Abstract
The GABAA receptor-mediated inhibitory transmission in prefrontal cortex (PFC) is implicated in cognitive processes such as working memory. Our previous study has found that GABAAR current is subject to the regulation of dopamine D4 receptors, a PFC-enriched neuromodulator critically involved in various mental disorders associated with PFC dysfunction. In this study, we have investigated the cellular mechanism underlying D4 modulation of GABAARs. We found that the density of surface clusters of GABAAR β2/3 subunits was reduced by D4, suggesting that the D4 reduction of GABAAR current is associated with a decrease in functional GABAARs at the plasma membrane. Moreover, the D4 reduction of GABAAR current was blocked by the actin stabilizer phalloidin and was occluded by the actin destabilizer latrunculin, suggesting that D4 regulates GABAAR trafficking via an actin-dependent mechanism. Cofilin, a major actin depolymerizing factor whose activity is strongly increased by dephosphorylation at Ser3, provides the possible link between D4 signaling and the actin dynamics. Because myosin motor proteins are important for the transport of vesicles along actin filaments, we also tested the potential involvement of myosin in D4 regulation of GABAAR trafficking. We found that dialysis with a myosin peptide, which competes with endogenous myosin proteins for actin-binding sites, prevented the D4 reduction of GABAAR current. These results suggest that D4 receptor activation increases cofilin activity presumably via its dephosphorylation, resulting in actin depolymerization, thus causing a decrease in the myosin-based transport of GABAAR clusters to the surface.
Prefrontal cortex (PFC),2 a brain region strongly associated with cognitive and emotional processes (1), is particularly critical for working memory, a mechanism for encoding and maintaining newly acquired, task-relevant information (2). Working memory relies on the coordinated sustained firing of PFC pyramidal neurons between the temporary presentation of a stimulus cue and the later initiation of a behavioral response (2). The synchronization of pyramidal neuron activity during working memory processes is controlled by GABAergic interneurons (3, 4). Impairments in GABA-mediated inhibition in the PFC have been considered a major mechanism for working memory disturbances in schizophrenia (5).
GABAergic neurotransmission is mediated by GABAA receptors, the heteropentameric ligand-gated ion channels located at inhibitory synapses at soma and proximal dendrites (6). After being assembled in endoplasmic reticulum, the GABAAR complex is targeted and clustered at synapses by receptor-associated proteins via unclear mechanisms (7). Postsynaptic GABAARs undergo constitutive endocytosis via a clathrin-mediated dynamin-dependent pathway (8). Depending on the subunit composition, GABAARs are internalized to peripheral endosomal compartments or perinuclear late endosomes (9, 10). Alterations in the assembly, trafficking, or function of GABAARs can lead to changes in GABAergic inhibition, which is often linked to the pathophysiology of various neurological disorders (11). For example, the GABAAR α2 subunit in the axon initial segment of PFC pyramidal neurons is up-regulated in schizophrenia (12). Schizophrenic patients show altered ratios of alternatively spliced transcripts of GABAAR γ2 subunit in PFC (13). Decreased GABAAR clustering results in enhanced anxiety (14).
PFC is a major target of dopaminergic input from the ventral tegmental area (15, 16), and dopamine plays a key role in regulating PFC functions such as working memory (17, 18). Evidence suggests that the dopamine D4 receptor, which is highly enriched in PFC (19, 20), is critically involved in neuropsychiatric disorders associated with PFC dysfunction. A D4 gene polymorphism that weakens D4 receptor function is strongly linked to attention deficit-hyperactivity disorder (21, 22). Elevated D4 receptor expression has been demonstrated in schizophrenic patients (23), and D4 receptors have high affinity for atypical antipsychotic drugs (24, 25). D4 receptor antagonists can alleviate cognitive deficits induced by stress (26) or long term treatment with the psychotomimetic drug phencyclidine (27, 28). D4 receptor-deficient mice show reduced novelty seeking and cortical hyperexcitability (29, 30).
To understand the mechanism of D4 actions in PFC, it is important to identify its cellular targets key to PFC functions such as working memory. One of our previous studies has demonstrated that GABAA receptors are subject to D4 regulation in PFC pyramidal neurons (31). In this study, we have revealed the mechanism underlying this regulation.
EXPERIMENTAL PROCEDURES
Acute Dissociation Procedure and Primary Culture Preparation—PFC neurons from young adult (3–4 weeks postnatal) rats were acutely dissociated using procedures described previously (32, 33). All of the experiments were carried out with the approval of State University of New York at Buffalo Animal Care Committee. After incubation of brain slices in NaHCO3-buffered saline, PFC was dissected and placed in an oxygenated chamber containing papain (0.8 mg/ml; Sigma) in HEPES-buffered Hanks' balanced salt solution (Sigma) at room temperature. After 35 min of enzyme digestion, the tissue was rinsed three times with a low Ca2+ saline and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was then plated into a 35-mm Lux Petri dish, which was then placed on the stage of a Zeiss Axiovert S100 inverted microscope.
Rat PFC cultures were prepared as previously described (34). Briefly, PFC was dissected from 18-day rat embryos, and the cells were dissociated by incubating with 0.25% trypsin for 30 min and subsequent trituration through a Pasteur pipette cells. The cells were plated on coverslips (coated with poly-l-lysine) in Dulbecco's modified Eagle's medium with 10% fetal calf serum at a density of 0.75 × 105 cells/cm2. When neurons attached to the coverslip within 4 h, the medium was changed to Neurobasal with B27 supplement. The neurons were maintained for 2–3 weeks before being used.
