Abstract
Haemophilus somni causes bovine pneumonia as well as septicemia and its sequelae but mechanisms of virulence and protective immunity are poorly understood. Since surface immunoglobulin binding proteins are virulence factors, we addressed their role as protective antigens in a mouse model of H. somni septicemia. Immunoglobulin binding protein A (IbpA), has homology to Bordetella pertussis filamentous hemagglutinin and other large bacterial exoproteins. IbpA is a major surface antigen encoded by the ibpA gene with many domains that may be important in pathogenesis and immune protection. Three IbpA recombinant protein subunits, IbpA3, IbpA5 and IbpADR2 were chosen for study because of putative functional domains and motifs. These recombinant GST fusion subunit proteins were compared with GST (negative control), formalin-killed H. somni (commercial vaccine control), live H. somni (to induce convalescent immunity) and H. somni culture supernatant (containing IbpA shed from the bacterial surface). In vaccination/challenge studies, both live H. somni (convalescent immunity) and supernatant protected equally but formalin-killed H. somni and GST did not protect against septicemia. The DR2 and A3 subunits protected moderately well and induced antibody responses against supernatant antigen and the homologous subunit in ELISA but not against whole cell antigens. Supernatant immunization protected better than the IbpA subunit antigens and induced high antibody activity against both whole cells and supernatant antigens. The results indicate that culture supernatant antigens or perhaps recombinant IbpA subunits may be useful in H. somni vaccines. These studies also provide insight into the contribution of IbpA domains to pathogenesis of H. somni septicemia.
Keywords: Histophilus somni, Immunoglobulin binding proteins, IbpA, mouse H. somni septicemia model
1. Introduction
Histophilus somni, also called Haemophilus somnus [1], is a major etiologic agent of the bovine respiratory disease complex [2,3]. It also causes septicemia, thrombotic meningoencephalitis, myocarditis, arthritis, infertility, and abortion [4–10]. Infection is associated with vasculitis, thrombosis and macrophage degeneration [2]. In addition, infected cattle are often H. somni carriers without clinical signs[11]. Some strains from carriers (carrier or avirulent strains) do not express surface immunoglobulin binding proteins (IgBPs) [12] whereas all tested pathogenic invasive strains of H. somni have IgBPs, which bind bovine IgG2 by the Fc portion [13,14]. These carrier strains were serum sensitive but the virulent strains were serum resistant [13,15,16]. The IgBPs are associated with resistance to complement mediated killing of H. somni [12,13]. This indicates that serum resistance and IgBPs may be related factors. Furthermore, cattle with disease due to H. somni develop high antibody titers to IgBPs [17]. Thus, IgBPs may be candidates for subunit vaccines. There is a need for new, more effective vaccines for H. somni disease because the efficacy of available vaccines is variable and controversial [18]. Even today most vaccines are composed of killed whole bacteria, which may be deficient in IgBPs because they are largely shed into the culture supernatant [14].
The IgBPs consist of a series of high molecular weight (HMW) proteins and a 76 kDa surface protein (p76) detected by SDS-PAGE and Western blotting [16]. Our original cloning studies showed that the HMW IgBPs and p76 were genetically linked [19]. When the whole DNA insert encoding the HMW IgBPs and the linked p76 was sequenced, only one open reading frame (ORF) was found [13,19,20]. This large (12.2 kb) gene, immunoglobulin binding protein A, (ibpA) has multiple initiation sites, many of which are functional, partially accounting for the proteins of different sizes [21,22]. The HMW IgBPs consist of a fibrillar network on the bacterial surface [16] and are shed in culture supernatant as well as being detected in the whole cell pellet [23]. The p76 IgBP is a surface exposed, peripheral outer membrane protein [16]. Sequencing of clones expressing p76 revealed a single 3.9 kb ORF containing tandem 1.2 kb direct repeats (DR1 and DR2) [21]. Later, several upstream domains in IbpA were defined, including IbpA3 and IbpA5 [20].
Current commercially available vaccines for H. somni utilize killed whole H. somni cells and have been only moderately successful [24–26]. Whole cell vaccines also have a reputation for occasionally causing severe adverse reactions in cattle [27]. Most vaccines still consist of whole killed H. somni, so better vaccines are needed. We showed that H. somni convalescent phase calf serum passively protected calves against experimental H. somni pneumonia [28]. Antigens recognized by this protective antiserum may be critical for immune protection. Our recent studies of a series of recombinant truncated IbpA subunits showed that glutathione S-transferase (GST)-fused recombinant subunits IbpA3 (aa 972–1515) and IbpA5 (aa 2071–2730) reacted strongly with protective calf convalescent phase serum [20], so it was hypothesized that these protein subunits may be protective. In addition, preliminary studies showed that bovine H. somni convalescent phase serum or rabbit antiserum to IbpA DR2 passively protected mice against H. somni septicemia [R. Kruger, JE Dixon, LB Corbeil, unpublished data]. Therefore, the purpose of this study was to evaluate the protective ability of the IbpA3, IbpA5, and IbpA DR2 recombinant subunits in comparison with crude native IbpA in culture supernatant. A mouse model of H. somni septicemia was used because bovine H. somni disease is mostly due to septicemia and it’s sequelae [4–10, 29]. The model includes preincubation of H. somni in fetal calf serum for 5 minutes to bind bovine transferrin [29]. This enhances virulence of H. somni for mice and simulates bovine septicemia more closely [29]. The level of protection was compared with vaccines composed of killed whole cells, live virulent organisms, and native IgBPs found in culture supernatant.
