Abstract
Exophytic condylomata acuminata of the external genitalia of 40 patients were analyzed for human papillomavirus (HPV) DNA by the Southern blot and hybrid capture methods. All lesions were initially analyzed by the Southern blot method by using a mixture of HPV type 6, 11, 16, and 18 whole genomic probes. Southern blots demonstrated characteristic PstI restriction patterns of HPV type 6, 11, or 16 in all but one lesion. HPV 6 subtypes accounted for 28 of 39 HPV-positive lesions. Twenty-seven of these 28 lesions contained HPV type 6a, and 1 lesion contained HPV type 6c. Eight lesions contained HPV type 11 and three contained HPV type 16. Two of the three condylomata acuminata containing HPV type 16 were obtained from solid-organ transplant recipients receiving immunosuppressive medications. The third lesion containing HPV type 16 was a typical exophytic condyloma acuminatum from a woman with previously resected vulvar carcinoma. The hybrid capture assay detected HPV DNAs in all lesions except the Southern blot-negative lesion. Twenty-five lesions were positive for the A probe only (HPV types 6 and 11 and related types). All of these lesions were found to contain HPV type 6 or 11 sequences in the Southern blot assay. The remaining 14 lesions were positive for both the A probe and the B probe (HPV types 16 and 18 and related types). The strongest signal in these 14 lesions by the hybrid capture assay was consistent with the result of the Southern blot assay in all but one case. We conclude that (i) HPV type 6a is the most common type found in these lesions, (ii) HPV type 16 may be present more often in exophytic condylomata acuminata from immunosuppressed individuals, (iii) hybrid capture is a useful tool for documenting the presence of HPV sequences in DNAs from exophytic condylomata acuminata, and (iv) in samples containing multiple HPV types, hybrid capture allows detection of minority HPV types.
Full text
PDF




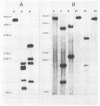
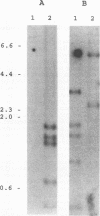
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann A. M., Sherman K. J., Myerson D., Daling J. R., McDougall J. K., Galloway D. A. Comparative virologic studies of condylomata acuminata reveal a lack of dual infections with human papillomaviruses. J Infect Dis. 1991 Feb;163(2):393–396. doi: 10.1093/infdis/163.2.393. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Burk R. D., Lancaster W. D., Pfister H., Schiffman M. H. Inter-laboratory variation as an explanation for varying prevalence estimates of human papillomavirus infection. Int J Cancer. 1989 Feb 15;43(2):260–262. doi: 10.1002/ijc.2910430216. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Bryan J. T., Rodriguez M., Katz B. P. Factors associated with detection of human papillomavirus E4 and L1 proteins in condylomata acuminata. J Infect Dis. 1992 Sep;166(3):512–517. doi: 10.1093/infdis/166.3.512. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Chin M. T., Strike D. G. Identification of human papillomavirus type 11 E4 gene products in human tissue implants from athymic mice. Virology. 1988 Jul;165(1):262–267. doi: 10.1016/0042-6822(88)90680-0. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Fife K. H. Human papillomavirus infections of the genital tract. Med Clin North Am. 1990 Nov;74(6):1455–1485. doi: 10.1016/s0025-7125(16)30490-4. [DOI] [PubMed] [Google Scholar]
- Campion M. J., Singer A., Clarkson P. K., McCance D. J. Increased risk of cervical neoplasia in consorts of men with penile condylomata acuminata. Lancet. 1985 Apr 27;1(8435):943–946. doi: 10.1016/s0140-6736(85)91724-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- DiLorenzo T. P., Tamsen A., Abramson A. L., Steinberg B. M. Human papillomavirus type 6a DNA in the lung carcinoma of a patient with recurrent laryngeal papillomatosis is characterized by a partial duplication. J Gen Virol. 1992 Feb;73(Pt 2):423–428. doi: 10.1099/0022-1317-73-2-423. [DOI] [PubMed] [Google Scholar]
- Evans B. A., Bond R. A., MacRae K. D. A colposcopic case-control study of cervical squamous intraepithelial lesions in women with anogenital warts. Genitourin Med. 1992 Oct;68(5):300–304. doi: 10.1136/sti.68.5.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., zur Hausen H. Partial characterization of viral DNA from human genital warts (Condylomata acuminata). Int J Cancer. 1980 May 15;25(5):605–609. doi: 10.1002/ijc.2910250509. [DOI] [PubMed] [Google Scholar]
- Kasher M. S., Roman A. Characterization of human papillomavirus type 6b DNA isolated from an invasive squamous carcinoma of the vulva. Virology. 1988 Jul;165(1):225–233. doi: 10.1016/0042-6822(88)90676-9. [DOI] [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Reid R., Jenson A. B., Greenberg M. D., Lancaster W., Kurman R. J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992 Mar;79(3):328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Penn I. Cancers of the anogenital region in renal transplant recipients. Analysis of 65 cases. Cancer. 1986 Aug 1;58(3):611–616. doi: 10.1002/1097-0142(19860801)58:3<611::aid-cncr2820580303>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987;48:113–147. doi: 10.1016/s0065-230x(08)60691-0. [DOI] [PubMed] [Google Scholar]
- Porreco R., Penn I., Droegemueller W., Greer B., Makowski E. Gynecologic malignancies in immunosuppressed organ homograft recipients. Obstet Gynecol. 1975 Apr;45(4):359–364. [PubMed] [Google Scholar]
- Roman A., Fife K. H. Human papillomaviruses: are we ready to type? Clin Microbiol Rev. 1989 Apr;2(2):166–190. doi: 10.1128/cmr.2.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutton G. P., Stehman F. B., Ehrlich C. E., Roman A. Human papillomavirus deoxyribonucleic acid in lesions of the female genital tract: evidence for type 6/11 in squamous carcinoma of the vulva. Obstet Gynecol. 1987 Oct;70(4):564–568. [PubMed] [Google Scholar]
- Walker P. G., Singer A., Dyson J. L., Oriel J. D. Natural history of cervical epithelial abnormalities in patients with vulval warts. A colposcopic study. Br J Vener Dis. 1983 Oct;59(5):327–329. doi: 10.1136/sti.59.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkinshaw S. A., Dodgson J., McCance D. J., Duncan I. D. Risk factors in the development of cervical intraepithelial neoplasia in women with vulval warts. Genitourin Med. 1988 Oct;64(5):316–320. doi: 10.1136/sti.64.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Mounts P. Heterogeneity in mRNA of human papillomavirus type-6 subtypes in respiratory tract lesions. Virology. 1989 Jan;168(1):1–12. doi: 10.1016/0042-6822(89)90397-8. [DOI] [PubMed] [Google Scholar]
- Wilbur D. C., Reichman R. C., Stoler M. H. Detection of infection by human papillomavirus in genital condylomata. A comparison study using immunocytochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988 Apr;89(4):505–510. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]