Abstract
Introduction:
Unilateral neglect after acute right hemispheric stroke significantly impedes poststroke recovery. We studied patients with right hemispheric stroke to determine whether increasing age was associated with more frequent or more severe neglect.
Methods:
Eight neglect tests within 5 days of symptom onset (and within 24 hours of admission) were administered to 204 subjects with acute right hemispheric stroke. Size of infarct was measured, and neglect tests were scored as percent error. “Any neglect” was defined by an elevated neglect test score, standardized relative to a group of normal controls.
Results:
When tested for neglect soon after acute stroke admission, 69.6% of subjects older than 65 years had “any neglect” (defined by comparison to a group of normal controls), compared with 49.4% of subjects aged 65 years and younger (p = 0.008). For every additional 10 years of age, patients were 1.83 times as likely to have neglect, even after adjusting for diffusion-weighted imaging (DWI) infarct volume and NIH Stroke Scale (NIHSS) score (95% CI 1.38–2.43). In addition, DWI volume and NIHSS independently predicted neglect. Score on virtually all of the neglect tests worsened as an effect of age. Percentage error on a line cancellation task was 3.8% higher for every additional 10 years of age, after adjustment for DWI volume and NIHSS (p = 0.006). Similar results were found for other neglect tests.
Conclusions:
Increasing age in patients with acute right hemispheric stroke significantly increases the odds of unilateral neglect as well as severity of neglect, independently of size of the stroke or NIH Stroke Scale score. The reason for this finding in older patients may be because they have more brain atrophy and may be less able to compensate for cerebral infarction, or because they tend to have more cardioembolic strokes, which may be more cortically based.
GLOSSARY
- CABG
= coronary artery bypass graft;
- DWI
= diffusion-weighted imaging;
- NIHSS
= NIH Stroke Scale;
- OR
= odds ratio.
Patients who experience unilateral neglect after acute right hemispheric stroke have a more difficult recovery course than do patients without neglect.1–4 Patients with neglect have longer stays in rehabilitation facilities4 and continue to have worse functional status for at least a year after their strokes.3 Similarly, advanced age is a risk factor for poor functional outcome after acute stroke.3,5 It is not clear, however, whether some of the poor outcome in the elderly is due to higher rates of symptoms such as neglect.
Older patients experience neglect at higher rates than younger patients. In one series, only 5% of patients aged 18 to 50 years had unilateral neglect, compared with 18% of subjects older than 80 years.6 In the Copenhagen Stroke Study, patients with neglect were on average almost 4 years older than equivalent patients without neglect.7 In contrast, in a series of 388 first-ever stroke patients in whom testing was performed for both unilateral neglect and anosognosia, patients with unilateral neglect were no different in age than subjects without neglect (mean age 76.6 years for those with neglect vs 75 years for those without neglect, p = 0.26). However, in this study, patients with anosognosia were older (82.6 years for those with anosognosia compared with 73.8 years for those without, p = 0.001).8
We studied patients with acute right hemispheric stroke to determine 1) whether increasing age was associated with neglect at a higher frequency, 2) whether increasing age was associated with more severe neglect, and 3) whether these relationships were independent of differences in stroke size and severity. In addition, we compared their relationships between age and neglect performance with those in a group of controls with vascular disease but without stroke.
METHODS
Subjects.
Subjects were a subset of a consecutive series of patients with acute right hemispheric ischemic stroke symptoms who were admitted to Johns Hopkins Hospital or Johns Hopkins Bayview Medical Center. Each subject met the following inclusion criteria: 1) able to provide informed consent for the study or indicate a family member who provided informed consent; 2) no contraindication to MRI (e.g., implanted ferrous metal); and 3) no prior diagnosis of dementia, previous symptomatic stroke, or other neurologic disease, significant hearing loss, or blindness. Exclusion criteria were as follows: 1) presence of acute left hemispheric infarct on diffusion-weighted imaging (DWI) MRI; 2) presence of acute infratentorial stroke; 3) presence of intraparenchymal hemorrhage (other than small petechial hemorrhage into an ischemic stroke); 4) absence of acute infarct on DWI MRI; and 5) left-handedness. All patients provided informed consent using forms approved by the institutional review board, and within 24 hours of admission (within the first 5 days after stroke onset, but usually within 24 hours of stroke onset), all of these subjects underwent extensive cognitive batteries with an emphasis on neglect testing. In addition, patients included in this study underwent neuroimaging with DWI MRI within 24 hours of admission. We included only subjects who completed at least part of the neglect battery and also had DWI.
