Abstract
Objective
Asian adults are at greater risk for metabolic abnormalities (insulin resistance, dyslipidemia) at the same BMI than are Caucasians. Elevated FFA and decreased adiponectin are linked with these same metabolic abnormalities.
Research Design and Methods
We tested the hypothesis that fasting plasma FFA are greater and adiponectin concentrations are lower in Korean that Caucasian adults matched for age, sex and BMI. Plasma FFA and adiponectin concentrations were analyzed using a microflourometric assay and radioimmunoassay, respectively.
Results
Fasting plasma FFA concentrations were not different (P=0.51) between Korean and Caucasian subjects 208 (183–232) vs. 215 (168–262) (median and 95% CI). Despite similar body composition in the two groups, the plasma adiponectin concentrations in Koreans were significantly lower than Caucasians in men, women and total subgroups (adjusted mean ± SEM; 4.9 ± 0.8 vs. 9.1 ± 0.8 μg/ml, P=0.004, 8.9 ± 1.0 vs. 13.2 ± 1.0 μg/ml, P=0.006, and 6.5 ± 0.6 vs. 11.1 ± 0.6 μg/ml, P<0.001), respectively, after adjustment for differences in height, weight and fat free mass as covariates. Men had lower plasma adiponectin concentrations than women in both Korean (P=0.041) and Western adults (P<0.001).
Conclusion
Plasma adiponectin levels are lower in Korean than age-, sex- and body mass index-matched Caucasian adults whereas fasting plasma FFA are not different. To the extent adipogenic factors account for ethnic differences in metabolic disease risk, our data suggests that differences in the regulation of adiponectin may predispose towards greater metabolic abnormalities in Asians than Caucasians at comparable BMI levels.
Keywords: ethnicity, body composition, lipolysis
INTRODUCTION
Different cut points have been suggested for the diagnosis of obesity and upper body obesity according to race or gender [1], because the risk of co-morbidities is greater for Asians than Caucasians at comparable body mass index (BMI), waist circumference (WC) and intra-abdominal fat (IAF) [2–5]. It is not yet known why Asians are more vulnerable to obesity-related diseases at similar BMI as Caucasians as regards potential pathophysiologic mechanisms.
Excess free fatty acids (FFA) are thought to contribute to at least some of the metabolic abnormalities associated with central or upper-body obesity, including dyslipidemia and insulin resistance [6]. Unfortunately, although the ethnic differences in amounts of visceral adipose tissue had been already evaluated, direct comparison of plasma total FFA concentrations in different ethnic groups has not yet been reported. Kamei et al. [7] reported no significant difference in fasting FFA concentration between native Japanese and Japanese Americans. Japanese Americans in this study, however, had older, higher BMI, and higher percent body fat than native Japanese. Adiponectin is another adipogenic factor linked to metabolic outcomes in obesity, metabolic syndromes and cardiovascular complications of these disorders [8]. In general, Caucasians seem to have greater plasma concentrations of adiponectin than other ethnic populations [9–14] and Kadowaki et al. [9] showed that even though American men were heavier, they had higher adiponectin concentrations than Japanese men. We could find no direct comparison of plasma FFA and adiponectin concentrations between Caucasian and Asian men and women who are matched for BMI and percent body fat.
We hypothesized that plasma total FFA and adiponectin concentrations are different in Asians and Caucasians after matching for age, sex, and BMI. We tested whether plasma FFA concentrations would be greater and adiponectin concentrations would be less in matched Korean and Caucasian adults.
RESEARCH DESIGN AND METHODS
Subjects and Experimental Design
Forty seven healthy Koreans and 47 age, sex, and BMI-matched Caucasians with a BMI ranging from 18.9 to 30.3 kg/m2 participated in this study, which was approved by both the Pusan National University Institutional Review Board and the Mayo Clinic Institutional Review Board. Height and weight were measured by an automatic height-weight scale to the nearest 0.1 cm and 0.1 kg, respectively, and BMI was calculated by dividing weight (kg) by height squared (m2). Percentage body fat and total fat mass were measured by bioelectric impedance analysis (Inbody 3.0, Biospace Co., Ltd, Korea) for Korean subjects, by dual energy x-ray absorptiometry (DPX-IQ, Lunar Radiation Corp, Madison, WI) for the Caucasian subjects. Blood samples were obtained from each subject after a 12-hour overnight fast by evacuation from an antecubital vein into vacutainer tubes.
