that present antigen to CD8+ T cells20. The induction and function of these regulatory T cells requires CD94/NKG2A receptors for Qa-121. Cells lacking Qa-1 are not suppressed by CD8+ regulatory T cells22. We therefore hypothesized that the suppression of the DTH reaction in immunized mice by AC-induced CD8+ regulatory T cells transferred to immunized mice requires compatibility between the Qa-1 haplotype of recipient mice and the donor of the regulatory spleen cells that suppress the DTH reaction.
To investigate the role of Qa-1 antigen compatibility in the suppression of a DTH reaction by CD8+ AC-SPL cells, we used an adoptive transfer assay in which AC-SPL cells were injected into immunized mice at a site challenged with antigen to elicit a DTH reaction. Our results (i) confirm that the antigen-specific suppression of a DTH reaction in the immunized recipients of AC-SPL cells is mediated by splenic CD8+ regulatory cells6,7,13,14; (ii) The suppression of antigen-induced swelling in the DTH reaction by CD8+ AC-SPL cells requires compatibility of the Qa-1 (but not the H-2) haplotype between the immunized recipient and the donor of regulatory T cells; (iii) also indicate that the suppression of the DTH reaction in immunized mice by AC-SPL cells is not due to bystander suppression and is associated with an absence of recruited monocytic cells.
RESULTS
Local antigen-specific suppression of the DTH reaction by CD8+ AC-SPL cells
The selective ability of CD8+ regulatory AC-SPL cells to suppress a DTH reaction is well documented6,7,13,14. Whether these cells suppress the DTH reaction directly and/or by bystander suppression is not known. Therefore, we investigated this issue using an adoptive transfer assay in which the AC-SPL cells are injected into immunized mice at the site challenged with antigen. This Local Transfer of Suppression (LTS) measures the regulation of a DTH reaction in vivo within 24hr after the challenge with antigen. To extend demonstrations of the selective ability of AC-induced CD8+ regulatory spleen cells to suppress locally a DTH reaction in immunized mice, AC-SPL cells recovered from donors that received an intracameral injection of TNP-BSA were separated into CD8+ and CD8− populations using immunomagnetic beads. Flow cytometry showed that the CD8+ population was approximately 83–95% CD8+and the CD8− population was <3% CD8+ (data not shown). The AC-SPL cells were injected into footpads of TNP-BSA-immunized mice immediately after the footpads were challenged with epicutaneous PCl and the swelling of the footpads measured 24hr later. The swelling of the challenged footpads was reduced significantly in the recipients of unseparated or CD8+ AC-SPL cells but not in the recipients of CD8− AC-SPL cells (Fig 1).
Figure 1. Suppression of the DTH reaction by CD8+ AC-SPL cells.
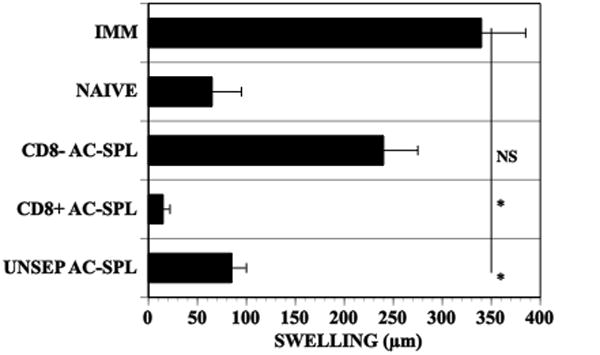
One week after BALB/c mice received an injection of TNP-BSA into an anterior chamber, the mice were immunized with TNP-BSA and CFA. One week after the intracameral injection spleen cells (AC-SPL cells) were recovered and the AC-SPL cells separated into CD8+ and CD8− populations by immunomagnetic beads. The footpads of mice immunized with TNP-BSA and CFA 9 days previously received id 25000 AC-SPL cells immediately after the footpad was challenged with epicutaneous PCl. Swelling was measured 24hr later. The data is pooled from two separate experiments 6–7 mice/group. NAÏVE: non-immunized mice, IMM: immunized mice that did not receive AC-SPL cells, UNSEP ACSPL: immunized mice that received unseparated AC-SPL cells, CD8+ AC-SPL: immunized mice that received CD8+ AC-SPL cells, CD8− ACSPL: immunized mice that received CD8− AC-SPL cells.* p<0.01.
Histologic examination of the challenged footpads demonstrated an infiltration of inflammatory cells and edema (Fig 2a). Challenged footpads of mice receiving an intracameral injection of TNP-BSA (Fig 2b) or CD8+ AC-SPL cells (Fig 2c) showed an absence of the infiltrated cells and resembled unchallenged footpads of immunized recipients or the challenged footpads of naïve mice (data not shown).
Figure 2. Histology of footpads of immunized mice challenged with antigen +/− intracameral antigen or CD8+ AC-SPL cells.