A separate comparison group of subjects also underwent some of the cognitive testing. These participants were TIA patients or patients scheduled to undergo coronary artery bypass graft (CABG) surgery, and all had no stroke on MRI obtained the day of testing. A subset of the cognitive tests was administered to the subjects.
Testing.
For all stroke patients, a part of or the entire neglect battery was administered, including eight tests:
Oral reading: reading 30 words, displayed over two columns, and five sentences on a single sheet of paper, read without time limits.
Copy scene: copying the “Ogden scene”: a house, a fence, and two trees.9 There are 16 total components to the picture, so each missing component is thus scored as an error. Grossly misplaced components are scored as half an error. Total errors are divided by total number of components for percent error.
Clock copying: subjects are instructed to copy an analog clock, reading a certain time; this is also scored by omitted components as errors, and percent of the total components.
Vertical line bisection: drawing a short horizontal line at the middle of a single vertical 10-inch line.
Horizontal line bisection: drawing a short vertical line at the middle of a single horizontal 10-inch line, presented both at midline and at 45 degrees both right and left of midline. The distance to the right of center averaged over these three trials provided the final score.
Line cancellation: crossing out all of 28 vertical lines shown on a piece of paper, presented at center, 45 degrees right of midline, and 45 degrees left of midline, with percent error (omissions) averaged over these three trials.10
Visual extinction, two tests: a) percent errors in detecting finger wiggling in the contralateral visual field over 12 trials of simultaneous bilateral stimulus presentation and b) the ratio of errors in detecting finger wiggling alone in the contralesional field to errors detected in finger wiggling in the left field during bilateral stimulation.
Tactile extinction, two tests: the same as 7 but with light touch stimulus.
Patient age was recorded at the time of testing, in years. Error rates were recorded as percent error, with the exception of the two ratio tests (7b and 8b, above), which were recorded as ratios.
For comparison subjects, all of the same neglect tests were administered, with the exception of 7a and 8a, and comparison subjects only had horizontal line bisection presented at midline. All testing was performed preoperatively for the CABG patients, or when patients no longer had transient stroke symptoms in the case of the TIA patients.
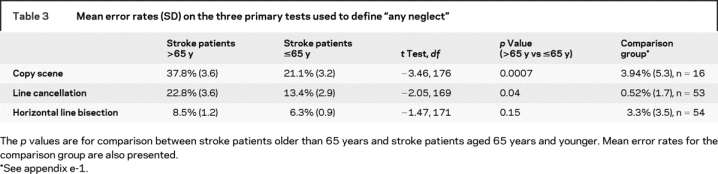
We defined a variable, “any neglect,” as positive if the subject had a Z score >2 on at least one of the following tests: horizontal line bisection, line cancellation, or copying the Ogden scene (the tests with the most complete data). Z scores were created based on the comparison individuals who were undergoing preoperative testing before CABG surgery or who had TIAs. A second, stricter definition of “any neglect” was created, in which an individual was required to have a Z score ≥2 on at least two of the three tests listed above.
The NIH Stroke Scale (NIHSS) was calculated for each patient by chart review. For some patients, the NIHSS was directly recorded in the chart at the time of admission, whereas for others, the previously validated retrospective technique in calculating the NIHSS was used.11
Volumetric analysis.