Whole Cell Recording of Ionic Currents—Pyramidal neurons located in the intermediate and deep layers (III–VI) of the rat PFC were recorded. Recordings of whole cell GABAAR-mediated currents used standard voltage clamp techniques (31). The internal solution consisted of 180 mm N-methyl-d-glucamine, 40 mm HEPES, 4 mm MgCl2, 0.5 mm 1,2-bis(2-aminohenoxy)ethane-N,N,N′,N′-tetraacetic acid, 12 mm phosphocreatine, 2 mm Na2ATP, 0.2 mm Na3GTP, 0.1 mm leupeptin, pH 7.3, 270 mosm/liter. The external solution consisted of 135 mm NaCl, 20 mm CsCl, 1 mm MgCl2, 10 mm HEPES, 5 mm BaCl2, 10 mm glucose, 0.001 mm tetrodotoxin, pH 7.3, 300 mosm/liter. Recordings were obtained using an Axopatch 200B amplifier that is controlled and monitored with a computer running pClamp 8 with a DigiData 1320 series interface. Electrode resistances were typically 2–4 MΩ in the bath. After seal rupture, series resistance (4–10 MΩ) was compensated (70–90%) and periodically monitored. The cell membrane potential was held at 0 mV. GABA (50 μm) was applied for 2 s every 30 s to minimize desensitization-induced decrease of current amplitude. Drugs were applied using a gravity-fed “sewer pipe” system. The array of application capillaries (∼150-μm inner diameter) was positioned a few hundred micrometers from the cell being recorded. Solution changes were affected by the SF-77B fast-step solution stimulus delivery device (Warner Instruments, Hamden, CT).
Data analyses were performed with Clampfit (Axon Instruments, Sunnyvale, CA) and Kaleidagraph (Albeck Software, Reading, PA). For analysis of statistical significance, ANOVA tests were performed to compare the differential degrees of current modulation between groups subjected to different treatments.
Electrophysiological Recording of Synaptic Currents—Recording of miniature inhibitory postsynaptic currents (mIPSC) in cultured PFC neurons (days in vitro 12–14) used the whole cell patch technique. Electrodes (3–5 MΩ) were filled with the following internal solution: 100 mm CsCl, 30 mm N-methyl-d-glucamine, 10 mm HEPES, 4 mm NaCl, 1 mm MgCl2 5 mm EGTA, 5 mm MgATP, 0.5 mm Na2GTP, 12 mm phosphocreatine, 0.2 mm leupeptin, 2 mm QX-314, pH 7.2–7.3, 265–270 mosm/liter. Oxygenated artificial cerebral spinal fluid (130 mm NaCl, 3 mm KCl, 5 mm MgCl2, 1 mm CaCl2, 26 mm NaHCO3, 1.25 mm NaH2PO4, 10 mm glucose, pH 7.4, 300 mosm/liter) was used as the external solution. Tetrodotoxin (0.5 μm), D-AP5 (20 μm), and 6,7-dinitroquinoxaline-2,3-dione (20 μm) were added to cultures to block action potentials N-methyl-d-aspartic acid and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors, respectively. The cell membrane potential was held at –70 mV. A mini analysis program (Synaptosoft, Leonia, NJ) was used to analyze the spontaneous synaptic events. For each different condition, mIPSC recordings of 8 min were used for analysis. Statistical comparisons of the amplitude and frequency of mIPSC were made using the Kolmogorov-Smirnov test.
Recording of evoked IPSC in PFC slices used the same internal solution as what was used for mIPSC recording in cultures. The slice (300 μm) was placed in a perfusion chamber attached to the fixed-stage of an upright microscope (Olympus) and submerged in continuously flowing oxygenated artificial cerebral spinal fluid containing D-AP5 (20 μm) and DNQX (20 μm). The cells were visualized with a 40× water immersion lens and illuminated with near infrared light, and the image was detected with an infrared-sensitive CCD camera (Olympus, Center Valley, PA). A Multiclamp 700A amplifier was used for slice recordings (Axon Instruments). Tight seals (2–10 GΩ) from visualized pyramidal neurons were obtained by applying negative pressure. The membrane was disrupted with additional suction and the whole cell configuration was obtained. The access resistances ranged from 13 to 18 mΩ and were compensated 50–70%. The cells were held at –70 mV. Clampfit (Axon Instruments) was used to analyze evoked synaptic activity.
The agents used such as N-(methyl)-4-(2-cyanophenyl)piperazinyl-3-methybenzamide maleate (PD168077; Tocris, Ballwin, MO), colchicine, phalloidin, latrunculin B (Calbiochem, San Diego, CA), dynamin inhibitory peptide (Tocris, Ballwin, MO), p-cofilin peptide, cofilin peptide, and a scrambled peptide were made up as concentrated stocks in water or Me2SO and stored at –20 °C. The final Me2SO concentration in all applied solutions was <0.1%. No change on GABAAR currents has been observed with this concentration of Me2SO. Stocks were thawed and diluted immediately before use. The amino acid sequence for the myosin peptide is KLFNDPNIGKKGARGKKGKKGRAQKGAN.
Immunocytochemical Staining—After treatment, the cultures were fixed in 4% paraformaldehyde for 20 min and incubated in 5% bovine serum for 1 h. For GABAAR surface expression, cultured neurons (nonpermeabilized) were incubated with an antibody against GABAAR β2/3 extracellular region (1:50; Chemicon, Billerica, MA) for 2 h at room temperature. After washing, the neurons were permeabilized with 0.1% Triton for 10 min and then incubated with MAP2 antibody (1:500; Santa Cruz, Santa Cruz, CA) for 2 h at room temperature. Following washing, the neurons were incubated with Alexa 488-conjugated secondary antibody (1:200; Invitrogen) and Alexa 594-conjugated secondary antibody (1:500; Invitrogen) for 1 h at room temperature. After washing, the coverslips were mounted on slides with VECTASHIELD mounting media (Vector Laboratories, Burlingame, CA).
Fluorescent images were obtained using a 100× objective with a cooled charge-coupled device camera mounted on a Nikon microscope. All of the specimens were imaged under identical conditions and analyzed with identical parameters using ImageJ software. Control and PD168077-treated neurons with similar MAP2 staining were selected for analysis. To define dendritic clusters, a single threshold was chosen manually, so that clusters corresponded to puncta of at least 1.5-fold intensity of the diffuse fluorescence on the dendritic shaft. Three to four independent experiments for each of the treatments were performed. On each coverslip, the cluster density, cluster size, and cluster fluorescence intensity of several neurons (two or three dendritic segments of at least 20 μm in length/neuron) were measured. Quantitative analyses were conducted blindly (without knowledge of experimental treatment).
Western Blots—After treatment, equal amounts of protein from culture homogenates were separated on 7.5% acrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk for 1 h at room temperature and then were incubated with the anti-p-cofilin (1:250; Cell Signaling, Danvers, MA), anti-cofilin (1:250; Cell Signaling), or anti-actin (1:500; Cell Signaling) for 3 h at room temperature. After washing, the blots were incubated with the horseradish peroxidase-conjugated anti-rabbit antibody (1:1000; Amersham Biosciences) for 2 h at room temperature. After washing, the blots were exposed to the enhanced chemiluminescence substrate. Quantification was obtained from densitometric measurements of immunoreactive bands on films using National Institutes of Health Image software.