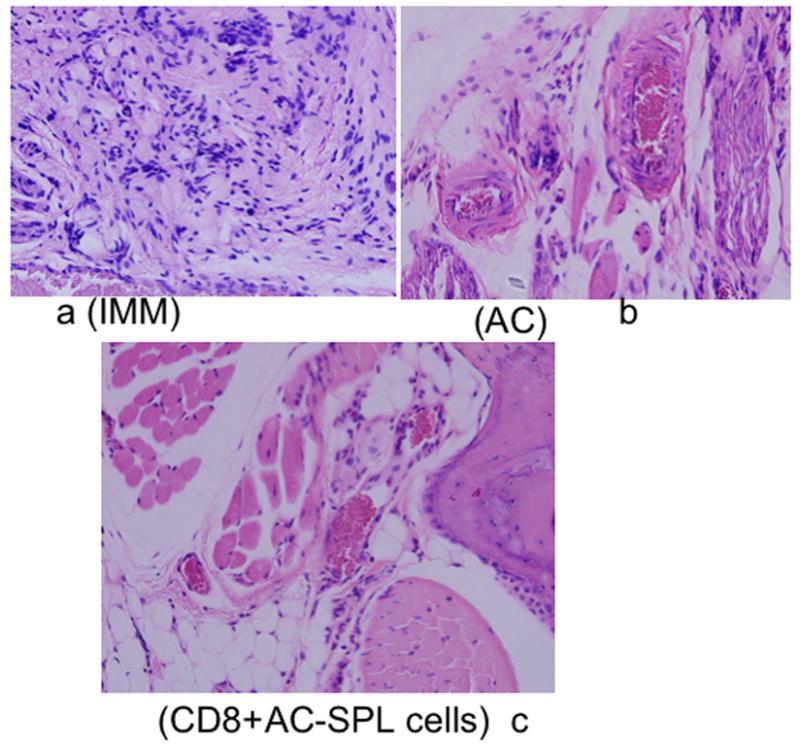
Twenty-four hr after the footpads of TNP-BSA-immunized mice were challenged with epicutaneous PCl, the feet of the euthanized mice were excised, fixed in formalin and stained with hemotoxylin/eosin. Representative digital fields at 25X magnification from immunized (a IMM) mice, immunized mice that received an intracameral injection of TNP-BSA before immunization (b AC) or immunized mice whose footpad received an injection of 25000 CD8+ AC-SPL cells immediately after the footpad received epicutaneous PCl (c, CD8+ AC-SPL). Data is representative of each of 3 mice/group and one of three experiments.
The antigen specificity of the reduction in DTH-induced footpad swelling in the LTS by CD8+ AC-SPL cells is demonstrated by the injection of CD8+ AC-SPL cells from mice receiving intracameral TNP-BSA or BSA into the footpads of TNP-BSA and/or BSA-immunized mice. CD8+ AC-SPL cells from mice receiving intracameral TNP-BSA reduced footpad swelling in TNP-BSA-immunized recipients challenged with PCl but not in BSA-immunized recipients challenged with BSA (Fig. 3A). Conversely, CD8+ AC-SPL cells from mice receiving intracameral BSA reduced the DTH-induced footpad swelling of BSA but not TNP-BSA-immunized recipients challenged with PCl. Similar results were obtained with unseparated AC-SPL cells recovered from mice that received an intracameral injection of TNP-BSA or OVA (Fig 3B). AC-SPL cells recovered from mice receiving intracameral TNP-BSA or OVA suppressed DTH only in TNP-BSA or OVA-immunized mice respectively.
Figure 3. Antigen-specific suppression of the DTH reaction by AC-SPL cells.
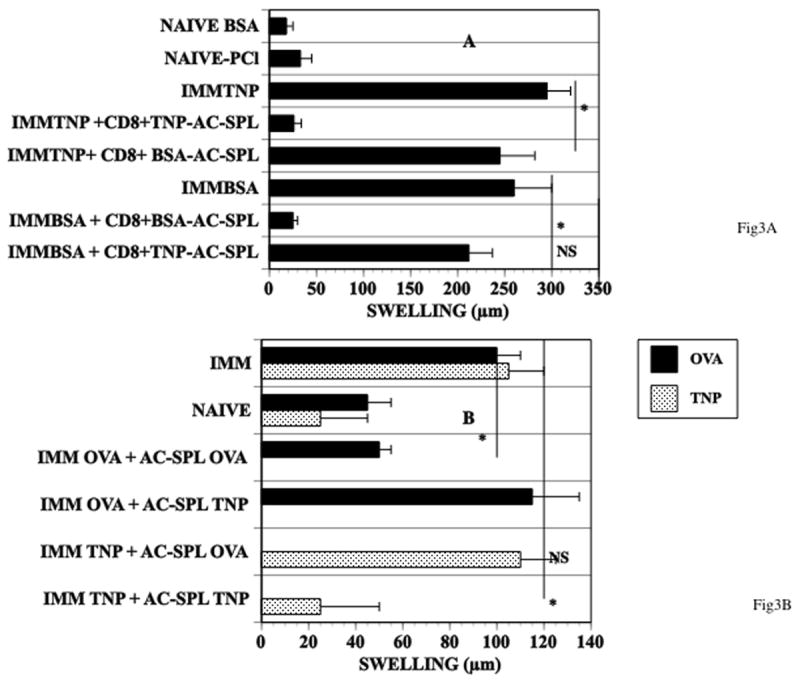
(A, CD8+ cells) Naïve mice received an intracameral injection of TNP-BSA or BSA. Seven days after the intracameral injection the mice were immunized with TNP-BSA or BSA (respectively). AC-SPL cells were recovered 7 days after the AC-injected mice were immunized. AC-SPL cells were separated by immunomagnetic beads and 25000 CD8+ AC-SPL cells were injected into the footpads of mice immediately after the mice were challenged with epicutaneous PCl or id BSA. The data is pooled from two separate experiments 6–7 mice/group. NAÏVE: non-immunized mice, IMM: immunized mice that did not receive AC-SPL cells, UNSEP: immunized mice that received unseparated AC-SPL cells, CD8+: immunized mice that received CD8+ AC-SPL cells, CD8−: immunized mice that received CD8− AC-SPL cells.* p<0.01. (B. unseparated AC-SPL cells) One week after mice received an injection of TNP-BSA or OVA into an anterior chamber the mice were immunized with TNP-BSA/CFA or OVA/CFA respectively. One week after immunizing, spleens were recovered and AC-SPL cells from mice that received an intracameral injection of TNP-BSA (AC-SPL- TNP) or OVA (AC-SPL-OVA ) were injected into the footpads immediately after TNP-BSA- immunized mice were challenged with epicutaneous PCl or the footpads of OVA-immunized mice were injected sc with OVA. Swelling was measured 24hr after challenge. The data is the mean swelling +/− S.E.M. of data pooled from two experiments, 7–8 mice/group. * p< 0.02.
