Abstract
Background
Nanotechnology-based drug delivery approaches may help increase therapeutic efficacy and decrease side effects of chemotherapeutics. We investigated expression levels of folate receptor (FR) in squamous cell carcinoma of the head and neck (SCCHN) to evaluate FR as a target for nanotherapy.
Methods
FR expression levels in archival SCCHN tissues were analyzed by immunohistochemistry and correlated with clinical parameters.
Results
FR was detected in 45% of primary tumors and 40% of corresponding lymph node metastases (LNM). FR expression in primary tumors of the metastatic group strongly correlated with the corresponding LNM (p=0.0002). FR expression was inversely correlated with disease-free survival in non-metastatic (p=0.0048), metastatic (p=0.0127), and LNM (p <0.001) groups, and with overall survival in the LNM group (p <0.0001).
Conclusions
FR is expressed in a significant proportion of primary SCCHN and corresponding LNM tissues, and correlates with worse clinical outcome. These findings provide support for FR-mediated nanotherapeutics in SCCHN.
Keywords: folate receptor, nanotechnology, head and neck cancer, squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common cancer worldwide and the most common neoplasm in the upper aerodigestive tract. In the United States, head and neck cancer accounts for 3 to 5 percent of all cancers. Approximately 40,000 new cases and more than 11,000 deaths are estimated in 2006 [1]. Despite advances in conventional therapies, including surgery, radiation, and chemotherapy, the overall survival rate for SCCHN has not significantly improved in the past three decades, and the majority of patients with SCCHN stage III or IV disease will die from their disease [2, 3]. Newer treatment modalities are therefore needed to improve outcomes for this group of patients.
The folate receptor (FR) is a confirmed tumor-associated antigen that binds folate and folate–drug conjugates with very high affinity and shuttles these bound molecules inside cells via an endocytic mechanism [4]. Expression of FR is significant in different types of human carcinomas including carcinomas of the kidney, ovary, endometrium, lung, breast, bladder and pancreas, making the development of new FR-targeted therapies, and the need to quantify functional FR levels in different tissue specimens, a topic of interest [4]. Folic acid, unlike antibodies, hormones or other ligands, is internalized to fulfill essential cellular functions, and hence remains retained in endocytic compartments or is released with its attachments into the cytoplasm rather than being trafficked to lysosomes [5]. It has been known for more than a decade that simple covalent attachment of folic acid (or a pteroate-based ligand) to virtually any macromolecule produces a conjugate that can be internalized into FR-bearing cells in an identical fashion to that of free folic acid [6].
There are potential advantages of receptor-targeted drug delivery over the conventional use of systemic therapy in that the receptor-mediated approach can reduce drug exposure to normal tissues and hence reduce potential toxicities, in addition to potentially enhancing drug uptake by malignant cells. Indeed, studies have shown that a significant advantage of FR-mediated delivery is the surprisingly low level of toxicity to normal tissues. Studies of a folate-targeted immunotherapeutic drug currently in phase 1 clinical trials have not shown evidence of toxicity in several animal species, even in some cases at supratherapeutic doses delivered over prolonged times [7].
There has been a wide range of successful applications of folate-drug conjugate delivery, including that of proteins and protein toxins [6, 8], radiopharmaceutical agents [9, 10], low molecular weight chemotherapeutic agents [11, 12], antisense oligonucleotides and ribozymes [13, 14], liposomes with entrapped drugs [15, 16], and finally drug-loaded nanoparticles [17-19].
Recent developments in nanotechnology have provided researchers with new tools for detection, imaging, and treatment of human cancer. This technology has enabled the development of nanoscale devices that can be conjugated with several functional molecules simultaneously, such as tumor-specific ligands, antibodies, anticancer drugs, and imaging probes. Since these nanodevices are 100-1000 fold smaller than cancer cells, they can easily move in and out of leaky blood vessels and interact with receptors both on the surface of and inside tumor cells [20]. Therefore, their application as tumor cell-specific delivery vehicles for imaging probes and therapeutics is ideal.