Analytical Methods
Total plasma FFA concentrations were measured using automated enzymatic techniques on the Cobas Fara centrifugal analyzer (Cobas Mira, Roche Diagnostics Ltd, Switzerland) at 340 nm (for FFA: FFA-C test kit; Wako Chemicals, Neuss, Germany). The FFA standard curve was prepared in our laboratory, containing 22 % linoleic acid, 28 % oleic and palmitic acid, 6% stearic and myristic acid, and 3% each of elaiadic acid, palmitoleic acid, linolenic acid and arachadonic acid bound to fatty acid-free bovine serum albumin. This was because the oleic acid standard provided with the kit resulted in total FFA concentrations 30% below those measured by HPLC, likely because of the kinetics of the reaction differ between different FFA species. All samples were analyzed in the same assay and standards were run at the beginning, middle, and end of the run. Samples from Korean and Caucasian volunteers were intermixed to avoid potential ordering effects. Plasma adiponectin concentration was determined with a double antibody radioimmunoassay kit (Linco Research, Inc. St. Charles, MO). Intra-assay coefficient of variation was 23%, 6.6% and 5.1% at 2.8, 28.5 and 64.1 ng/mL, respectively.
Statistical analysis
The mean and standard deviation of fasting plasma FFA and adiponectin concentrations from previous studies were used to develop the statistical power for this study. To test the hypothesis that fasting plasma FFA and adiponectin concentrations in Koreans would be ≥ 20% greater than Caucasians (one-sided test) a sample size of 47 subjects per group provides 94% power to detect this difference with an alpha of 5%. Relations between continuous variables were analyzed by means of the calculation of Pearson or partial correlation coefficients or multivariate analysis as appropriate. Results are presented as mean and SD, SEM, or median and 95% CI, as appropriate. We used analysis of covariance (ANCOVA) to determine differences in adjusted mean adiponectin between Koreans and Caucasians after controlling for several covariates. A p-value <0.05 was deemed statistically significant. SPSS 11.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
RESULTS
By design, there was no difference in age, sex, and BMI, between Korean and Caucasian volunteers. Although Korean participants were shorter, weighed less, and had less fat free mass compared with Caucasian subjects, percent body fat was not different between the two groups (Table 1). 80% of the volunteers were in the BMI range 18.9–24.9 kg/m2, 18% had a BMI 25–29.9 kg/m2 and only 2% of the subjects had a BMI ≥30 kg/m2.
Table 1.
Characteristics of Korean and Caucasian groups
| Korean (N = 47) | Caucasian (N = 47) | P value | |
|---|---|---|---|
| Age (yrs) | 39.8 ± 6.9 | 38.7 ± 6.7 | 0.140 |
| Sex (M:F) | 26: 21 | 26: 21 | 1.000 |
| Height (cm) | 164.9 ± 8.0 | 174.2 ± 10.8 | <0.001 |
| Weight (Kg) | 63.4 ± 11.0 | 70.7 ± 15.1 | <0.001 |
| BMI (Kg/m2) | 23.1 ± 2.8 | 23.1 ± 2.9 | 0.344 |
| Percent body fat (%) | 24.3 ± 4.3 | 25.7 ± 7.4 | 0.079 |
| Fat free mass (Kg) | 44.3 ± 8.3 | 52.3 ± 12.7 | <0.001 |
| Total plasma FFA (μmol/l) | 208 (183–232) | 215 (168–262) | 0.563 |
| Adiponectin (ug/ml) | 7.1 ± 4.1 | 10.8 ± 4.4 | <0.001 |
Data were expressed as mean ± SD excepting sex (number) and total plasma FFA (median and 95% CI). By paired t-test except McNemar’s test for sex, Wilcoxon signed-rank test for total plasma FFA comparison
Percent body fat was negatively correlated with height and fat free mass. Fat free mass was positively correlated with height, weight, and BMI. Women had a tendency to have a higher body fat percent and higher fat free mass than men (data not shown).