To determine whether AC-SPL cells would suppress the DTH reaction to another antigen in the presence of the antigen that induced the AC-SPL regulatory cells, AC-SPL cells recovered from donors that received an intracameral injection of TNP-BSA (AC-SPLTNP) or OVA (AC-SPLOVA) were injected into the foopads of mice immunized to TNP-BSA or OVA. These mice were challenged with the immunizing antigen (PCl or OVA) and the antigen used to generate the AC-SPL cells. AC-SPL TNP cells suppressed the DTH reaction in TNP-BSA-immunized mice but did not suppress the DTH reaction in OVA-immunized mice even if PCl was included with the challenge OVA. Similarly, AC-SPLOVA cells that suppressed the OVA-initiated DTH reaction in OVA-immunized mice did not suppress the DTH reaction in TNP-BSA-immunized mice even if OVA was included with the challenge PCl (Fig. 4A,B).
Figure 4. AC-SPL cells suppress the DTH reaction directly.

TNP-BSA (A) or OVA- immunized (B) mice received id AC-SPL cells recovered from mice that received an intracameral injection of TNP-BSA (AC-SPL-TNP) or OVA (AC-SPL-OVA ) immediately after the mice were challenged with epicutaneous PCl or id OVA. TNP-BSA-immunized mice receiving AC-SPL- OVA cells after challenge with PCl also received id OVA. OVA-immunized mice receiving AC-SPL-TNP cells received epicutaneous PCl after id challenge with OVA. Data is pooled from 2 experiments, 8 mice/group. *p<0.05.
The suppression of the DTH reaction in the LTS by regulatory AC-SPL cells requires compatibility in the Qa-1 haplotype between the regulatory T cell donor and the recipient
Qa-1 antigens have been implicated as an integral for the suppressive activity of CD8+ regulatory T cells1,2,5,9,22 and for the induction of anterior chamber-associated immune deviation (ACAID) by F4/80+ cells activated by antigen and TGF-β20. To determine whether the suppression of DTH by AC-SPL cells following local transfer is associated with compatibility between the Qa-1 haplotype of the donor of AC-SPL cells and the immunized recipient of the AC-SPL cells we injected TNP-BSA-induced AC-SPL cells from Balb/c mice (H-2d,Qa-1b) into the footpads of TNP-BSA-immunized C3H (H-2k,Qa-1b) mice immediately after the footpad was challenged with epicutaneous PCl. The Balb/c AC-SPL cells suppressed DTH in C3H mice even though they are mismatched at the H2 locus (Table 1). TNP-BSA-induced AC-SPL cells from SJL (H-2s, Qa-1a) mice were injected into the footpads of TNP-BSA-immunized C57BL10.s (H-2s, Qa-1b) or SJL mice. SJL AC-SPL cells did not suppress DTH in C57BL10.s mice even though these mice are matched at the H2 locus. Balb/c AC-SPL cells also suppressed the DTH reaction when injected into the footpads of immunized C57BL/6 mice at the time of challenge (data not shown). Moreover, The number of CD4+, Qa-1+cells increased in the spleens of C57BL/6 (Fig 5) or DBA/NCR mice immunized with OVA and CFA (data not shown). This increase in CD4+, Qa-1+ cells did not occur in the spleens of immunized mice that received an intracameral injection of OVA before immunization.
Table 1.
Compatibility in Qa-1 haplotype between AC-SPL cells and the immunized recipient is necessary for the Suppression of the DTH reaction in the LTS
| IMMUNIZED | H2 | Qa-1 | ACSPL | H2 | Qa-1 | IMM (μm) | IMM+AC-SPL cells (μm) | NAÏVE (μm) |
|---|---|---|---|---|---|---|---|---|
| C3H | k | b | BALB/c | d | b | 360+/−40 | 120+/−18 | 56+/−10 |
| C57BL10.s | s | b | SJL | s | a | 229+/−41 | 300+/−57 | 15+/−5 |
| SJL* | s | a | SJL | s | a | 105+/−20 | 17+/−10 | 3+/−10 |
positive control for C57BL10.s
IMM=immunized
Twenty five thousand AC-SPL cells recovered from TNP-BSA-immunized BALB/c or SJL mice one week after the injection of TNP-BSA into the AC were injected id into a footpad of TNP-BSA- immunized C3H, C57BL10.s or SJL mice immediately after the footpad was challenged with epicutaneous PCl. Swelling was measured 24hr after challenge. Data represents the mean swelling ± SEM of 3 experiments, 9–12 mice/group.