2. Materials and methods
2.1. Animals and experimental design
Female, 5–6 weeks old, NIH Swiss Webster mice obtained from the National Cancer Institute (Fredrick, MD) through Charles River Labs were housed in groups of 4–5 in individual ventilated cages. Immunization experiments were conducted in three separate trials. In each experiment, 4 or 5 animals per group were immunized with antigens and adjuvant twice at a three week interval and challenged intraperitoneally 2 to 2.5 weeks later, as described below.
2.2. Bacterial strains and culture
The virulent H. somni strain, 2336, (from a pneumonic calf) has been previously described and used to produce pneumonia in calves [2,13,15,20,28] and septicemia in mice [29]. For mouse challenge experiments and Western blots to whole cell antigens, the organism was grown on brain heart infusion (BHI) agar (Difco Laboratories, Detroit, MI) supplemented with 5% bovine blood in candle jars at 37 °C. At 18 hours of growth, the organism was scraped off the blood agar plate and suspended in PBS to a 75% light transmittance at 610 nm (Coleman Jr. Spectrophotometer). Based on past experience, a 75%T suspension of this 18 hour culture contains about 8 × 108 H. somni cfus/ml. The actual counts were confirmed by plate counts each time a suspension was made. For production of culture supernatant or whole cells for ELISA analysis (see below), BHI broth (Difco Laboratories) was supplemented with 0.1% trizma base and 0.01% thiamine monophosphate (BHITT) [30]. The broth was inoculated with H. somni and incubated with shaking at 37 °C for 6–18 h. The culture suspension was centrifuged at 8000 × g for 10 min and the supernatant was filtered through a 0.2 µM filter. The whole cell pellet was washed 3 times with PBS and used in the ELISA as whole cell antigen.
2.3. Recombinant IbpA subunit fusion protein production
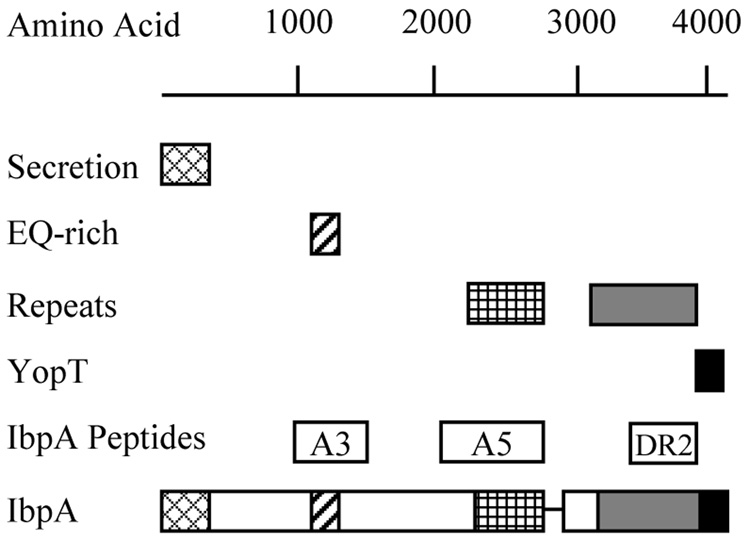
The approximate location of each of the IbpA subunits is shown in Figure 1. Previously described plasmids pHS 140 and pHS 134 [13,20,21] were used for cloning the A3 and A5 coding regions of the IbpA gene of H. somni. pHS 139 was used as a template for the DR2 sequence. The forward and reverse primers were as follows: A3: AGCTGGATCCAGTGAACGAATCACTGTAGG, AGCTGAATTCGAAGCTTTCACAAAACCTGTTTTA; A5: AGCTGGATCCGATTTGGATCTTGTAGCGG, AGCTGAATTCGAGGCATAAATATGATCTGCCG; DR2: AGCTGGATCCATCGAAAAGTTAAATCATGGATTA, AGCTGAATTCAGATTATTTTTTTTGTAGTTGACCAC. The PCR-amplified A3 and A5 fragments were ligated into pET-GSTx and the amplified DR2 fragment was ligated into pET41a (Novagen, Darmstadt, Germany) using the Rapid DNA Ligation Kit (Roche, Nutley, NJ). Both vectors encoded a glutathione-S-transferase (GST) tag at the N-terminus of the inserted DNA sequence, and a His tag at the C-terminus. Escherichia coli BL21 DE3 codon plus cells transformed with each of the plasmids was used to inoculate 1 liter of LB medium containing ampicillin (GSTx) or kanamycin (pET41a) and chloramphenicol and were grown at 37 °C to an OD600 of 0.6, at which time protein expression was induced by addition of 0.4 mM isopropyl-D-thiogalactopyranoside (IPTG). After induction the culture was left at 25 °C overnight and harvested by centrifugation. The cells were resuspended in 50 ml of PBS containing 1% triton and 5 mM DTT (lysis buffer) and were lysed using a French Press. The lysate was cleared by centrifugation at 15,000 × g for 30 min at 4 °C and the GST tagged proteins were attached to the glutathione agarose (Sigma, St. Louis, MO) by rotation for one h at 4 °C. The protein-loaded beads were washed by rotation at 4 °C with lysis buffer (4 × 10 ml) and wash buffer, 50 mM Tris pH 8.0, 100 mM NaCl, 5 mM DTT, (3 × 10 ml) for 15 min. The protein was eluted by twice treating with 20 mM glutathione in wash buffer for 30 min at 4 °C. Protein concentrations were determined with the BioRad Protein Assay (BioRad Laboratory Inc., Hercules, CA) and purity evaluated by SDS-PAGE followed by Coomassie staining.