We are currently developing a nanoparticle formulation of paclitaxel conjugated to a tumor targeting folate acid (FA) ligand, with the objective of achieving greater drug delivery, and possibly improved pharmacokinetics, To that end, we have examined whether the folate receptor (FR) is a good candidate for tumor-targeted agents in SCCHN, by investigating the FR expression status of SCCHN tissue samples. We retrospectively examined the clinical characteristics of patients with SCCHN treated at the Winship Cancer Institute and the Emory Clinic, and correlated disease free survival and overall survival with FR expression.
Material and Methods
Archival paraffin-embedded tissue specimens from primary SCCHN cases with (48 cases) and without (47 cases) corresponding lymph node metastases were used for immunohistochemical studies. The clinical characteristics of the patients were retrieved from the files in the Department of Pathology and Laboratory Medicine at Emory University. The histological grading of SCCHN was determined by one pathologist (SM) using H&E stained sections according to published criteria. All tissue specimens and appropriate clinical information were obtained under the guidelines and approval of the Institutional Review Board of Emory University School of Medicine, Atlanta, GA.
Immunohistochemistry
Folic acid receptor (FR) expression in tissue specimens was determined using a goat anti-human FR polyclonal antibody (sc-16387, 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Goat IgG at 1:100 dilution was used as a negative control. Immunohistochemical analysis on formalin-fixed, paraffin-embedded human specimens was performed according to a modified procedure. In brief, after deparaffinization with xylene and rehydration with EtOH, endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide with methanol for 15 minutes. To retrieve the antigens, the tissue slides were heated in a microwave oven in 100M sodium citrate buffer (pH 6.0) for 10 minutes and then allowed to remain at room temperature for 20 minutes. After being washed in PBS, the slides were incubated with serum blocking solution (2.5% normal horse serum, Vector Laboratories, Burlingame, CA) containing Avidin (Blocking Kit, Vector Laboratories) for 30 minutes to decrease the background signal. Next, the slides were incubated with a 1:100 dilution of anti-FR primary antibody at 4°C overnight, and washed with PBS. Then the slides were incubated with a biotinylated secondary antibody for 20 minutes at room temperature and with biotin-avidin peroxidase conjugate (ABC kit, Vector Laboratories) for 15 minutes at room temperature. The substrate (0.1% 3.3′-diaminobenzidine solution, Sigma Chemical Co., St. Louis, MO, in PBS with 0.01% hydrogen peroxide) was then added for 5 minutes. Finally, the slides were counterstained with hematoxylin for 50 seconds (Vector laboratories) and observed by light microscopy.
Evaluation of folate receptor expression
All tissue sections were evaluated by a single pathologist (SM). Adjacent normal epithelium was also evaluated and graded. The level of FR expression was considered positive when characteristic membrane and cytoplasm immunoreactivity was present. Cases with no staining were scored a 0, cases with weak staining (<25% of cells) were scored a 1, and 2+ when >25% cell staining was noted (Figure 1). This scoring system was adopted from a recent publication examining FR expression in archival paraffin-embedded tissue from colorectal cancer [21]. Ten bone marrow aspiration cell blocks were used as a control.
Figure 1.