Fig 5. Expression of Qa-1 antigens by CD4+ splenocytes in immunized and immunized, AC-injected mice.
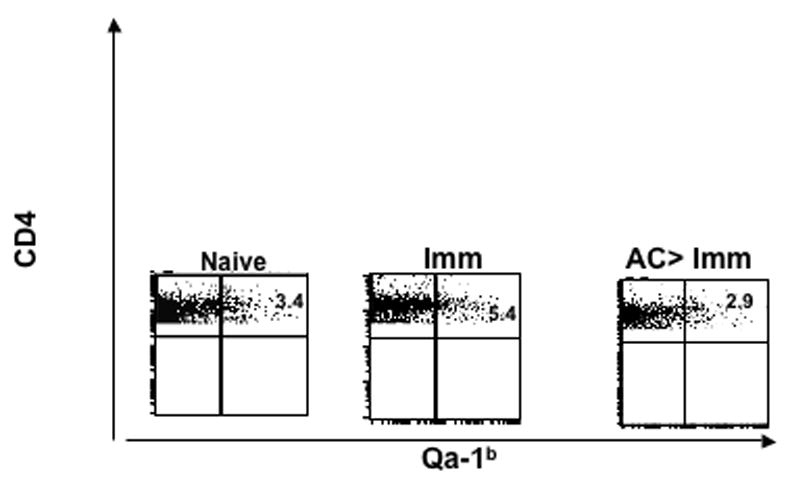
Splenic CD4+Qa-1b+ cells from naive, OVA immunized (Imm) and naïve C57BL/6 mice that received an intracameral injection of OVA before immunization (AC>Imm ) were stained with allophycocyanin anti-Qa-1b and FITC anti-CD4 antibodies. cells analyzed were CD4+ gated cells. Percent positive values are given for the respective dot-plot flow cytometric analysis.
To investigate further a role for the expression of Qa-1 antigens in the AC-SPL cell-induced reduction in swelling at the site challenged by antigen, BALB/c AC-SPL cells were mixed with antibodies to Qa-1b or, H-2Kd,Dd or control myeloma protein MOPC 21 and injected into the footpad of TNP-BSA-immunized mice immediately after the footpad receive a challenge with epicutaneous PCl. The PCl-induced swelling was reduced significantly in the recipients of AC-SPL cells +/− control MOPC 21 or anti-MHC class I. In contrast, the footpad swelling of the recipients of AC-SPL cells + anti-Qa-1b was not significantly different from that of control mice that received no AC-SPL cells +/− antibodies (Fig 6). Collectively these data demonstrate that suppression of the DTH reaction in the footpad by the AC-SPL regulatory T cells is associated with the Qa-1 but not the H2 haplotype of the recipient of AC-SPL suppressor effector cells.
Figure 6. Antibodies to Qa-1 antigens inhibit the suppression of the DTH reaction by AC-SPL cells.
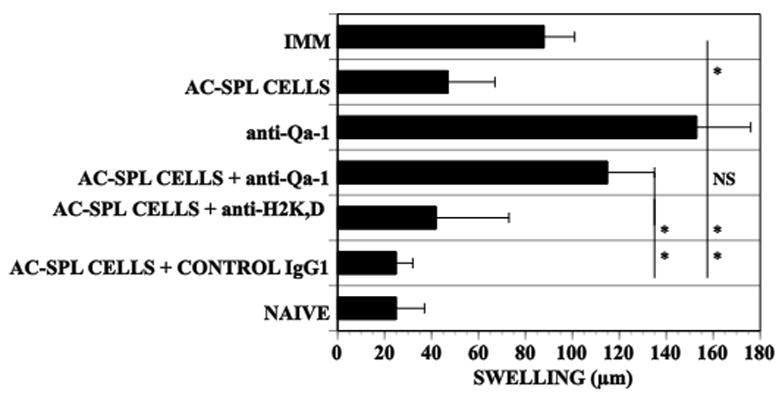
Mice were immunized with TNP-BSA one week after they received an intracameral injection of TNP-BSA. One week after immunizing spleens were recovered and AC-SPL cells prepared. The AC-SPL cells only or mixed with 10μg anti-Qa-1, anti-MHC H-2Kd,Dd or (isotype control for anti-Qa-1) MOPC-11 IgG were injected into footpads of recipient immunized mice immediately after the footpad was challenged with epicutaneous PCl. Data represents the mean swelling +/−SEM of data pooled from three experiments, 9–10 mice/group. p< 0.01.