Fig. 1.
Sequence structure diagram for H. somni IbpA. The secretion domain, EQ-rich region, repeat units, and YopT homology domain are shown. The position of IbpA3, IbpA5 and IbpA DR2 are indicated (Modified from reference 20).
2.4. Mouse vaccination and challenge
Experiment 1
Mice were immunized with 3.3 µl of an 18 h culture supernatant concentrated to 1/10th of the original volume using a Centricon 10,000 mw concentrator (Amicon, Beverly, MA), 2 × 108 formalin-killed organisms, or PBS as a negative control. Each of the antigens was diluted in PBS to 100 µl, then mixed with 10µg Quil A (Accurate Chemical and Scientific, Westbury, NY) in 100 µl PBS before being injected subcutaneously (SQ) in the mice. A positive H. somni infection convalescent phase control group received an intraperitoneal (IP) inoculation of approximately 108 live H. somni organisms (in PBS without FCS, thus not enchancing virulence [29]). Three weeks later, all mice received a second immunization. The convalescent control group received a second immunization by infection, as in the first immunization.
Experiments 2 and 3
In order to test IbpA subunit vaccines, mice were immunized with 40 µg of the recombinant IbpA subunits DR2, A3, or A5 proteins fused to GST in two separate experiments, with 4 mice/group in experiment 2 and 5/group in the experiment 3. In experiment 2, positive control (supernatant vaccine) and negative control mice received 3.3 µl of an 18 h concentrated culture supernatant or PBS respectively. Experiment 3 was modified to remove broth constituents from the supernatant preparation and substituted GST for PBS to control for the GST tag on recombinant IbpA subsunits. Thus, positive (supernatant vaccine) control mice received 30 µl of supernatant from a 6 h culture that was dialyzed overnight through a 10,000 MWCO snakeskin dialysis bag (Pierce, Rockford, IL) with PBS to remove excess BHI medium. Negative control mice received 13.3 µg of recombinant GST because it constituted about a third of the recombinant subunit fusion proteins. All antigens were diluted with PBS to 100 µl and mixed with 10µg Quil A in 100 µl PBS before being injected SQ. All immunizations were repeated three weeks after the first inoculation, as in experiment 1.
Protection was determined 14–17 days after the second immunization in all three experiments, by challenging with approximately 108 virulent H. somni organisms pre-incubated with fetal calf serum (FCS) to enhance virulence [29]. 125 µl containing 108 H. somni in PBS was incubated with 125 µl of FCS for 5 min at room temperature. The mixture was diluted with PBS to 1.0 ml and inoculated IP into mice. Mice were monitored by R Geertsema, DVM, every 8–12 hours for signs of septicemia and assigned a clinical score, as described below. Groups were not coded (not a “blinded study”). Any mouse found to be moribund was euthanized and surviving mice were euthanized at 48–72 hours, as indicated in the results. Mice were weighed every 24 hours. Clinical scores were based on general appearance and activity according to following scale: 0= normal; 1= ruffled fur, normal activity; 2= hunched, eyes closed, reduced activity; 3= hunched, eyes closed, cold ears, and reluctant to move when prodded; and 4= moribund or dead. Blood was collected after euthanasia from moribund mice or surviving mice at 48–72 h. In immunization experiments 2 and 3, approximately 50 µl of blood was collected from the tail vein 14 days after the second immunization and the mice were challenged 3 days later. After clotting, the blood was centrifuged at 4000 × g for 20 min and the serum collected for antibody analysis.