Volumetric analysis was performed by a technician or investigator with the assistance of ImageJ software, using DWI images.12 Lesions were traced on individual slices, and volumes of infarct were calculated based on the slice thickness and were recorded in cubic centimeters. Patients with left hemispheric acute infarcts were excluded, as were patients with any infratentorial infarct. For a subsample of 10% of the total sample, volumetric analysis was repeated by another investigator, and an intraclass correlation coefficient was calculated for this subset.
Statistical analysis: Stroke patients.
Stata version 8.0 for Macintosh was used for all analyses.13 We used descriptive statistics to characterize the study population. We analyzed age both as a continuous variable (as an independent variable in regression models) and as a categorical variable, comparing patients younger than 65 years with those older than 65 years. As a dependent variable in these regression models, we used “any neglect” (both definitions) in a logistic regression model to assess prediction of neglect frequency, and we also used percent error on the various tests described above in linear regression models to determine severity of neglect. Finally, in the multivariate models, we included infarct size (measured using DWI images, as described above) and admission NIHSS as covariates. For these regression models, variables were removed one at a time to calculate a likelihood ratio test to determine the level of significance of the variable in question. Goodness of fit was assessed with the Hosmer–Lemeshow statistic for the logistic regression. Collinearity was assessed with the variance inflation factor. We also used χ2 testing for univariate categorical comparisons (dichotomized age vs any neglect) and Student t tests for univariate continuous comparisons (e.g., mean age for subjects with and without any neglect).
Statistical analysis: Comparison group.
The relationship between age and error rate on various neglect tests was studied in a comparison group, as in the cases (stroke patients), using linear regression using both a continuous variable (for age) and a categorical variable, comparing patients younger than 65 years with those older than 65 years. For comparison with the normal controls, a separate model was created that included both populations as well as a variable for case status. The effect of case status on the association between age and neglect performance was explored through an interaction term.
RESULTS
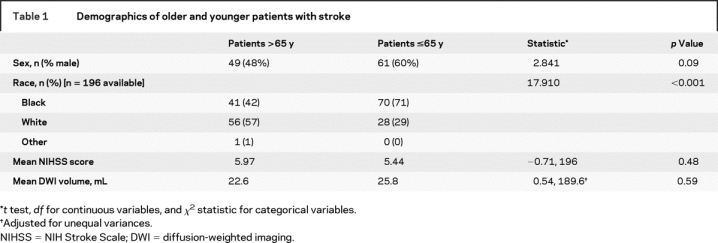
Of 329 potentially eligible patients, 125 were excluded: 60 had TIAs, 32 had some infratentorial lesion on acute MRI, 29 had left hemispheric acute stroke, and 4 had intracerebral hemorrhage on MRI. Thus, a total of 204 stroke patients were included in this study and were studied within 24 hours of admission and within 5 days of symptom onset. The median age was 65 years, with a range from 26 to 95 years; the mean age was 64.3 years (SD 14 years). Further demographic information about these age groups is provided in table 1. The mean stroke volume for stroke patients older than 65 years was 22.6 mL, compared with 25.8 mL for younger patients (p = 0.59). Information about completeness of testing data is supplied in table 1.
Table 1 Demographics of older and younger patients with stroke
For a subsample of 10% of the total sample of cases, volumetric measurements were duplicated by the lead investigator. For these 21 cases (ranging in volume from 0.097 to 306.012 mL), the intraclass correlation coefficient was excellent, at 0.978 (95% CI, 0.958–0.997).
Data are first presented using “any neglect” defined by at least one Z score ≥2 on the three tests described above. A total of 69.6% of subjects older than 65 years had “any neglect,” as defined above, whereas only 49.4% of subjects aged 65 and younger had neglect (p = 0.008). Subjects with neglect had an average age of 66 years, whereas those without neglect were aged 60 years, on average (p = 0.005). In a multivariate logistic regression analysis, for every additional 10 years of age, patients were 1.54 times as likely to have any neglect by this definition, even after adjusting for DWI infarct volume and NIHSS score (adjusted odds ratio [OR] 1.54, 95% CI 1.18–2.01; p = 0.0008; crude OR 1.37, 95% CI 1.09–1.71). DWI volume and initial NIHSS were also independent predictors of having any neglect. Per additional 10 mL of infarct volume, by DWI, subjects were 32% more likely to have any neglect in this adjusted model (OR 1.32, 95% CI 1.06–1.64). Per additional point on the NIHSS, there was a 13% increase in odds of having any neglect (adjusted OR 1.13, 95% CI 1.03–1.25) after adjustment for DWI volume and age.