DISCUSSION
Splenic CD8+ T cells induced by the intracameral injection of antigen suppress the transfer of DTH in vivo by sensitized T cells6,7,12,14 and antigen-induced T cell proliferation and interferon-γ production in vitro23–26. The LTS assay measures the suppression of a DTH reaction in immunized recipients by the transfer of CD8+ regulatory T cells but not naïve spleen cells to the site challenged by antigen. We used this in vivo demonstration of the suppression of DTH by injected AC-SPL cells because this is analogous to the suppression of DTH reaction by the injection of antigen into the anterior chamber. Histological examination of the footpads of immunized mice that were challenged with PCl and immunized mice that received an intracameral injection of TNP-BSA or footpads that received CD8+ AC-SPL cells after challenge with PCl revealed that the recruitment of monocytic cells is absent in both immunized mice that received an intracameral injection of antigen and those that had a small number of AC-induced CD8+ T cells introduced into the site challenged with antigen. CD8+AC-SPL cells suppress the antigen-induced production of IFN-γ23 by immunized lymph node T cells and thereby could prevent the recruitment of monocytic cells to the site challenged by antigen by inhibiting the production of chemokines induced by IFN-γ produced by T cells responding to the challenge antigen. CD8+ AC-SPL cells do not suppress by cytotoxic mechanisms6 and no apoptotic cells were observed at the DTH reaction site suppressed by intracameral injection of antigen or AC-SPL cells. The mechanism of suppression of a DTH reaction by the CD8+ regulatory-effector AC-SPL cells is not known. However, there are reports that suggest that CD8+ regulatory-effector cells produce immunosuppressive TGF-β23,30–32. The suppression of TCR-mediated proliferation of T cells in vitro by CD8+ regulatory cells induced by the intracameral injection of antigen is partially inhibited by antibodies to TGF-β132. We have observed that antibodies to TGF-β 1,2,3 completely inhibit the suppression of DTH by CD8+ AC-SPL cells in the LTS (Cone, R.E. et al submitted for publication). TGF-β inhibits the production of IFN-γ by activated T cells33. Therefore, it is probable that TGF-β produced by CD8+ AC-SPL cells inhibits the production of IFN-γ-induced chemokines, thereby preventing the recruitment of monocytic cells to the challenge site. We are currently investigating this hypothesis. However, because several types of CD8+ regulatory T cells have been described29, perhaps several distinct types of suppressive mechanisms exist in this group of regulatory T cells.
The CD4+, CD8− T cells induced by the AC injection of antigen participate in the induction of CD8+ regulatory spleen cells and also inhibit the antigen-induced proliferation of T cells in vitro12,13,20. Therefore, although the CD4+ T cells transfer the suppression of the induction of DTH they do not themselves suppress the DTH reaction in immunized, challenged recipients. Similarly, in vivo naturally occurring CD4+, CD25+ regulatory T cells are not required to participate in the induction of ACAID24 but they can serve an afferent in vitro function in the induction of splenic CD8+ regulatory T cells26, and in the induction of ACAID25,27. In addition, in contrast to the CD8+ regulatory T cells induced by the intracameral injection of antigen, CD4+/CD25+, FoxP3+ regulatory T cells do not suppress activated T cells (4). Therefore, It is unlikely that CD4+ regulatory T cells would suppress the function of activated T cells within 24hrs in the LTS. CD8+ AC-SPL cells did not suppress the DTH reaction to an irrelevant antigen even when their apparent cognate antigen is present. This local regulatory T cell-mediated suppression of the DTH reaction in immunized mice may therefore require cell contact32 or the nearby presence of the activated T cell and be directed towards T cell receptor peptides presented by Qa-1 proteins8,9,29..
It has been reported that the CD8+ T cell -mediated suppression of DTH induced by intracameral antigen is H-2-restricted28. However, those studies focused on the induction of ACAID that could be restricted by MHC class II antigens. In studies implicating MHC class I, H-2 incompatible afferent peripheral blood cells that failed to induce ACAID when transferred to recipient hosts were also incompatible for Qa-1 alleles. β-2 microglobulin −/− B cells do not induce ACAID28. However, Qa-1 proteins are also associated with β2 microglobulin. In contrast to previous investigations, the investigations herein focus on the effector stage of CD8+ regulatory T cells induced by intracameral antigen. Our observations also support observations that the induction and activity of AC-induced CD8+ regulatory T cells is dependent on the expression of CD94/NKG2A receptors for Qa-121,26. We observed that regulatory T cells from AC-injected donors suppressed the DTH reaction in Qa-1-compatible recipients even if the regulatory cells and the recipients differed in the H-2 haplotype of class I antigens.
Moreover, antibodies to H-2K,D did not influence the CD8+ AC-SPL cell-mediated suppression of the DTH reaction. Therefore, the addition of anti-Qa-1 antibodies would not inhibit the suppression of the DTH reaction by sterically blocking H2K or D antigens. Because the suppression of DTH by the CD8+ AC-SPL cells occurs within 24hr of injection and in spite of an H-2 difference, it is unlikely that a host response to the AC-SPL cells would prevent the suppressive activity of the AC-SPL cells. Hu et al22 demonstrated that the proliferation of CD4+, Qa-1 −/− OT-2 cells to OVA is insensitive to cell-mediated suppression and these mice have an increased sensitivity to the induction of experimental autoimmune encephalomyelitis. These observations suggest that the lack of expression of Qa-1 antigens “protects” the mice from Qa-1-restricted suppression by CD8+ regulatory T cells. Consistent with the observations of Hu et al, our results herein and elsewwhere21 indicate that antibody blockade of Qa-1 antigens inhibits the suppression of a DTH reaction effected by CD8+ AC-SPL cells. The cutaneous DTH reaction can be initiated by one antigen-specific T cell34 that induces the recruitment of non-specific monocytic cells including T cells35,36. Because we observed that the suppression of the DTH reaction is associated with the absence of recruited monocytic cells, the “target” of suppression could be a rare antigen-specific T cell induced by a dendritic cell presenting TCR peptides26,27. Therefore we have not sought to identify such an infrequent cell. However, immunization does induce an increase in splenic CD4+Qa-1+ cells that does not occur in mice that received an intracameral injection of antigen before immunization. Moreover, spleen cells from the immunized mice will transfer the DTH reaction when injected into the footpad of naïve mice (data not shown). Although the data suggests strongly that the suppression of the DTH reaction by CD8+ AC-SPL regulatory cells is associated with Qa-1, the possibility of other influencing elements is not ruled out. The use of mice congenic for Qa-1a or Qa-1b or Qa-1 −/− 22 mice would define the apparent Qa-1 dependence as Qa-1 restriction. Unfortunately, these mice are not readily available. Nevertheless, the suppression of the DTH reaction by AC-SPL cells transferred into mice that differ from the AC-SPL regulatory T cells in H2 antigens but not Qa-1 antigens buttresses our view that the suppression of the DTH reaction by CD8+ AC-SPL cells is not dependent on compatibility in H-2 antigens between the donor of the regulatory T cells and the recipient.