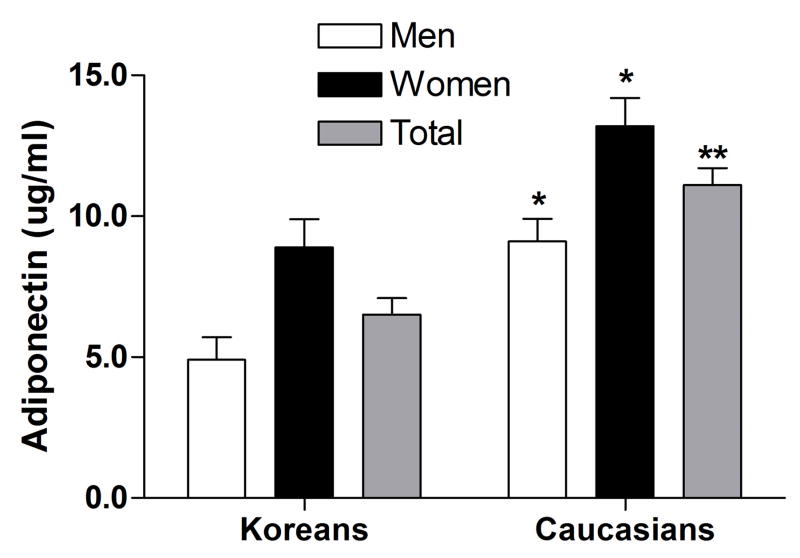
We did not find a significant difference in plasma FFA concentrations between Korean and Caucasian subjects (Table 1, P=0.51). We found no significant correlation between plasma FFA and BMI or percent body fat in either Korean or Caucasian people (data not shown). Plasma adiponectin concentrations were lower in Korean than Caucasian adults (P<0.001, Table 1). Men had lower adiponectin levels than women in both Korean (P=0.041) and Western population (P<0.001, Fig. 1). By simple regression analysis adiponectin was significantly and negatively correlated with BMI and free fat mass (P<0.05) in Korean adults and negatively correlated only with BMI (P<0.05) in Caucasian adults. No other variable was significantly associated with adiponectin. When two group were combined, ethnicity (P=0.001) was the only independent predictors of plasma adiponectin in a multivariate analysis. The main effects of ethnicity on plasma adiponectin concentrations remained significant (P<0.001), after controlling for height, weight and fat free mass as covariates with ANCOVA. The plasma adiponectin concentrations in Koreans were significantly lesser than with Caucasians in men, women and total subgroups (adjusted mean ± SEM; 4.9 ± 0.8 vs. 9.1 ± 0.8 μg/ml, P=0.004, 8.9 ± 1.0 vs. 13.2 ± 1.0 μg/ml, P=0.006, and 6.5 ± 0.6 vs. 11.1 ± 0.6 μg/ml, P<0.001), respectively.
Figure 1.
Comparison of plasma adiponectin concentration in men (white bars), women (dark grey bars) and total (light grey bars) subjects between Koreans and Caucasians. Values represent adjusted means ± SEM after adjustment for BMI and fat free mass as covariates (additional adjustment for sex in total). *P<0.005, **P<0.001 by ANCOVA
DISCUSSION
This is the first study, to our knowledge, to compare the concentration of two important adipogenic factors in metabolic disease - plasma FFA and adiponectin - directly among Koreans with similar age, sex, and BMI as Caucasians. Previous studies indicate ethnic differences in the amount of IAF, a factor associated with abnormal FFA and adipokines, between Asians and Caucasians at the same WC levels [4, 5]. A lower threshold of WC to define abdominal adiposity\disease risk has been proposed for south Asian populations compared with Caucasian populations [1]. It seemed likely to us that there might be differences in some key adipogenic factors that relate to metabolic complications of obesity. We found that, although fasting FFA concentrations were not different, adiponectin concentrations were lower in Korean than Caucasian men and women.
Plasma FFA, the major circulating lipid fuel in postabsorptive humans, originates from adipose tissue lipolysis. Adiponectin is an adipocyte-derived hormone which is thought to have anti-inflammatory and anti-atherogenic effects [12, 15] and is found at relatively high concentrations in human plasma ranging from 5 to 30 μg/ml. The adiponectin mRNAs and its plasma levels are reduced in obesity and its related co-morbidities. Recently, racial and ethnic differences have been reported in plasma adiponectin levels between Caucasian and other racial or ethnic groups [9–14]. To our knowledge, only a single study evaluated ethnic difference in adiponectin concentration between Eastern Asians who remain in their country of origin and Caucasians [9]. However, this study reported results only men, unmatched for age and BMI. Other studies have included mainly overweigh/obese men or women or patients with diabetes mellitus or coronary artery disease [10–14]. Therefore, we hypothesized racial difference in fasting plasma FFA or adiponectin concentrations could partially explain why Asians generally tended to have a higher risk of metabolic abnormalities at a lower WC and BMI than Caucasians.