When “any neglect” was defined more strictly, as defined above, 31% of the total sample of stroke patients had neglect by this definition: a Z score ≥2 on at least two of the three included tests. With this definition, of those older than 65 years, 40% of individuals had neglect, compared with 23.5% of younger individuals (p = 0.025). For every 10 additional years, in the model adjusted for NIHSS and DWI volume, individuals were 1.36 times as likely to have neglect by this definition (adjusted OR 1.36, 95% CI 1.01–1.83).
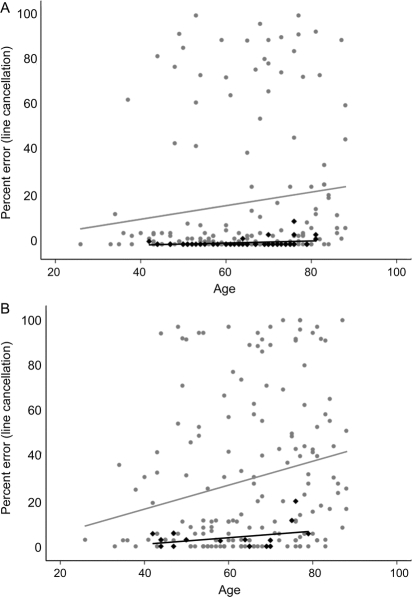
Scores on most of the neglect tests worsened as an effect of age. Percent error on a line cancellation task was 3.8% higher (with a minimum of 0% and maximum possible of 100%) for every additional 10 years of age after adjustment for DWI volume and NIHSS (p = 0.006) (figure). Similar results were found for the copy scene task (β = 6.5, p < 0.0001) and copying of a clock (β = 4.1, p = 0.002), each per 10 additional years of age. See tables 2 and 3 for additional results. These results are suggestive of worse performance on most tests for individuals with increasing age. Performance by the comparison patients is found in appendix e-1 on the Neurology® Web site at www.neurology.org.
Figure Error rates on two neglect tests, by age, in normal controls (black diamonds, black line) and cases (gray circles, gray line)
(A) Line cancellation percent error. (B) Copy scene percent error.
Table 2 Difference in error rates on each of the neglect tests, per additional 10 years of age, for patients with right hemisphere stroke
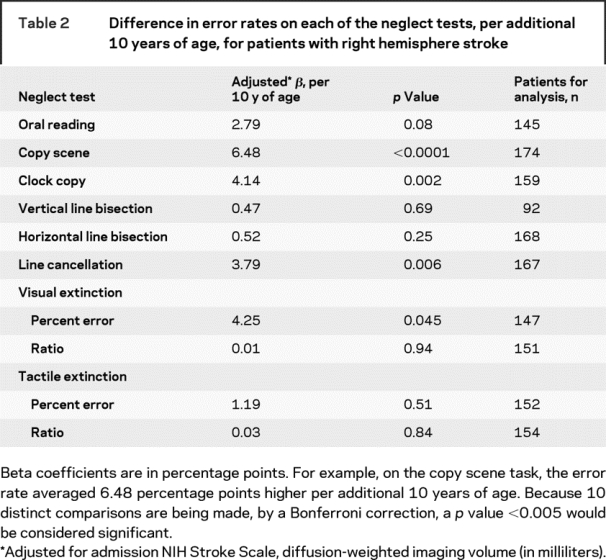
Table 3 Mean error rates (SD) on the three primary tests used to define “any neglect”
DISCUSSION
We have found that, among patients with acute right hemispheric stroke, neglect occurs at higher frequency and at increasing severity in older patients. This is independent of the size of the stroke and the severity of other presenting clinical symptoms. We found similar results across multiple neglect tests and by analyzing age and neglect as either continuous or categorical variables. The relationship persisted when a stricter definition of neglect was used. In addition, infarct volume, defined by DWI volume, and severity of stroke, defined by NIHSS score, were each independent predictors of presence of neglect. Although not all individual neglect test error rates were significantly associated with increasing age, they all were in the direction of higher error rates for older individuals.