RESULTS
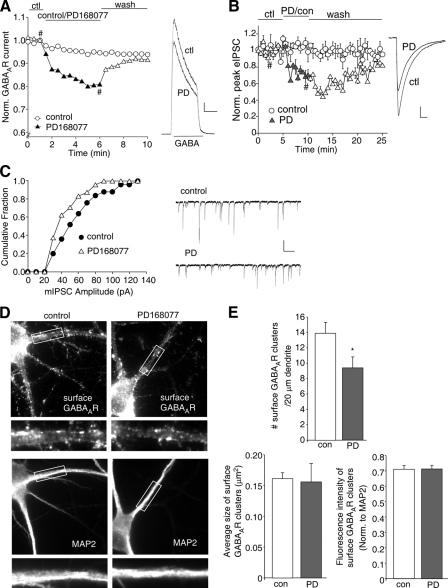
Activation of D4 Receptors Reduces GABAAR Channel Currents and Surface Expression in PFC Pyramidal Neurons—To examine the impact of dopamine D4 receptors on GABAergic signaling in PFC, we first tested the effect of PD168077, a highly selective D4 receptor agonist (35), on whole cell ionic currents mediated by both synaptic and extrasynaptic GABAARs in acutely dissociated PFC pyramidal neurons. GABA (50 μm) application evoked a partially desensitizing outward current in neurons (held at 0 mV) that could be completely blocked by the GABAAR antagonist bicuculline (30 μm; data not shown). As shown in Fig. 1A, application of PD168077 (30 μm) caused a reversible reduction of GABAAR current amplitudes in dissociated PFC pyramidal neurons (16.8 ± 1.7%, n = 15). Consistent with our previous findings (31), this effect of PD168077 was blocked by the specific D4 antagonist L-74172 (10 μm, data not shown), suggesting the mediation by D4 receptors.
FIGURE 1.
D4 receptors reduce GABAAR currents and surface expression in PFC pyramidal neurons. A, plot of normalized peak GABA (50 μm)-evoked current as a function of time in the absence and presence of D4 agonist (PD168077, 30 μm) application in dissociated PFC pyramidal neurons. Inset, representative current traces (at time points denoted by #). Scale bar, 500 pA, 1 s. B, plot of normalized evoked IPSC as a function of time and agonist application in PFC slices. Inset, representative IPSC traces (at time points denoted by #). Scale bar, 25 pA, 20 ms. C, cumulative plot of the distribution of mIPSC amplitudes before (control) and after PD168077 application in a cultured PFC pyramidal neuron. Inset, representative mIPSC traces. Scale bar, 50 pA, 2 s. D, immunocytochemical images of surface GABAAR β2/3 subunits and MAP2 staining in PFC cultures either untreated (control) or treated with PD168077 (30 μm, 10 min). E, quantitative analysis of surface GABAAR β2/3 clusters along the dendrites (density, size, and normalized intensity) in control versus PD168077-treated neurons. *, p < 0.05, ANOVA. ctl or con, control; PD,. PD168077.
To examine the impact of D4 receptors on GABAergic synaptic transmission, we further measured IPSC evoked by electrical stimulation of synaptic GABAA receptors. As shown in Fig. 1B, bath application of PD168077 (40 μm) to PFC slices caused a reversible reduction of IPSC amplitudes (34.6 ± 2.6%, n = 7), whereas IPSC amplitudes remained stable in control neurons when no PD168077 was applied. Moreover, we measured miniature IPSC, a response from quantal release of single GABA vesicles. As shown in Fig. 1C, PD168077 (30 μm) caused a reversible reduction of mIPSC amplitudes in cultured PFC pyramidal neurons (17.8 ± 3.5%, n = 23). Taken together, these results suggest that D4 receptors down-regulate GABAAR function at the synapse.
Next, we tested whether the D4-induced down-regulation of GABAAR function was due to a decrease in GABAAR surface expression. We labeled surface GABAA receptors using an antibody that targets the extracellular region of GABAAR β2/3 subunit in PFC cultures. Neurons were co-stained with MAP2, a dendritic marker. As illustrated in Fig. 1D, surface GABAARs were clustered around the soma and proximal dendrites. In cells treated with PD168077 (30 μm, 10 min), GABAAR surface clusters were substantially reduced. Quantification of immunocytochemical images (Fig. 1E) indicates that the density of GABAAR surface clusters (number of clusters/20 μm dendrite) was significantly reduced by PD168077 (control: 13.9 ± 1.4, n = 12; PD168077: 9.4 ± 1.4, n = 12, p < 0.05, ANOVA). PD168077 did not cause a significant change in the size of GABAAR surface clusters or the fluorescence intensity (normalized to MAP2 immunofluorescence) of GABAAR surface clusters. These results suggest that D4 receptor activation leads to a decrease of GABAAR surface cluster density, which is associated with the D4-induced reduction of whole cell GABAAR current, evoked IPSC, and miniature IPSC amplitude.
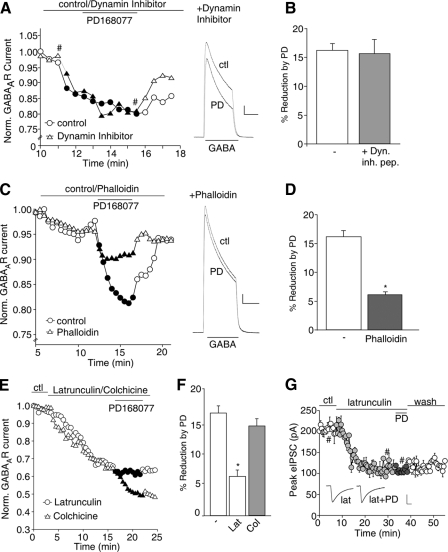
The Actin Cytoskeleton Is Involved in D4 Regulation of GABAAR Currents—Next, we examined the underlying mechanism for D4 reduction of GABAARs at the cell surface. Previous studies have shown that GABAA receptors are removed from the plasma membrane mainly by clathrin/dynamin-mediated endocytosis (36, 37). To test whether D4 receptor activation induces GABAAR endocytosis, we dialyzed neurons with a dynamin inhibitory peptide, which competitively blocks dynamin from binding to amphiphysin, thus preventing endocytosis (38). The effectiveness of this peptide to block GABAAR endocytosis has been demonstrated in our previous studies (39). As shown in Fig. 2 (A and B), PD168077 reduced GABAAR current in the presence of dynamin inhibitory peptide (50 μm, 15.6 ± 2.5%, n = 6), which was similar to the effect of PD168077 in the absence of this peptide (16.8 ± 1.7%, n = 6). These results suggest that D4 reduction of GABAAR current is not through increased endocytosis of GABAARs.