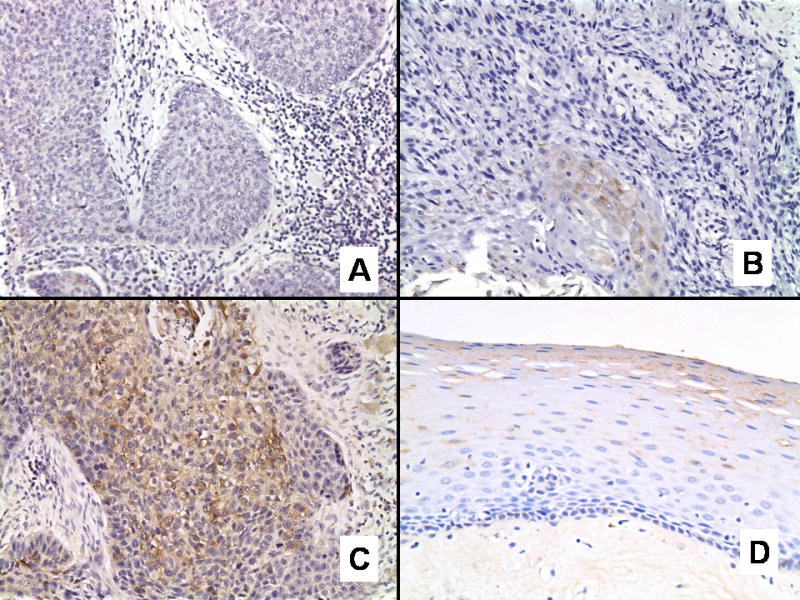
Folate receptor expression in head and neck squamous cell carcinoma exhibiting (A) negative, (B) weak (1+), and (C) strong (2+) immunohistochemical staining. (D) Adjacent normal epithelium exhibiting negative staining for folate receptor.
Statistical analysis
McNemar test was used to compare FR expression (expressed as binary) between the primary and lymph node metastatic groups [22] and Fisher exact test was used to compare FR expression between the no metastatic group and primary metastatic and no metastatic group and lymph node metastatic group [23]. Kaplan-Meier curves and log-rank tests were used to correlate FR expression with disease free survival (DFS) and overall survival (OS) in each group [24] and stratified log-rank test was used to correlate FR to DFS/OS by tumor group. A p-value < 0.05 was considered statistically significant in all analyses.
Results
Patient characteristics
A total of 143 specimens were available for analysis from 95 patients with SCCHN. Of these, 47 patients (49%) had non-metastatic and 48 (51%) had metastatic disease. The median age was 62 years (yrs) (22-88 yrs) and the male/female ratio was 3/1. The tumor grades were defined as moderately differentiated in 62 (65%), well differentiated in 17 (18%), and poorly differentiated in 16 (17%) patients (Table 1).
Table 1.
Patients Characteristics
| Patients with Non metastatic tumors N(%) | Patients with metastatic tumors N(%) | ||
|---|---|---|---|
| Total | 47 | 48 | |
|
| |||
| Sex | |||
| M | 31(66) | 9(19) | |
| F | 16(34) | 39(81) | |
|
| |||
| Grade | |||
| WD | 15(32) | 2(4) | |
| MD | 30(64) | 36(75) | |
| PD | 2(4) | 10(21) | |
|
| |||
| Site | |||
| OP | 5(11) | 24(50) | |
| L | 16(34) | 7(15) | |
| OC | 26(55) | 13(27) | |
| HP | 0(0) | 4(8) | |
|
| |||
| FR | |||
| 0 | 29(62) | 24(50) | |
| +1 | 7(15) | 10(21) | |
| +2 | 11(23) | 14(29) | |
Footnote: Abbreviations: M=male, F=female, WD=well differentiated, MD=moderately differentiated, PD=poorly differentiated, OP=oropharynx, L=larynx, HP= hypopharynx, OC=oral cavity, FR=folate receptor, N=numbers
Clinical characteristics
The median overall survival for all patients was 4.8 years. The 3 and 5 year overall survival rates were 68% and 43%, respectively (Figure 2). The median disease free survival was not reached, with a 3 and 5 yr disease free survival of 65% and 57%, respectively (Figure 2).
Figure 2.