MATERIALS AND METHODS
Mice
Female or male BALB/c, C57BL/6, SJL, C57BL10.s or C3H mice, 8–10 weeks old, were purchased from Charles River/NCI Laboratories (Wilimington, MA). The mice were maintained at the Center for Laboratory Animal Care of the University of Connecticut Health Center. All work with animals was approved previously by the University of Connecticut Health Center Animal Care Committee (ACC-2004-098,2007-369). All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antigens
2,4,6, Trinitrobenzene sulphonic acid, bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from Sigma Chemical Co. (St. Louis, MO). Trinitrophenylated BSA (TNP-BSA) was prepared as described7. Picryl chloride (PCl), 2-chloro-1,3,5-trinitorobenzene (the antigenic equivalent of TNP used to elicit contact sensitivity) was purchased as 2-chloro-5-trypthtane from Chemical Alta Ltd (Edmonton, Alberta, Canada). Mice were immunized by a single sc injection of 200 μg TNP-BSA or 200–400 μg OVA in 50 μl Freund’s Complete Adjuvant (CFA, Sigma) to a flank. In general, the mice were challenged to induce Delayed-Type Hypersensitivity (DTH) to trinitrophenol (TNP) or OVA 7–10 days after the mice were immunized.
DTH reaction
TNP
Contact sensitivity (CS) to TNP in TNP-BSA-sensitized or naïve mice was induced by the epicutaneous application of 15 μl 1% PCl in acetone:olive oil 4:1 to a footpad. The CS response was usually measured approximately 24,48 hr after the application of PCl by measuring footpad thickness with an engineer’s digital micrometer (Mitatoyo, Tokyo, Japan). Mice were anesthetized with ketamine/xylazine (please see below) and the thickness of each footpad measured before challenge with antigen. One footpad was then challenged with PCl and the other with vehicle only. Twenty-four-48 hr later the mice were anesthetized and the thickness of each footpad was measured.
Ovalbumin, bovine serum albumin
Mice were immunized by an sc injection into a flank of 50 μl Complete Freund’s Adjuvant/200 μg BSA or OVA. Seven-9 days after immunization, BSA or OVA-immunized or naïve mice were anesthetized with ketamine/xylazine and footpad thickness was measured before challenge with antigen. One footpad was then injected id with 30μl phosphate-buffered saline (PBS,pH 7.2) containing 50μg OVA or BSA. Twenty-four -48 hr later the mice were anesthetized with ketamine/xylazine and the thickness of the footpad was measured.
Footpad swelling was computed as μm antigen-challenged footpad at time of challenge-μm antigen-challenged footpad before challenge minus μm vehicle-challenged footpad at time of challenge minus μm vehicle-challenged footpad before challenge. After swelling was assessed, the mice were euthanized and antigen-challenged and vehicle-challenged feet removed and fixed with 10% buffered formalin. The feet were processed by the Department of pathology Research Histology laboratory and stained with hemotoxylin and eosin. The sections were examined by one of us (REC) and, in blinded fashion, by Dr. Thiruchandurai Rajan, department of Pathology and Laboratory Medicine. University of Connecticut Health Center. Representative areas were imaged digitally.
Injection of antigen into the anterior chamber6 ( intracameral injection)
Mice were anesthetized with an intraperitoneal (ip) injection of ketamine (75mg/kg)/xylazine (15mg/kg) as described. Under a dissecting microscope a 32g needle attached to a cannula attached to a manually controlled Hamilton syringe (Stoelting Co, Woodale, IL) was inserted into the anterior chamber (AC). Approximately 3 μl PBS containing 4 μg TNP-BSA or 50μg OVA or BSA was injected into the AC. The mice recovered 15–30 min after the intracameral injection, took water and food normally. Mice receiving an intracameral injection of OVA, BSA or TNP-BSA were immunized with TNP-BSA or OVA or BSA one week after the intracameral injection of antigen.