FIGURE 2.
D4 reduction of GABAAR currents is through an actin-dependent mechanism. A, plot of normalized peak GABAAR current as a function of time and PD168077 (30 μm) application in neurons dialyzed with or without the dynamin inhibitory peptide (50 μm). Inset, representative current traces (at time points denoted by #). Scale bar, 500 pA, 1 s. B, cumulative data (means ± S.E.) showing the percentage of reduction of GABAAR current by PD168077 in a sample of neurons in the absence (control) or presence of the dynamin inhibitory peptide. C, plot of normalized peak GABAAR current as a function of time and PD168077 application in neurons dialyzed with the actin stabilizer phalloidin (12.5 μm). Inset, representative current traces (at time points denoted by #). Scale bar, 500 pA, 1 s. D, cumulative data (mean ± S.E.) showing the percent reduction of GABAAR current by PD168077 in the absence or presence of phalloidin in a sample of neurons tested. *, p < 0.005, ANOVA. E, plot of normalized peak GABAAR current as a function of time and PD168077 application in neurons dialyzed with the actin depolymerizer latrunculin B (5 μm) or perfused with the microtubule depolymerizer colchicine (30 μm). F, cumulative data (mean ± S.E.) showing the percent reduction of GABAAR current by PD168077 in the absence or presence of latrunculin B or colchicine in a sample of neurons tested. *, p < 0.005, ANOVA. G, plot of evoked IPSC as a function of time and PD168077 application in a PFC slice perfused with latrunculin B. Inset, representative IPSC traces (at time points denoted by #). Scale bar, 50 pA, 20 ms. ctl, control; PD, PD168077; Dyn. inh. pep., dynamin inhibitory peptide; Lat, latrunculin B; Col, colchicine.
Previous studies have suggested the involvement of cytoskeleton proteins in regulating GABAA receptor current and surface stability (40, 41); thus we investigated the potential role of microtubules and/or actin in D4 regulation of GABAAR current. As shown in Fig. 2C, dialysis with the actin stabilizing compound phalloidin (12.5 μm) largely blocked the capability of PD168077 to reduce GABAAR current. Phalloidin itself had little effect on basal GABAAR current (5.1 ± 1.0%, n = 5). As summarized in Fig. 2D, the effect of PD168077 was significantly (p < 0.005, ANOVA) smaller in phalloidin-loaded neurons (6.1 ± 0.5%, n = 4), compared with control neurons (16.1 ± 1.2%, n = 6). Conversely, application of latrunculin B, an actin depolymerizing compound, caused a decline of GABAAR current (31.1 ± 2.0%, n = 7) and largely occluded the effect of subsequently applied PD168077 (Fig. 2E). However, the microtubule destabilizing compound colchicine, which reduced basal GABAAR current (27.2 ± 3.0%, n = 8), failed to alter the reducing effect of PD168077 (Fig. 2E). As summarized in Fig. 2F, neurons dialyzed with latrunculin B showed a significantly (p < 0.005, ANOVA) smaller effect of PD168077 (6.1 ± 1.1%, n = 6), compared with control neurons (16.1 ± 1.2%, n = 6) or neurons perfused with colchicine (14.1 ± 1.2%, n = 14). Consistently, bath application of latrunculin B also occluded the effect of PD168077 on evoked IPSC in PFC slice recordings (Fig. 2G, 6.2 ± 2.0%, n = 5). These results suggest that D4 reduces GABAAR current via an actin-dependent mechanism.
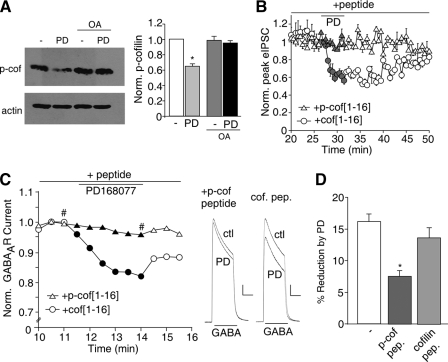
D4 Reduction of GABAAR Current Is Dependent upon the Actin Depolymerizing Factor Cofilin—Next, we investigated the link between D4 receptor signaling and actin cytoskeleton. The dynamics of actin assembly is regulated by cofilin, a major actin depolymerizing factor (42). The actin depolymerizing activity of cofilin is greatly increased by dephosphorylation at Ser3 (43, 44). In vitro studies have shown that protein phosphatase 1 (PP1) can lead to the dephosphorylation and activation of cofilin (45). Our previous study has found that D4 regulation of GABAAR current depends on activation of the anchored PP1 (31). Thus, we speculated that D4 activation might induce actin depolymerization by dephosphorylating cofilin via PP1, thus leading to the reduced GABAAR synaptic trafficking along actin cytoskeleton. To test this, we first examined the impact of D4 on cofilin activity using a Ser3 phospho-cofilin antibody in cultured PFC neurons. As shown in Fig. 3A, application of PD168077 (30 μm, 10 min) significantly reduced the level of Ser3-phosphorylated (inactive) cofilin (65.1 ± 3.1% of control, n = 5; p < 0.005, ANOVA), and this effect was blocked by pretreatment with the PP1 inhibitor okadaic acid (1 μm, 40 min, 95.3 ± 3.1% of control, n = 3; p > 0.05, ANOVA). The level of total cofilin or actin was not changed. These results suggest that D4 activation leads to the dephosphorylation and activation of cofilin through a PP1-dependent mechanism.
FIGURE 3.