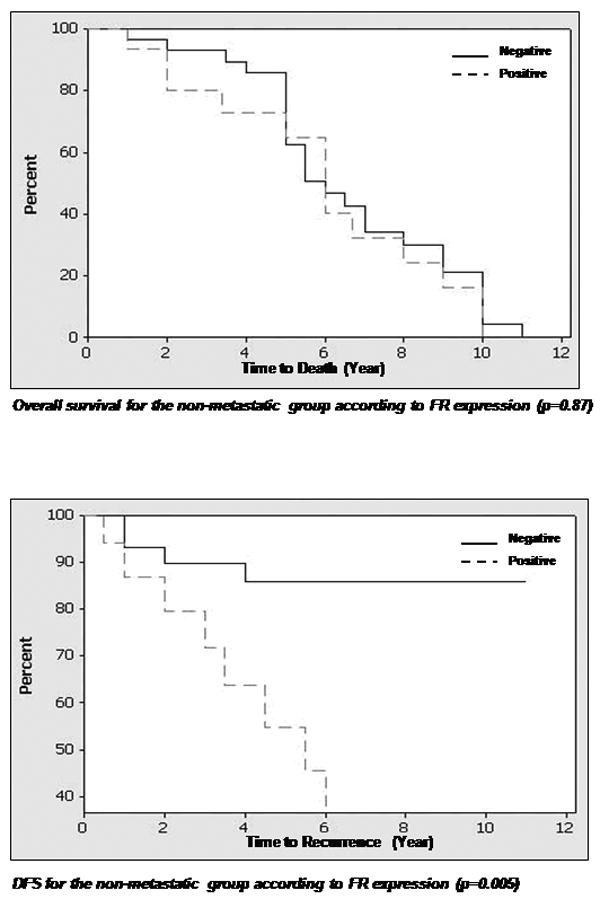
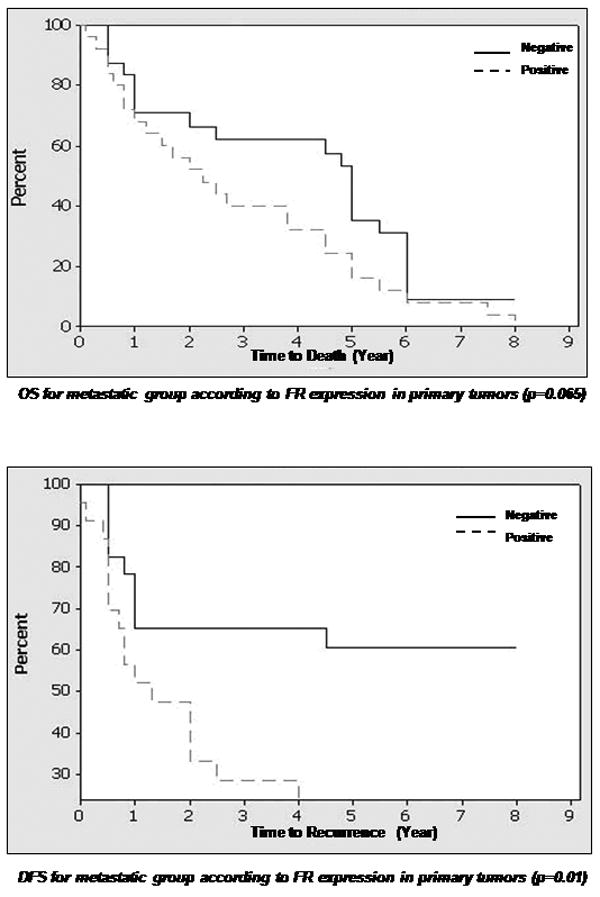
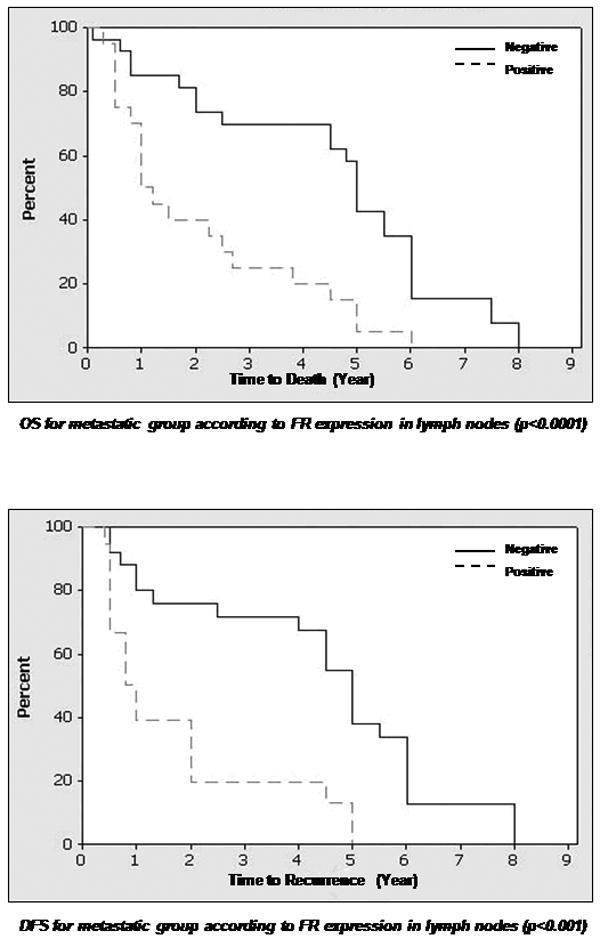
Percentage of patients with disease free survival and overall survival (in years) in relation to folate receptor expression (negative or positive) in primary and metastatic tissues.
Footnote: Abbreviations: DFS=disease free survival, OS=overall survival Legend: line= Zero FR expression, dashed line = any positive expression (+1 to +2), FR=folate receptor
Folate receptor expression
FR expression of 1+ or more was detected in 43 (45%) primary tumors and 19 (40%) lymph node metastases. An expression level of 2+ or more was detected in 24 (25%) primary tumors and 11 (23%) lymph node metastases. Both cytoplasmic and membrane staining were noted in the tumor cells. No statistical differences were noted between patients in the 1+ and 2+ expression levels of FR. In our data analysis that correlated expression with clinical parameters, the expressers were separated from the non-expressers and the analysis compared these two groups only. There was a correlation between positive FR expression in primary tumors of the metastatic group and the corresponding lymph node metastasis (p=0.0002). Positive FR expression was also correlated with disease free survival in the non-metastatic group (p=0.0048) and the metastatic group (p=0.0127). Furthermore, a correlation was noted between positive FR expression in lymph node metastases and overall survival (low expression associated with longer survival, p <0.0001), as well as disease free survival (high expression associated with earlier recurrence, p <0.001). No correlation was