Preparation of splenic regulatory-effector cells(AC-SPL cells)7
Seven days after after intracameral injection the AC-injected mice were immunized. Seven days after immunizing,i the mice were euthanized, spleens recovered, diced and expressed through a 40mm nylon mesh into phosphate –buffered saline (PBS, pH 7.2) using the plunger of a 10ml syringe. The cell suspension was washed 2 times with PBS and resuspended in PBS. To separate the spleen cells based on the expression of CD8, cells suspended in PBS or Becton-Dickonson (BD), BD™ IMag separation buffer Rockville, MD). were separated by immunomagnetic beads into CD8+ and CD8− populations with a BD™ (Becton-Dickinson) CD8+ T lymphocyte enrichment set according to the manufacturers protocol. Enrichment was assessed by immnofluorescent staining of the cells using BD™ FITC-anti-CD8α antibodies. Stained cells were analyzed by flow cytometry.
Adoptive transfer (Local Transfer of Suppression, LTS)
AC-SPL cells were suspended in PBS, quantified with a Coulter counter at 1 × 108/ml. Twenty-five thousand (25000 )AC-SPL cells were injected sc into the footpad of immunized mice immediately following epicutaneous challenge with PCl. When mice were challenged with BSA or OVA AC-SPL cells were suspended in PBS containing 6.6mg/ml BSA or OVA. A footpad received 30 μl of this mixture id. Swelling was measured 24hr after injection. In antibody inhibition assays, the AC-SPL cells were mixed with 10μg monoclonal anti-Qa-1b (BD Biosciences (San Jose, CA, USA) anti-mouse H-2Kd,Dd (Ebioscience,, San Diego, CA. clone 34-1-25) or control MOPC-21 IgG (Sigma) in 15 μl PBS and the mixture injected into the footpad.
Flow Cytometry
Allophycocyanin anti-Qa-1b, FITC anti-CD4 and unlabeled anti-CD16/CD32 blocking antibody were obtained from BD biosciences, San Jose, CA. Cell were incubated in PBS, 1% fetal calf serum, 0.1% sodium azide with 0.5μg blocking antibody for 10 min. Then fluorochromed antibodies (0.5μg) or FITC-streptavidin were added and the cells held for 30min. The cells were washed 3X and added to a FACS calibur analyzer. Data was analyzed by CelllQuest software (BD biosciences). A total of at least 70,000 cells were collected for each sample.
Statistics
Statistical significance was calculated by one-way ANOVA or Student’s t Test. P-values were determined by the Student-Neuman-Keuls test for ANOVA or by the t test and p values <0.05 were considered significant.
Acknowledgments
We are most appreciative of the evaluation of histology by Dr. T.V. Rajan, Department of Pathology and Laboratory Medicine, University of Connecticut Health Center and resolution of the images by Sourojit Bhowmick or Dr. Zhifang Hao, Department of Immunology, University of Connecticut Health Center.. This work was supported by USPHS grants EY017537, EY017289, National Eye Institute and the Connecticut Lions Eye Research Foundation (to J.O.R.).
Footnotes
Abbreviations: AC: anterior chamber;AC-SPL cells: spleen cells produced by mice receiving an intracameral injection of antigen;BSA:bovine serum albumin;DTH: delayed-type hypersensitivity; LTS:local transfer of suppression; MHC: major histocompatibility complex; OVA:ovalbumin; PBS:phosphate-buffered saline;PCl:picryl chloride; TCR: T cell receptor for antigen; TGF:transforming growth factor.
References
- 1.Jiang H, Chess L. An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv in Immunol. 2004;83:253–288. doi: 10.1016/S0065-2776(04)83008-6. [DOI] [PubMed] [Google Scholar]
- 2.Cantor H. Reliving suppression? Nature Immunology. 2004;5:347–9. doi: 10.1038/ni0404-347. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Flood PM, Gershon RK. Immunoregulatory T cell pathways. Ann Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Brook MO, Carvalo Caspar M, Zhang J, Ramon HE, Sayegh MH, Wood KJ. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Nat Acad Sci (USA) 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Nat Acad Sci (USA) 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cone RE, Li X, Sharafieh R, O’Rourke J, Vella AT. The suppression of delayed-type hypersensitivity by CD8+regulatory T cells requires interferon-γ. Immunology. 2006;120:112–119. doi: 10.1111/j.1365-2567.2006.02486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Wang Y, Urso D, O’Rourke J, Cone RE. Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the antigen-induced production of immunoglobulin G1 antibodies. Immunology. 2004;113:44–56. doi: 10.1111/j.1365-2567.2004.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Chess L. The specific regulation of immune responses by CD8+ regulatory T cells restricted by the MHC class 1b molecule, Qa-1. Annu Rev Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Maricic I, Kumar V. Anti-TCR antibody treatment activates a novel population of nonintestinal CD8α,α+TCRα,β+ regulatory T cells and prevents experimental autoimmune encephalomyelitis. J Immunol. 2007;178:6043–6050. doi: 10.4049/jimmunol.178.10.6043. [DOI] [PubMed] [Google Scholar]
- 10.Kezuka T, Streilein JW. In vitro generation of regulatory CD8+ T cells similar to those found in mice with anterior chamber-associated immune deviation. Investigative Ophthalmology & Visual Science. 2000;41:1803–1811. [PubMed] [Google Scholar]
- 11.Kapp JA, Honjo Kazuhito, Kapp Linda M, Xu Xiao yan, Cozier Alana, Bucy R Pat. TCR transgenic CD8+ T cells activated in the presence of TGF-β express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. Int Immunol. 2006;18:1549–1562. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 12.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Critical Reviews in immunology. 2002;11:13–46. [PubMed] [Google Scholar]