The actin depolymerizing factor, cofilin, is involved in D4 reduction of GABAAR currents. A, left panel, Western blots of p-cofilin and actin in cultured PFC neurons incubated with PD168077 (30 μm, 10 min) in the absence or presence of okadaic acid (1 μm, 40 min pretreatment). Right panel, quantification showing the normalized level of p-cofilin with different treatments. *, p < 0.005, ANOVA. B, plot of evoked IPSC as a function of time and PD168077 application in neurons dialyzed with the Ser3-phosphorylated cofilin peptide p-cof[1–16] (100 μm) or nonphosphorylated cofilin peptide cof[1–16] (100 μm). C, plot of normalized peak GABAAR current as a function of time and PD168077 application in neurons dialyzed with p-cof[1–16] or cof[1–16]. Inset, representative current traces (at time points denoted by #). Scale bar, 500 pA, 1 s. D, cumulative data (means ± S.E.) from acutely dissociated PFC neurons showing the percent reduction of GABAAR current by PD168077 in a sample of neurons dialyzed with different peptides. *, p < 0.005, ANOVA. OA, okadaic acid; PD, PD168077; ctl, control; pep., peptide; scram., scrambled.
To further test the involvement of cofilin, we dialyzed neurons with the cofilin peptides consisting of 1–16 residues of cofilin with or without Ser3 phosphorylation (46, 47). The Ser3-phosphorylated cofilin peptide, p-cof[1–16] (MApSGVAVSDGVIKVFN), serves as an inhibitor of endogenous cofilin, because it binds to cofilin phosphatases and thus prevents the dephosphorylation and activation of endogenous cofilin. The nonphosphorylated cofilin peptide, cof[1–16], serves as a negative control. As shown in Fig. 3B, in cells dialyzed with p-cof[1–16] (50 μm), the D4-induced decrease of IPSC was largely blocked (5.3 ± 2.1%, n = 5), whereas the control peptide cof[1–16] (50 μm) did not alter the D4 effect on IPSC (30.5 ± 2.2%, n = 6). Similarly, in acutely dissociated PFC neurons (Fig. 3, C and D), the D4 effect on GABAAR current was significantly (p < 0.05, ANOVA) blocked by dialysis with p-cof[1–16] peptide (8.2 ± 1.6%, n = 13), but not cof[1–16] peptide (13.8 ± 1.5%, n = 8), compared with control conditions (16.1 ± 1.2%, n = 6). These results suggest that D4 suppresses GABAAR current via a mechanism requiring cofilin activity.
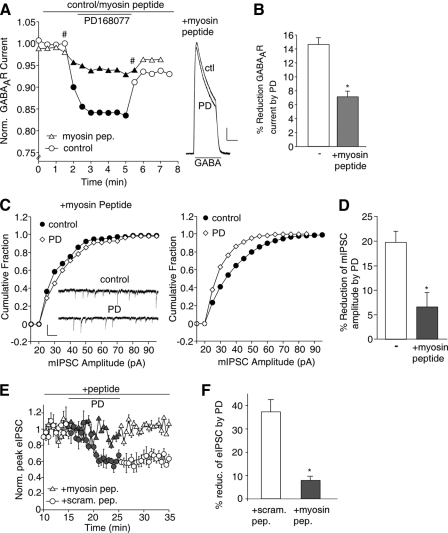
The Actin Motor Protein, Myosin, Is Involved in D4 Regulation of GABAAR Current—Given the actin dependence of D4 regulation of GABAAR current, we further examined the potential involvement of actin-based motor proteins. Myosin, a family of motor proteins that move on F-actin, has been found to be critical for the trafficking of AMPARs (48–50); however, its involvement in GABAAR trafficking is unknown. Thus, we dialyzed neurons with a synthetic peptide derived from the conserved actin-binding site of myosin proteins, which competes with endogenous myosin for actin binding and therefore impairs myosin-based trafficking along actin filaments (51). As shown in Figs. 4 (A and B), in the presence of the myosin peptide, the effect of D4 on GABAAR current in dissociated PFC neurons was significantly (p < 0.005, ANOVA) smaller (7.1 ± 0.8%, n = 14) compared with control conditions (14.6 ± 1.0%, n = 14).
FIGURE 4.
The actin motor protein, myosin, is involved in D4 reduction of GABAAR currents. A, plot of normalized peak GABAAR current as a function of time and PD168077 (30 μm) application in neurons dialyzed with or without the myosin peptide (25 μm). Inset, representative current traces (at time points denoted by #). Scale bar, 200 pA, 1 s. B, cumulative data (mean ± S.E.) showing the percentage of reduction of GABAAR current by PD168077 in a sample of neurons dialyzed with or without the myosin peptide. *, p < 0.005, ANOVA. C, cumulative plots of the distribution of mIPSC amplitudes before (control) and after PD168077 (30 μm) application in cultured PFC neurons dialyzed with or without the myosin peptide (25 μm). Inset, representative mIPSC traces. Scale bar, 50 pA, 1 s. D, cumulative data (mean ± S.E.) showing the percentage of reduction of mIPSC amplitudes by PD168077 in the absence and presence of the myosin peptide. E, plot of normalized peak evoked IPSC as a function of time and PD168077 application in PFC neurons dialyzed with the myosin peptide (25 μm) or a scrambled control peptide (25 μm). F, cumulative data (means ± S.E.) showing the percentage of reduction of evoked IPSC amplitude by PD168077 in the absence and presence of the myosin peptide. *, p < 0.005, ANOVA. PD, PD168077; ctl, control; pep., peptide.
Next, we examined the impact of the myosin peptide on D4 modulation of GABAergic transmission. As shown in Fig. 4C, bath application of PD168077 to PFC cultures caused a significant (p < 0.001; Kolmogorov-Smirnov test) reduction of mIPSC amplitudes, as indicated by a leftward shift on the mIPSC distribution; however, this effect was prevented by the myosin peptide. As summarized in Fig. 4D, the effect of D4 on mIPSC amplitude was significantly (p < 0.005, ANOVA) reduced in neurons dialyzed with the myosin peptide (6.6 ± 2.9%, n = 6), compared with control conditions (19.8 ± 2.2%, n = 7). Similarly, the myosin peptide, but not a scrambled control peptide, significantly (p < 0.005, ANOVA) blocked the reducing effect of D4 on evoked IPSC in PFC slices (Fig. 4, E and F, with myosin peptide, 7.8 ± 1.8%, n = 8; with scrambled peptide, 37.1 ± 5.3%, n = 8). These data suggest that D4 affects myosin-mediated transport of GABAARs along actin filaments.