found between FR expression in primary tumors of the non-metastatic and metastatic groups (p=0.563). No correlation existed between FR expression in primary tumors of non-metastatic group and lymph node metastases of the metastatic group (p= 0.81). Adjacent normal epithelium was present in 23 of 48 primary tumors in the metastatic group, and only 2 cases showed FR expression. The staining was focal, weak and confined to the parakeratin and spinous layer. In the non-metastatic group, 28 of 47 cases had adjacent normal epithelium to examine, with 4 cases exhibiting 1+ staining similar to that described for the metastatic group. No expression was noted in the normal tissues of the mucosa. None of the 10 bone marrows examined from cancer-free patients expressed FR.
Discussion
Our findings demonstrate that FR is expressed in a significant proportion of SCCHN tissues, both in primary tumors and the corresponding lymph node metastases, and appears to be correlated with clinical outcome. FR expression was an important clinical predictor whether a few (1+) or a majority (2+) of tumor cells had positive FR expression. FR expression appears to be retained in metastatic lymph nodes, suggesting a strong potential for FR as a target for drug delivery in both primary and metastatic SCCHN. Our data suggest a correlation between FR expression and earlier recurrence, indicating that FR expression may be a marker for a more aggressive tumor phenotype. Negative FR expression in our study was associated with longer survival. This further justifies the use of FR-targeted therapies in a subset of SCCHN that seems to exhibit a more aggressive behavior.