Asians consume less fat and more carbohydrate than Caucasians [16]. Our results suggest that there is maybe limited inter-racial variability in fasting plasma FFA concentrations between people with similar normal and overweight BMI. This suggests that the regulation of FFA availability may be similar in different ethnic groups, although food items or the amount of dietary composition consumed varies widely between populations. In this study, Caucasians were taller, weigh more, and free fat mass more compared with Koreans for the same BMI as in previous studies [17].
We found that Korean adults had significantly (P<0.001) lower adiponectin levels than Caucasians even after adjustment for confounding covariates. The prevalence of hypoadiponectinemia (<4.0 μg/mL) was 23.9% in Korean adults, which was higher than in Caucasian adults (6.5%). In addition, we observed that women had higher adiponectin levels than men in both Korean and Western population. This findings are consistent with those of most [12, 18], but not all [19], studies. Some of these sex differences in adiponectin may result from differences in the numbers and sizes of fat cells or sexual hormone [18, 19]. In addition, we found that fat free mass, not body fat percent was negatively correlated with plasma adiponectin concentration. However, some studies have shown lower adiponectin levels in subjects with higher body fat percent [20–22], whereas other studies did not show such a relationship [13, 23, 24], which are consistent with our study. This discrepancy may be attributed, in part, to the differences in BMI, co-morbidity, age distribution or sample size amongst the subjects of previous studies. Possible mechanisms underlying the ethnic difference in adiponectin concentrations include differences in fat distribution, genetic polymorphisms in genes regulating adiponectin synthesis, secretion or degradation, and diet [2, 10, 13, 14, 16].
Our study has some limitations, including the lack of information regarding visceral fat, which could be an important predictor of adiponectin. We used, bioimpedance analysis (BIA) for body composition for Koreans and dual energy X-ray absorptiometry (DXA) methods for Caucasians in this study, although BIA has been shown to be accurate in comparison to DXA [25]. Although the generalizabilty of our study to all Asians and Caucasians is uncertain (all of the participants were Korean and Americans), nevertheless recent reports of similar differences between Caucasians and other ethnic groups [9–14] suggest this phenomenon is not limited to Korean and American Caucasians. Finally, we do not know whether there are ethnic differences in postprandial FFA concentrations or differences in other adipokines between these populations.
In conclusion, we found significant difference in plasma adiponectin, but not total plasma FFA concentrations between Koreans and Caucasians at the same BMI. Our findings suggest that total FFA concentrations are well-regulated within the normal range in the overnight postabsorptive state even in different ethnic groups consuming different diets. However, ethnic difference in adiponectin concentrations is consistent with the greater vulnerability of Asians to obesity-related diseases than Caucasian [26].
Table 2.
Correlation coefficients (r) of plasma adiponectin levels, versus body mass index, percent body fat, free fat mass, and total plasma free fatty acid in both race with the adjustment for age and gender
| Variables | Korean (N=47) |
Caucasian (N = 47) |
||
|---|---|---|---|---|
| r | P value | r | P value | |
| Body mass index (kg/m2) | −0.34 | 0.028 | −0.36 | 0.018 |
| Percent body fat (%) | −0.20 | 0.209 | −0.15 | 0.351 |
| Fat free mass (Kg) | −0.37 | 0.017 | −0.29 | 0.065 |
| Free fatty acid (μmol/l) | 0.17 | 0.277 | 0.05 | 0. 733 |
Statistics were tested by partial correlation analysis
Acknowledgments
We would like to thank Deborah Harteneck for technical assistance with this study.