- 13.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immne deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:393–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Streilein JW, Niederkorn JY. Characterization of the suppressor cell (s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. Jour Immunol. 1985;134:1381–7. [PubMed] [Google Scholar]
- 15.Jiang L, Yang P, Hao H, Lin B, Hou X, Shengping Z, Hongyan Huang X, Kijlstra A. Increased expression of Foxp3 in splenic CD8+ T cells from mice with anterior chamber-associated immune deviation. Molecular Vision. 2007;13:968–74. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Goldschneider I, O’rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–746. [PubMed] [Google Scholar]
- 17.Li X, Shen S, Urso D, Kalique S, Park SH, Sharafieh R, O’Rourke J, Cone RE. Phenotypic and immnoregulatory characteristics of monocytic iris cells. Immunol. 2006;117:566– 575. doi: 10.1111/j.1365-2567.2006.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Orazio TG, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the production of anterior chamber-associated immune deviation. Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonoda K-H, Exley M, Snapper S, Balk ST, Stein-Streilein J. CD1-reactive natural killer T cells are required for the development of systemic tolerance through an immune privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Orazio TJ, Mayhew E, Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen II. Evidence for presentation by Qa-1. J Immunol. 2001;166:26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, O’Rourke J, Cone RE. Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular –induced splenic CD8+ regulatory T cells. Int Immunol. 2008;20:509–516. doi: 10.1093/intimm/dxn008. [DOI] [PubMed] [Google Scholar]
- 22.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nature Immunology. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Ghali WE, Pingle P, Traboulsi A, Dalal T, O’Rourke J, Cone RE. Splenic T cells from mice receiving intracameral antigen suppress in vitro antigen-induced proliferation and interferon gamma production by sensitized lymph node cells. Ocular immunol Inflamm. 2003;11:39–52. doi: 10.1076/ocii.11.1.39.15578. [DOI] [PubMed] [Google Scholar]
- 24.Keino H, Takeuchi M, Kezuka T, Hattori T, Usui M, Taguchi O, Streilein JW, Stein-Streilein J. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Investigative Ophthalmology & Visual Science. 2006;47:1047–1055. doi: 10.1167/iovs.05-0110. [DOI] [PubMed] [Google Scholar]
- 25.Skelsey ME, Mayhew E, Niederkorn JY. CD25+, Interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology. 2003;11:18–29. doi: 10.1046/j.1365-2567.2003.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao H, Peizeng Y, Liqiong J, Junfeng Z, Chen L, Xiaomin L, Hongyan Z, Kijlstra A, Zhang C. Upregulation of CD94 on CD8+T Cells in Anterior Chamber-Associated Immune Deviation. BMC Immunology. 2008;9:53– 62. doi: 10.1186/1471-2172-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Yang P, Zhou H, Li B, Huang X, Meng Q, Wang L, Kijlstra A. CD4+CD25+Tregs express an increased LAG-3 and CTLA-4 in anterior chamber-associated immune deviation. Graefes Arch for Clin and Exp Ophthalmol. 2007;245:1549–57. doi: 10.1007/s00417-007-0591-8. [DOI] [PubMed] [Google Scholar]
- 28.Ashour HM, Niedcerkorn JY. Peripheral tolerance via the anterior chamber of the eye: role of B cells in the MHC class I and II presentation. J Immunol. 2006;176:5950–5957. doi: 10.4049/jimmunol.176.10.5950. [DOI] [PubMed] [Google Scholar]
- 29.Smith TRF, Kumar V. Revival of CD8+Treg-mediated suppression. Trends in Immunology. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Myers LB, Croft BS, Kwon RS, Mittler, Vella AT. Peptide-specific CD8 regulatory cells use IFN-γ to elaborate TGF-β suppression. J Immunol. 2003;174:7625–7632. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 31.Menoret A, Myers LM, Lee S-J, Mittler RS, Rossi RJ, Vella AT. TGFβ protein processing and activity through TCR triggering of primary CD8+T regulatory cells. J Immunol. 2006;177:6091–6097. doi: 10.4049/jimmunol.177.9.6091. [DOI] [PubMed] [Google Scholar]
- 32.Jiang L, He H, Yang P, Lin X, Zhou H, Huang X, Kijlstra A. Splenic CD8+ T cells secrete TGF-β1 to exert suppression in mice with anterior chamber-associated immune deviation. Graefes archive for experimental and clinical ophthalmology. 2008;(8) doi: 10.1007/s00417-008-0947-8. On Line First:October. [DOI] [PubMed] [Google Scholar]
- 33.Chen JW, Wahl SM. Engagement of cytotoxic lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGFbeta)production by murine CD4 (+) T cells. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchal G, Seman M, Milon G, Truffa-Bachi P, Zilberfarb V. Local adoptive transfer of skin delayed-type hypersensitivity initiated by a single T lymphocyte. Journ Immunol. 1982;129:954–958. [PubMed] [Google Scholar]
- 35.McCluskey RT, Benacerraf B, McCluskey J. Studies on the specificity of the cellular infiltrate in delayed hypersensitivity reactions. Jour Immunol. 1963;90:466–77. [PubMed] [Google Scholar]
- 36.Yazdi A, Goreschi K, Rocken M. Inflammasome activation in delayed-type hypersensitivity reactions. Journ of Investi Dermat. 2007;127:1853–1855. doi: 10.1038/sj.jid.5700815. [DOI] [PubMed] [Google Scholar]