It is unclear why the overall survival difference was seen only with the group that had lymph node metastases. Obviously other clinical factors that were not controlled for may have affected the overall survival in the non metastatic group and made it such that the difference between the groups with detectable versus undetectable FR expression translated only into a disease free survival, and not an overall survival difference. This could also indicate that FR expression may have a more significant prognostic importance once the disease is metastatic to regional lymph nodes, which may be an interesting subject for reexamination in other studies.
Considering the potential toxicity to hematopoietic cells that may result from delivery of cytotoxic agents through the FR, bone marrows from 10 cancer free patients were examined for FR expression. None of the marrow specimens expressed FR.
Understanding the biological principles that determine the presence and level of FR expression in SCCHN and other malignancies could open the door for better understanding of carcinogenesis and tumor progression. It is still unknown whether high expression of FR is responsible for initiation of the carcinogenic process or if malignancy is a precondition for elevated expression of FR. Positive FR receptor was associated with a worse clinical outcome in our study, and its known association with tumor stage and grade in other studies [25] raises questions regarding its role in tumor progression. Because FR is expressed and accessible in multiple malignancies and seems to be expressed selectively in pathologic cells, its targeting allows the delivery of non-specific drugs selectively into these cells, while normal tissues that lack FR are spared the toxicity of these drugs. Also, unlike other targeted therapies such as cetuximab or Herceptin that target markers linked specifically to a known disease, the targeting of FR allows delivery of non-specific drugs selectively into pathologic cells. What may add to the complexity of the picture however, is the heterogeneity of the FR as documented by studies confirming the existence of three distinct mutations of the FR in a squamous cell carcinoma cell line affecting the functional status of the receptor [26]. The observation that FR levels are high in malignant tumors of epithelial origin compared to normal cells, and are associated with tumor stage and grade, raises the question of its role in tumor etiology and progression [27]. FR mutations may reflect increased folate demands by tumors that offer a growth advantage; this has not been studied in SCCHN and deserves closer examination as it may affect any therapeutic applications using the FR as a target.
The recent interest in folate ternary nanoparticles conjugated to chemotherapeutic drugs, such as taxanes, has produced both in vitro and in vivo data that demonstrate many advantages over the unconjugated taxane counterpart. These advantages include tumor-specific targeting, decreased side-effects in normal cells, improved solubility and bioavailability, retained activity against multi-drug resistance, better antiangiogenic activity, and improved therapeutic index (unpublished data from Nie S, and Yang L). In this study, we have demonstrated the expression of FR in a substantial proportion of primary SCCHN tumors and the associated lymph node metastases, and correlated FR expression levels with clinical outcome. Although only 45% of tumors expressed FR in our study, this is still a very significant population that could potentially benefit from this technology, especially when compared with other targeted therapies that have become standard of care, such as Herceptin for breast cancer. Together, these findings open the door for the further development of FR-mediated nanotherapeutic drug delivery in SCCHN. Indeed, we plan to develop similar molecules using heparin or polyglutamic acid as the carrier and FA as the targeting ligand. These targeted nanotherapeutic agents have the potential advantages of reducing drug exposure to normal tissues, hence minimizing toxicities, and enhancing drug uptake by tumor cells.
Acknowledgments
Xu Wang's contribution to the study was equivalent to that of the first author. The study is supported in part by an NIH CCNE grant to DMS and SN and by GCC Scholars grants to DMS, SN and GC.