This study was supported by DK45343 and UL1 RR024150 from the U.S. Public Health Service, 7-07-DCS-03 from the American Diabetes Association and by the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James WP. Assessing obesity: are ethnic differences in body mass index and waist classification criteria justified? Obes Rev. 2005;6:179–81. doi: 10.1111/j.1467-789X.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–7. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 5.Han JH, Park HS, Kim SM, Lee SY, Kim DJ, Choi WH. Visceral adipose tissue as a predictor for metabolic risk factors in the Korean population. Diabet Med. 2008;25:106–10. doi: 10.1111/j.1464-5491.2007.02317.x. [DOI] [PubMed] [Google Scholar]
- 6.Koutsari C, Jensen MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Res. 2006;47:1643–50. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Kamei N, Yamane K, Nakanishi S, Ishida K, Ohtaki M, Okubo M, et al. Effects of a westernized lifestyle on the association between fasting serum nonesterified fatty acids and insulin secretion in Japanese men. Metabolism. 2005;54:713–8. doi: 10.1016/j.metabol.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Rabin KR, Kamari Y, Avni I, Grossman E, Sharabi Y. Adiponectin: linking the metabolic syndrome to its cardiovascular consequences. Expert Rev Cardiovasc Ther. 2005;3:465–71. doi: 10.1586/14779072.3.3.465. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Sekikawa A, Okamura T, Takamiya T, Kashiwagi A, Zaky WR, et al. Higher levels of adiponectin in American than in Japanese men despite obesity. Metabolism. 2006;55:1561–3. doi: 10.1016/j.metabol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 11.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004;53:1–3. doi: 10.1016/j.metabol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Tayebjee MH, Tan KT, Patel JV, Macfadyen RJ, Lip GY. Serum adiponectin in coronary heart disease: ethnic differences and relation to coronary artery disease severity. Heart. 2005;91:1605–6. doi: 10.1136/hrt.2004.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shand B, Elder P, Scott R, Poa N, Frampton CM. Comparison of plasma adiponectin levels in New Zealand Maori and Caucasian individuals. N Z Med J. 2007;120:U2606. [PubMed] [Google Scholar]
- 14.Martin M, Palaniappan LP, Kwan AC, Reaven GM, Reaven PD. Ethnic differences in the relationship between adiponectin and insulin sensitivity in South Asian and Caucasian women. Diabetes Care. 2008;31:798–801. doi: 10.2337/dc07-1781. [DOI] [PubMed] [Google Scholar]
- 15.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–74. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez B, Sakata K, et al. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: the INTERLIPID study. J Hum Hypertens. 2003;17:631–9. doi: 10.1038/sj.jhh.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Thornton JC, Heymsfield SB, Pierson RN., Jr The relationship between body mass index and body cell mass in African-American, Asian, and Caucasian adults. Acta Diabetol. 2003;40(Suppl 1):S305–S8. doi: 10.1007/s00592-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 18.Abbasi F, Chang SA, Chu JW, Ciaraldi TP, Lamendola C, McLaughlin T, et al. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol 2006. 2006;290(1):R139–R44. doi: 10.1152/ajpregu.00287.2005. [DOI] [PubMed] [Google Scholar]
- 19.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–31. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–8. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 21.Silha JV, Nyomba BL, Leslie WD, Murphy LJ. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care. 2007;30:286–91. doi: 10.2337/dc06-1073. [DOI] [PubMed] [Google Scholar]
- 22.Hara T, Fujiwara H, Nakao H, Mimura T, Yoshikawa T, Fujimoto S. Body composition is related to increase in plasma adiponectin levels rather than training in young obese men. Eur J Appl Physiol. 2005;94:520–6. doi: 10.1007/s00421-005-1374-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim C, Park J, Park J, Kang E, Ahn C, Cha B, et al. Comparison of body fat composition and serum adiponectin levels in diabetic obesity and non-diabetic obesity. Obesity. 2006;14:1164–71. doi: 10.1038/oby.2006.133. [DOI] [PubMed] [Google Scholar]
- 24.Sobngwi E, Effoe V, Boudou P, Njamen D, Gautier JF, Mbanya JC. Waist circumference does not predict circulating adiponectin levels in sub-Saharan women. Cardiovasc Diabetol. 2007;6:31. doi: 10.1186/1475-2840-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolanowski M, Nilsson BE. Assessment of human body composition using dual-energy x-ray absorptiometry and bioelectrical impedance analysis. Med Sci Monit. 2001;7:1029–33. [PubMed] [Google Scholar]
- 26.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110:267–78. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]