DISCUSSION
In this study, we have revealed that D4 receptor activation in PFC pyramidal neurons reduces GABAAR-mediated channel current and inhibitory transmission via a mechanism involving actin-based trafficking of GABAARs to the synaptic membrane. Our results suggest that D4 triggers the PP1-mediated dephosphorylation and activation of cofilin, the major actin depolymerizing factor, leading to the loss of actin stability. Consequently, the myosin motor-mediated transport of GABAAR-containing vesicles along F-actin is interrupted, resulting in reduced GABA responses.
The trafficking of functional GABAARs is fundamental for establishing and maintaining inhibitory transmission (52). There is evidence suggesting that newly assembled GABAARs are delivered to extrasynaptic sites and then rapidly imported to synaptic sites through lateral diffusion (53). Surface GABAARs are constitutively endocytosed from the cell surface via a dynamin/clathrin-dependent mechanism that is regulated by phosphorylation (8, 54). Internalized GABAARs are either rapidly recycled back to the cell surface or targeted for lysosomal degradation, and this sorting decision is regulated by a direct interaction of GABAARs with Huntingtin-associated protein 1 (55).
Using acutely dissociated neurons, primary cultures and brain slices, our electrophysiological data show that D4 receptor activation reduces functional GABAARs at both synaptic and extrasynaptic sites, which is consistent with the D4-induced reduction of surface GABAAR clusters on soma and processes illustrated by immunocytochemical results. The pharmacological experiments with agents disturbing actin dynamics suggest that D4 down-regulates GABAAR trafficking and function by reducing actin stability. In agreement with this, it has been shown that actin depolymerization can lead to a decrease in GABAAR clusters at the cell surface (40).
Several studies have demonstrated the role of actin cytoskeleton in regulating AMPA-type glutamate receptor cluster distribution (56), surface expression (57), and channel internalization (58, 59). However, the involvement of actin in anchoring and clustering GABAARs at inhibitory synapses is much less clear. The actin-binding protein radixin has been identified as the first directly interacting molecule that anchors GABAARs at cytoskeletal elements (41). Depletion of radixin expression or replacement of the radixin/F-actin binding motif interferes with GABAAR α5 cluster formation (41). Although radixin only associates with GABAAR α5 subunit, which mainly localizes at extrasynaptic sites and mediates tonic inhibition, other GABAAR subunits might be targeted to synapses via actin filaments by interacting with other actin-associated scaffolding proteins. It is possible that the D4-induced actin depolymerization disrupts the interaction of GABAARs with their anchoring proteins, leading to the loss of GABAARs at the synapse.
Actin filaments, which are enriched at synapses, undergo dynamic polymerization and depolymerization. Cofilin, the major actin depolymerizing factor (42), is inactivated by phosphorylation at Ser3 and reactivated by dephosphorylation of this site (43, 44). Thus, cofilin phosphorylation/dephosphorylation at Ser3 acts as a switch for actin assembly (F-actin stabilization) and disassembly (F-actin severing) (60, 61). Using a Ser(P)3 cofilin antibody, we have shown that D4 receptors decrease the level of phosphorylated (inactive) cofilin, suggesting that the actin-depolymerizing activity of cofilin is increased by D4 receptors in the PFC. Moreover, we have demonstrated that the D4 effect on cofilin phosphorylation requires PP1, a protein phosphatase that is able to dephosphorylate and activate cofilin in vitro (45). It is consistent with our previous finding about the involvement of PP1 in D4 regulation of GABAAR currents (31). By using a Ser3-phosphorylated cofilin peptide (46, 47) to inhibit the activation of endogenous cofilin, we have further demonstrated that the D4 regulation of GABAAR-mediated ionic current and inhibitory transmission requires the activation of cofilin.
Because D4 increases the actin-depolymerizing activity of cofilin, we would expect to see changes in F-actin organization by D4 activation. Indeed, PD168077 treatment led to a marked loss of F-actin clusters and a diffuse labeling pattern of F-actin in cultured PFC neurons (data not shown). These results suggest that D4 activation can alter actin dynamics, thus leading to changes in actin-based trafficking of receptors.
Myosin proteins are actin-associated motors whose major function is to control the transport of organelles along the actin filament (62). About 40 myosin genes (grouped into 12 distinct classes) have been identified. These motors are composed of a conserved N-terminal motor domain followed by a coiled-coil region and a globular C-terminal tail containing the cargo binding domain (63). Using a peptide against the actin-binding region that is conserved for most myosin proteins (51), we have demonstrated the role of myosin motor proteins in D4 regulation of GABAAR trafficking and function. Class V of myosins, which is thought to regulate the trafficking of organelles and associated proteins in neurons (62), has been implicated in AMPAR trafficking (48, 50). However, we found that blocking myosin V function with a specific antibody did not affect the D4 regulation of GABAAR current (supplemental Fig. S1, A and B), suggesting the lack of involvement of myosin V in GABAAR trafficking. In agreement with this, it has been shown that GABAAR-mediated IPSC is unaffected in neurons transfected with dominant-negative myosin V (50). Furthermore, we tested the effect of blebbistatin, a myosin II inhibitor (64), on D4 regulation of GABAARs. As shown in supplemental Fig. S1 (C–E), blebbistatin (2.5 μm) failed to alter the reducing effect of PD168077 on mIPSC amplitude (control, 19.8 ± 2.2%, n = 7; with blebbistatin, 19.1 ± 0.4%, n = 5), suggesting a lack of involvement of myosin II. It awaits to be identified which subtype of myosin proteins is involved in the regulation of actin-based GABAAR trafficking.
In the central nervous system, dopamine, by activating different receptors, regulates GABAA receptors via distinct mechanisms. D1 receptor has been shown to reduce GABAAR currents in neostriatum by activating a PKA/DARPP-32/PP1 signaling cascade to increase GABAAR β1 subunit phosphorylation (65). D3 receptor has been shown to suppress postsynaptic GABAAR currents in nucleus accumbens by increasing the phospho-dependent endocytosis of GABAA receptors (39). The present study has revealed that D4 receptors regulate GABAAR trafficking and function via an actin/cofilin/myosin-dependent mechanism in prefrontal cortex. These studies provide a framework for understanding the role of dopamine receptors in regulating the efficacy of GABAAR-mediated inhibitory synaptic transmission of diverse brain regions.
