Abstract
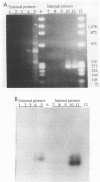
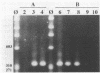
A sensitive assay based on amplification of a 319-bp DNA fragment of the intracellular bacterium of swine proliferative enteritis was developed for the detection of the organism in the feces of swine. A vernacular name, ileal symbiont intracellularis (IS-intracellularis), has recently been published for the intracellular bacterium, which was formerly known as a Campylobacter-like organism (C.J. Gebhart, S.M. Barnes, S. McOrist, G.F. Lin, and G.H.K. Larson, Int. J. Syst. Bacteriol. 43:533-538, 1993). As few as 10(1) IS-intracellularis organisms purified from intestinal mucosa, or 10(3) IS-intracellularis per g of feces, were detected. No amplification product was produced from a polymerase chain reaction performed on DNA extracted from the feces of healthy pigs. A 319-bp DNA fragment specific for IS-intracellularis was produced on amplification of DNA from the feces of pigs with experimental and naturally occurring proliferative enteritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstain J. M., Grimprel E., Lukehart S. A., Norgard M. V., Radolf J. D. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991 Jan;29(1):62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. H., Cullinane L. C. Monitoring the health of pigs in New Zealand abattoirs. N Z Vet J. 1990 Dec;38(4):136–141. doi: 10.1080/00480169.1990.35639. [DOI] [PubMed] [Google Scholar]
- Gebhart C. J., Barns S. M., McOrist S., Lin G. F., Lawson G. H. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993 Jul;43(3):533–538. doi: 10.1099/00207713-43-3-533. [DOI] [PubMed] [Google Scholar]
- Gebhart C. J., Lin G. F., McOrist S. M., Lawson G. H., Murtaugh M. P. Cloned DNA probes specific for the intracellular Campylobacter-like organism of porcine proliferative enteritis. J Clin Microbiol. 1991 May;29(5):1011–1015. doi: 10.1128/jcm.29.5.1011-1015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumerlock P. H., Tang Y. J., Meyers F. J., Silva J., Jr Use of the polymerase chain reaction for the specific and direct detection of Clostridium difficile in human feces. Rev Infect Dis. 1991 Nov-Dec;13(6):1053–1060. doi: 10.1093/clinids/13.6.1053. [DOI] [PubMed] [Google Scholar]
- Lawson G. H., McOrist S., Rowland A. C., McCartney E., Roberts L. Serological diagnosis of the porcine proliferative enteropathies: implications for aetiology and epidemiology. Vet Rec. 1988 Jun 4;122(23):554–557. doi: 10.1136/vr.122.23.554. [DOI] [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C., MacIntyre N. Demonstration of a new intracellular antigen in porcine intestinal adenomatosis and hamster proliferative ileitis. Vet Microbiol. 1985 Jun;10(4):303–313. doi: 10.1016/0378-1135(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Lomax L. G., Glock R. D. Naturally occurring porcine proliferative enteritis: pathologic and bacteriologic findings. Am J Vet Res. 1982 Sep;43(9):1608–1614. [PubMed] [Google Scholar]
- Plikaytis B. B., Gelber R. H., Shinnick T. M. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990 Sep;28(9):1913–1917. doi: 10.1128/jcm.28.9.1913-1917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stills H. F., Jr Isolation of an intracellular bacterium from hamsters (Mesocricetus auratus) with proliferative ileitis and reproduction of the disease with a pure culture. Infect Immun. 1991 Sep;59(9):3227–3236. doi: 10.1128/iai.59.9.3227-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. H., Andersen P. R., Green E., Hermon-Taylor J., McFadden J. J. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J Clin Microbiol. 1990 May;28(5):933–937. doi: 10.1128/jcm.28.5.933-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]