Footnotes
Paper was presented as a late breaking poster at the American Association of Cancer Research meeting in Los Angeles, April of 2007
References
- 1.Jemal A, et al. Cancer Statistics. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Munro AJ. An overview of randomised controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer. 1995;71:83–91. doi: 10.1038/bjc.1995.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker N, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Analytical biochemistry. 2005;338:284–93. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Turek JJ, Leamon CP, L PS. Low, endocytosis of folate protein conjugates: ultrastructural localization in KB cells. J Cell Sci. 1993;106:423–30. doi: 10.1242/jcs.106.1.423. [DOI] [PubMed] [Google Scholar]
- 6.Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci, USA. 1991;88:5572–6. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases. Advanced Drug Delivery Reviews. 2004;56(8):1055–8. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Leamon CP, Low PS. Membrane folate-binding proteins are responsible for folate-protein conjugate endocytosis into cultured cells. Biochem J. 1993;291:855–60. doi: 10.1042/bj2910855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linder KE, Wedeking P, R K. In vitro and in-vivo studies with a- and g-isomers of Tc-OXA-Folate show uptake of both isomers in folate receptor (+) KB cell lines. Proc 47th annual meeting Soc Nuc Med; 2000. p. 119P. [Google Scholar]
- 10.Leamon CP, et al. Synthesis and biochemical evaluation of EC20: a new folate derived Tc-based radiopharmaceutical. Bioconjug Chem. 2002;13(6):1200–10. doi: 10.1021/bc0200430. [DOI] [PubMed] [Google Scholar]
- 11.Ladino CA, et al. Folate-maytansinoids: target-selective drugs of low molecular weight. Int J Cancer. 1997;73:859–64. doi: 10.1002/(sici)1097-0215(19971210)73:6<859::aid-ijc16>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, et al. Synthesis and Evaluation of Taxol-Folic Acid Conjugates as Targeted Antineoplastics. Bioorganic and medicinal chemistry. 2002;10(7):2397–2414. doi: 10.1016/s0968-0896(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 13.Citro G, et al. Inhibition of leukaemia cell proliferation by folic acid-polylysine-mediated introduction of c-myb antisense oligodeoxynucleotides into HL-60 cells. Br J Cancer. 1994;69:463–7. doi: 10.1038/bjc.1994.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Huang L. Targeted delivery of antisense oligodeoxynucleotides by LPDII. J Liposome Res. 1997;7:63–75. [Google Scholar]
- 15.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269:3198–204. [PubMed] [Google Scholar]
- 16.Vogel K, Wang S, Lee RJ. Low, peptide-mediated release of folate-targeted liposome contents from endosomal compartments. J Am Chem Soc. 1996;118:1581–6. [Google Scholar]
- 17.Zhang Y, Kohler N, Z M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials Chem. 2002;13:1328–35. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 18.Oyewumi MO, Mumper RJ. Engineering tumor-targeted gadolinium hexanedione nanoparticles for potential application in neutron capture therapy. Bioconjug Chem. 2002;13:1328–35. doi: 10.1021/bc025560x. [DOI] [PubMed] [Google Scholar]
- 19.Oyewumi MO, Mumper RJ. Influence of formulation parameters on gadolinium entrapment and tumor cell uptake using folate-coated nanoparticles. Int J Pharm. 2003;251:85–97. doi: 10.1016/s0378-5173(02)00587-2. [DOI] [PubMed] [Google Scholar]
- 20.Hattori Y, Maitani Y. Folate-linked lipid-based nanoparticle for targeted gene delivery. Curr Drug Deliv. 2005;2(3):243–52. doi: 10.2174/1567201054368002. [DOI] [PubMed] [Google Scholar]
- 21.Shia J, et al. Immunohistochemical expression of folate receptor α incolorectal carcinoma: patterns and biological significance. Human Path. 2008;39:498–505. doi: 10.1016/j.humpath.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 22.McNemar Q. Review of “Statistical Inference: The Distribution-free Approach” by Eugene Edgington. Contemporary Psychology. 1970;15:651. [Google Scholar]
- 23.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85(1):87–94. [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 25.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119(2):243–50. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 26.O RB, Kamen BA. UMSCC38 cells amplified at 11q13 for the folate receptor synthesize a mutant nonfunctional folate receptor. Cancer Res. 1994;54(14):3905–11. [PubMed] [Google Scholar]
- 27.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119(2):243–50. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]