Supplementary Material
Acknowledgments
We thank Xiaoqing Chen for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants (to Z. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: PFC, prefrontal cortex; GABA, γ-aminobutyric acid; GABAA, GABA, type A; GABAAR, GABAA receptor; ANOVA, analysis of variance; IPSC, inhibitory postsynaptic currents; mIPSC, miniature IPSC; p-cof, p-cofilin; PP1, protein phosphatase 1.
References
- 1.Miller, E. K. (1999) Neuron 22 15–17 [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic, P. S. (1995) Neuron 1 477–485 [DOI] [PubMed] [Google Scholar]
- 3.Rao, S. G., Williams, G. V., and Goldman-Rakic, P. S. (2000) J. Neurosci. 20 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantinidis, C., Williams, G. V., and Goldman-Rakic, P. S. (2002) Nat. Neurosci. 5 175–180 [DOI] [PubMed] [Google Scholar]
- 5.Lewis, D. A., Hashimoto, T., and Volk, D. W. (2005) Nat. Rev. Neurosci. 6 312–324 [DOI] [PubMed] [Google Scholar]
- 6.Moss, S. J., and Smart, T. G. (2001) Nat. Rev. Neurosci. 2 240–250 [DOI] [PubMed] [Google Scholar]
- 7.Kittler, J. T., McAinsh, K., and Moss, S. J. (2002) Mol. Neurobiol. 26 251–268 [DOI] [PubMed] [Google Scholar]
- 8.Kittler, J. T., Delmas, P., Jovanovic, J. N., Brown, D. A., Smart, T. G., and Moss, S. J. (2000) J. Neurosci. 20 7972–7977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly, C. N., Wooltorton, J. R. A., Smart, T. G., and Moss, S. J. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 9899–9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly, C. N., Kittler, J. T., Thomas, P., Uren, J. M., Brandon, N. J., Smart, T. G., and Moss, S. J. (1999) J. Biol. Chem. 274 36565–36572 [DOI] [PubMed] [Google Scholar]
- 11.Luscher, B., and Keller, C. A. (2004) Pharmacol. Ther. 102 195–221 [DOI] [PubMed] [Google Scholar]
- 12.Volk, D., Pierri, J., Fritschy, J., Auh, S., Sampson, A., and Lewis, D. (2002) Cereb. Cortex 12 1063–1070 [DOI] [PubMed] [Google Scholar]
- 13.Huntsman, M., Tran, B., Potkin, S., Bunney, W. J., and Jones, E. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 15066–15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J. P., Belzung, C., Fritschy, J. M., Luscher, B., and Mohler, H. (1999) Nat. Neurosci. 2 833–839 [DOI] [PubMed] [Google Scholar]
- 15.Lewis, D. A., Campbell, M. J., Foote, S. L., and Morrison, J. H. (1986) Hum. Neurobiol. 5 181–188 [PubMed] [Google Scholar]
- 16.Berger, B., Trotteir, C., Verney, P., Gaspar, P., and Alvarez, C. (1988) J. Comp. Neurol. 273 99–119 [DOI] [PubMed] [Google Scholar]
- 17.Sawaguchi, T., and Goldman-Rakic, P. S. (1991) Science 251 947–950 [DOI] [PubMed] [Google Scholar]
- 18.Williams, G. V., and Goldman-Rakic, P. S. (1995) Nature 376 572–575 [DOI] [PubMed] [Google Scholar]
- 19.Mrzljak, L., Bergson, C., Pappy, M., Huff, R., Levenson, R., and Goldman-Rakic, P. S. (1996) Nature 381 245–248 [DOI] [PubMed] [Google Scholar]
- 20.Wedzony, K., Czyrak, A., Mackowiak, M., Fijal, K., and Czyrak, A. (2000) J. Physiol. Pharmacol. 51 205–221 [PubMed] [Google Scholar]
- 21.LaHoste, G. J., Swanson, J. M., Wigal, S. B., Glabe, C., Wigal, T., King, N., and Kennedy, J. L. (1996) Mol. Psychiatry 1 121–124 [PubMed] [Google Scholar]
- 22.Rowe, D. C., Stever, C., Giedinghagen, L. N., Gard, J. M., Cleveland, H. H., Terris, S. T., Mohr, J. H., Sherman, S., Abramowitz, A., and Waldman, I. D. (1998) Mol. Psychiatry 3 419–426 [DOI] [PubMed] [Google Scholar]
- 23.Seeman, P., Guan, H.-C., and Van Tol, H. H. M. (1993) Nature 365 441–445 [DOI] [PubMed] [Google Scholar]
- 24.Van Tol, H. H. M., Bunzow, J. R., Guan, H.-C., Sunahara, R., Seeman, P., Niznik, H., and Civelli, O. (1991) Nature 350 610–614 [DOI] [PubMed] [Google Scholar]
- 25.Kapur, S., and Remington, G. (2001) Annu. Rev. Med. 52 503–517 [DOI] [PubMed] [Google Scholar]
- 26.Murphy, B., Arnsten, A., Goldman-Rakic, P. S., and Roth, R. H. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1325–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jentsch, J. D., Redmond, D. E., Jr., Elsworth, J., Taylor, J. R., Youngren, K. D., and Roth, R. H. (1997) Science 277 953–955 [DOI] [PubMed] [Google Scholar]
- 28.Jentsch, J. D., Taylor, J. R., Redmond, D. E., Jr., Elsworth, J. D., Youngren, K. D., and Roth, R. H. (1999) Psychopharmacology 142 78–84 [DOI] [PubMed] [Google Scholar]
- 29.Dulawa, S., Grandy, D. K., Low, M. J., Paulus, M., and Geyer, M. (1999) J. Neurosci. 19 9550–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinstein, M., Cepeda, C., Hurst, R. S., Flores-Hernandez, J., Ariano, M. A., Falzone, T. L., Kozell, L. B., Meshul, C. K., Bunzow, J. R., Low, M. J., Levine, M. S., and Grandy, D. K. (2001) J. Neurosci. 21 3756–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, X., Zhong, P., and Yan, Z. (2002) J. Neurosci. 22 9185–9193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng, J., Cai, X., Zhao, J. H., and Yan, Z. (2001) J. Neurosci. 21 6502–6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, G., Greengard, P., and Yan, Z. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2596–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai, X., Gu, Z., Zhong, P., Ren, Y., and Yan, Z. (2002) J. Biol. Chem. 277 36553–36562 [DOI] [PubMed] [Google Scholar]