It could be hypothesized that performance on any cognitive test, including neglect tests, worsens with increasing age. Some of this age-related effect was found in a separate sample of comparison patients (other patients with vascular disease), but a significant effect was only found with one neglect test (line cancellation), and the size of the effect was much smaller (almost 10-fold smaller) than that found with this study population with stroke. The curve describing the relationship between age and neglect score was much steeper for patients with stroke than with controls, despite similar age distributions.
Older patients with stroke may be more likely to have had prior ischemic brain injury and thus may not be able to compensate as well for further injury (and may experience more cognitive sequelae, such as neglect, as a consequence). This is further supported by literature suggesting that other cognitive functions after stroke, such as language, are affected more frequently in older than in younger patients.14 Total brain volume could also explain some of the differences in extent of neglect. Even though we adjusted for infarct volume, if for the older patients this represents a larger percentage of total brain volume, the older patients might have more difficulty performing the neglect tests.
An alternative explanation for our findings is that older patients have specific brain changes that make them more vulnerable to neglect as a result of right hemisphere stroke. It has been proposed that there are two brain networks that work together to carry out visual attentional functions: the dorsal network, important for goal-directed selection of stimuli (located in the dorsal posterior parietal and frontal cortex bilaterally), and another, the ventral network, responsible for stimulus-driven control of attention and target detection, which is located primarily in the right hemisphere. Patients with neglect usually have lesions to the right hemisphere, damaging the ventral network (and thus have problems with stimulus detection), as well as the connections between the ventral and dorsal network on the same side, affecting goal-directed stimulus selection also.15 Dysfunction of both networks (by lesion or disconnection) leads to more severe neglect than does dysfunction of just one of the networks. Further evidence for the importance of intact connections between these two networks (dorsal and ventral) is supported by functional imaging data, showing that disconnection of the tracts between frontal and parietal cortices was associated with more severe neglect.16 Elderly patients may have more atrophy, for example, or more white matter disease in the bilateral frontal cortices (the dorsal network bilaterally), which may lead to impaired goal-directed selection of stimuli and may make them more vulnerable to developing neglect in the setting of a new right hemispheric ischemic lesion affecting the ventral network. Severe neglect requires dysfunction of both networks.
The primary limitation of the current study is the paucity of information on stroke location. Elderly patients might be more likely to have cardioembolic strokes, such as due to atrial fibrillation, and might be more likely to have strokes in the cortex, a location that would lead to more neglect.6 If this difference accounted for all of our findings, however, one would expect systematic differences in stroke volume in these populations. The infarct size was comparable in the older and younger patients (mean 22.6 mL vs 25.8 mL, not significant), and averaged even smaller in older patients. There was also no significant statistical relationship between age and DWI volume when each was examined as a continuous variable.
Another limitation is that there is no agreed-on definition of neglect. Some authors have advocated the use of several tests to increases sensitivity and specificity.17 Even with multiple definitions of neglect, older patients in our study had neglect more frequently than did younger patients.
We had a moderate amount of missing data on the components of our neglect battery (table 2). In a sensitivity analysis to determine whether those with missing data were otherwise different from those included in the study, the mean DWI volume for the 28 subjects who were unable to be rated for “any neglect” due to missing data was 27 mL, compared with 25 mL for those 181 participants who were able to be rated. The mean age of those with missing “any neglect” data was 62.4 years, compared with 63.9 years in the included group; both groups averaged 6 as their NIHSS. Based on these post hoc analyses, it does not seem that the excluded group is substantially different in reference to stroke size, severity, or age. In part, because the entire neglect battery is time-consuming, some subjects either stop tolerating testing of any type or are interrupted for clinical tests or therapies.