- 35.Glase, S. A., Akunne, H. C., Georgic, L. M., Heffner, T. G., MacKenzie, R. G., Manley, P. J., Pugsley, T. A., and Wise, L. D. (1997) J. Med. Chem. 40 1771–1772 [DOI] [PubMed] [Google Scholar]
- 36.Tehrani, M., and Barnes, E. J. (1993) J. Neurochem. 60 1755–1761 [DOI] [PubMed] [Google Scholar]
- 37.Kittler, J. T., and Moss, S. J. (2003) Curr. Opin. Neuorbiol. 13 341–347 [DOI] [PubMed] [Google Scholar]
- 38.Gout, I., Dhand, R., Hiles, I. D., Fry, M. J., Panayotou, G., Das, P., Truong, O., Totty, N. F., Hsuan, J., and Booker, G. W. (1993) Cell 75 25–36 [PubMed] [Google Scholar]
- 39.Chen, G., Kittler, J. T., Moss, S. J., and Yan, Z. (2006) J. Neurosci. 26 2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, D. K., Olenik, C., Hofmann, F., Barth, H., Leemhuis, J., Brunig, I., Aktories, K., and Norenberg, W. (2000) J. Neurosci. 20 6743–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loebrich, S., Bahring, R., Katsuno, T., Tsukita, S., and Kneussel, M. (2006) EMBO J. 25 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dos Remedios, C. G., Chhabra, D., Kekic, M., Dedova, I. V., Tsubakihara, M., Berry, D. A., and Nosworthy, N. J. (2003) Physiol. Rev. 83 433–473 [DOI] [PubMed] [Google Scholar]
- 43.Morgan, T. E., Lockerbie, R. O., Minamide, L. S., Browning, M. D., and Bamburg, J. R. (1993) J. Cell Biol. 122 623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agnew, B. J., Minamide, L. S., and Bamburg, J. R. (1995) J. Biol. Chem. 270 17582–17587 [DOI] [PubMed] [Google Scholar]
- 45.Ambach, A., Saunus, J., Konstandin, M., Wesselborg, S., Meuer, S. C., and Samstag, Y. (2000) Eur. J. Immunol. 30 3422–3431 [DOI] [PubMed] [Google Scholar]
- 46.Aizawa, H., Wakatsuki, S., Ishii, A., Moriyama, K., Sasaki, Y., Ohashi, K., Sekine-Aizawa, Y., Sehara-Fujisawa, A., Mizuno, K., Goshima, Y., and Yahara, I. (2001) Nat. Neurosci. 4 367–373 [DOI] [PubMed] [Google Scholar]
- 47.Zhou, Q., Homma, K. J., and Poo, M. M. (2004) Neuron 44 749–757 [DOI] [PubMed] [Google Scholar]
- 48.Lise, M.-F., Wong, T. P., Trinh, A., Hines, R. M., Liu, L., Kang, R., Hines, D. J., Lu, J., Goldenring, J. R., Wang, Y. T., and El-Husseini, A. (2006) J. Biol. Chem. 281 3669–3678 [DOI] [PubMed] [Google Scholar]
- 49.Osterweil, E., Wells, D. G., and Mooseker, M. S. (2005) J. Cell Biol. 168 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Correia, S., Bassani, S., Brown, T. C., Lise, M. F., Backos, D. S., El-husseini, A., Passafaro, M., and Esteban, J. A. (2008) Nat. Neurosci. 11 457–466 [DOI] [PubMed] [Google Scholar]
- 51.Furch, M., Geeves, M. A., and Manstein, D. J. (1998) Biochemisty 37 6317–6326 [DOI] [PubMed] [Google Scholar]
- 52.Michels, G., and Moss, S. J. (2007) Crit. Rev. Biochem. Mol. Biol. 42 3–14 [DOI] [PubMed] [Google Scholar]
- 53.Thomas, P., Mortensen, M., Hosie, A. M., and Smart, T. G. (2005) Nat. Neurosci. 8 889–897 [DOI] [PubMed] [Google Scholar]
- 54.Kittler, J. T., Chen, G., Honing, S., Bogdanov, Y. D., McAinsh, K., Arancibia-Carcamo, I., Jovanovic, J. N., Pangalos, M., Haucke, V., Yan, Z., and Moss, S. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittler, J. T., Thomas, P., Tretter, V., Bogdanov, Y. D., Haucke, V., Smart, T. G., and Moss, S. J. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 12736–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allison, D. W., Gelfand, V. I., Spector, I., and Craig, A. M. (1998) J. Neurosci. 18 2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen, L., Liang, F., Walensky, L. D., and Huganir, R. L. (2000) J. Neurosci. 20 7932–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, Q., Xiao, M., and Nicoll, R. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu, Z., Jiang, Q., Fu, A. K., Ip, N. Y., and Yan, Z. (2005) J. Neurosci. 25 4974–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bamburg, J. R. (1999) Annu. Rev. Cell Dev. Biol. 15 185–230 [DOI] [PubMed] [Google Scholar]
- 61.Huang, T. Y., DerMardirossian, C., and Bokoch, G. M. (2006) Curr. Opin. Cell Biol. 18 26–31 [DOI] [PubMed] [Google Scholar]
- 62.Bridgman, P. (2004) J. Neurobiol. 58 164–174 [DOI] [PubMed] [Google Scholar]
- 63.Karcher, R. L., Deacon, S. W., and Gelfand, V. I. (2002) Trends Cell Biol. 12 21–27 [DOI] [PubMed] [Google Scholar]
- 64.Limouze, J., Straight, A. F., Mitchison, T., and Sellers, J. R. (2004) J. Muscle Res. Cell Motil. 25 337–341 [DOI] [PubMed] [Google Scholar]
- 65.Flores-Hernandez, J., Hernandez, S., Snyder, G. L., Yan, Z., Fienberg, A. A., Moss, S. J., Greengard, P., and Surmeier, D. J. (2000) J. Neurophysiol. 83 2996–3004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.