Our findings, which incorporate infarct volume and stroke severity in assessing the relationship between age and neglect, suggest that elderly stroke patients may be more likely to experience poststroke neglect. This is beyond what would be expected as a result of age alone and highlights a particular problem for older stroke patients. Further studies are needed to determine whether stroke location or total brain volume account for much of this age effect. In addition, information about the extent of white matter burden of the brain will be critical in future studies to determine whether this explains some of the age effect. Particular care should be taken to identify any potential symptoms of neglect and to rehabilitate elderly patients with right hemispheric strokes.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by R.F.G.
Supplementary Material
Address correspondence and reprint requests to Dr. Rebecca F. Gottesman, Meyer 6-109, 600 N. Wolfe St., Baltimore, MD 21287 rgottesm@jhmi.edu
Supplemental data at www.neurology.org
Supported by NIH grant R01-NS047691 (to A.E.H.) and a Johns Hopkins Clinician Scientist Award (to R.F.G.).
Disclosure: The authors report no disclosures.
Received March 24, 2008. Accepted in final form July 20, 2008.
REFERENCES
- 1.Adams GF, Hurwitz LJ. Mental barriers to recovery from strokes. Lancet 1963;282:533–590. [DOI] [PubMed] [Google Scholar]
- 2.Jehkonen M, Laihosalo M, Kettunen JE. Impact of neglect on functional outcome after stroke: a review of methodological issues and recent research findings. Restorative Neurol Neurosci 2006;24 209–215. [PubMed] [Google Scholar]
- 3.Jehkonen M, Ahonen JP, Dastidar P, et al. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand 2000;101:195–201. [DOI] [PubMed] [Google Scholar]
- 4.Gillen R, Tennen H, McKee T. Unilateral spatial neglect: relation to rehabilitation outcomes in patients with right hemisphere stroke. Arch Phys Med Rehabil 2005;86:763–767. [DOI] [PubMed] [Google Scholar]
- 5.Wade DT, Langton Hewer R. Stroke: associations with age, sex, and side of weakness. Arch Phys Med Rehabil 1986;67:540–545. [PubMed] [Google Scholar]
- 6.Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 2004;63:468–474. [DOI] [PubMed] [Google Scholar]
- 7.Pederson PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Hemineglect in acute stroke- incidence and prognostic implications: the Copenhagen Stroke Study. Am J Phys Med Rehabil 1997;76:122–127. [DOI] [PubMed] [Google Scholar]
- 8.Appelros P, Karlsson GM, Hennerdal S. Anosognosia versus unilateral neglect: coexistence and their relations to age, stroke severity, lesion site and cognition. Eur J Neurol 2007;14:54–59. [DOI] [PubMed] [Google Scholar]
- 9.Ogden J. Contralesional neglect of constructed visual images in right and left brain-damaged patients. Neuropsychologia 1985;23:273–277. [DOI] [PubMed] [Google Scholar]
- 10.Albert ML. A simple test of visual neglect. Neurology 1973;23:658–664. [DOI] [PubMed] [Google Scholar]
- 11.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000;31:858–862. [DOI] [PubMed] [Google Scholar]
- 12.Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health, 2005. Available at: http://rsb.info.nih.gov/ij. Accessed August 18, 2008.
- 13.Stata Statistical Software: Release 8.0 [program]. College Station, TX: Stata Corporation, 2002. [Google Scholar]
- 14.Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379–1384. [DOI] [PubMed] [Google Scholar]
- 15.Corbetta M, Shulman GL. Control of goal-directed and stimulus-directed attention in the brain. Nat Rev Neurosci 2002;3:201–216. [DOI] [PubMed] [Google Scholar]
- 16.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007;53:905–918. [DOI] [PubMed] [Google Scholar]
- 17.Jehkonen M, Ahonen JP, Dastidar P, Koivisto AM, Laippala P, Vilkki J. How to detect visual neglect in acute stroke. Lancet 1998;351:727–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.