All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.4. ELISA of immunized mouse serum
Specific serum IgG antibodies were analyzed against different H. somni antigen preparations by ELISA. Briefly, 96-well plates (Costar-High Binding EIA/RIA one-half well plates, Corning Inc., Corning, NY) were coated overnight at room temperature with 50 µl of washed whole cell pellet, at 6 h of growth, diluted to 75%T, culture supernatant diluted to an equivalent level as the whole cells, or recombinant IbpA subunits at 100 ng/well (with GST at 33 ng because it constituted about a third of the subunit fusion proteins). After blocking overnight with 3% gelatin in PBS with 0.02% sodium azide, wells were incubated for 1.5 h in a humid chamber at 37 °C with mouse serum diluted to 1:10,000. Positive and negative controls consisted convalescent phase mouse serum (from mice recovered from H. somni septicemia) and naïve mouse serum (as well as PBS) respectively. Each serum was tested in duplicate. Plates were washed 3 times with PBS/0.05% Tween 20, and antibody level was detected by incubating for 1 h with horseradish peroxidase conjugated rabbit anti-mouse IgG (Zymed Labs, San Francisco, CA) at 1:1,000 followed by TMB (Tetramethylbenzidine/hydrogen peroxide) substrate (KPL, Gaithersburg, MD). After 15 min the reaction was stopped with 0.3 M H3PO4 and absorbance was determined by reading at A450/A650 in a dual wavelength microplate reader (Molecular Devices Corp., Menlo Park, CA). Data was reported as absorbance values + or − the standard errors.
2.5. Western blotting
H. somni whole cells scraped from a blood agar plate, supernatant from a 6 h culture, or recombinant IbpA subunits were boiled in sodium dodecyl sulfate (SDS) sample buffer and loaded on 10% polyacrylamide gels. After SDS-PAGE electrophoresis overnight, proteins were transferred to polyvinylidene fluoride membranes at 30V for 5 h followed by 50V for 1 h. After blocking for 1 h with 2% gelatin in Tris buffered saline (TBS)/0.05% Tween 20, blots were soaked for 2 h in the indicated immunized mouse or convalescent phase bovine serum at a dilution of 1:1,000. The calf serum was a 50:50 mixture of serum from calves E5 and E7 collected 6 weeks after experimental H. somni infection as previously described [28]. Negative control serum was from mice immunized with GST or from naïve mice. Convalescent phase (recovered from infection) mouse or calf serum was always included as a positive control. After washing 3 times, blots were incubated with alkaline phosphatase conjugated goat anti-mouse IgG & IgM or goat anti-bovine IgG (both from KPL) at a dilution of 1:16,000 for 1 h. Blots were washed 4 times and developed with nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indoly phosphate (BCIP) (Pierce) for 10 min.
2.6. Statistical Analysis
Protection against septicemia was analyzed by Fisher’s exact test on percent survival data.
3. Results
3.1. Immunization of mice with H. somni culture supernatant or killed cells and challenge with H. somni
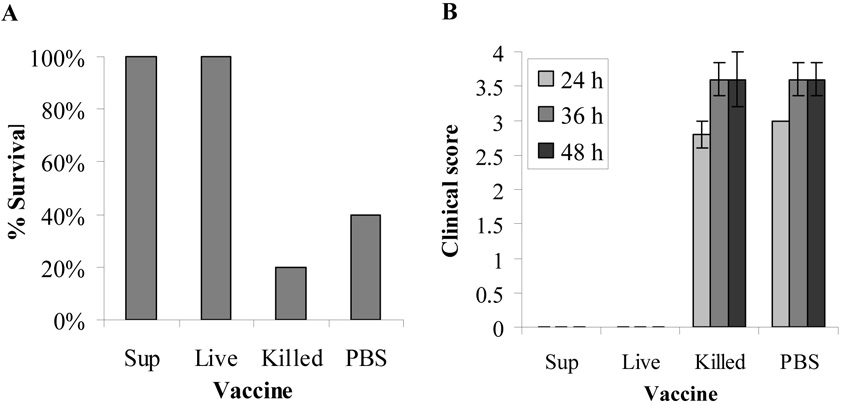
To determine if mice could be protected from H. somni septicemia, groups of 5 mice were immunized twice, 3 weeks apart, with culture supernatant or formalin-killed whole cells. Mice that had been challenged twice with live organisms but no FCS (convalescent mice) were used as a positive controls and mice that received the Quil A adjuvant with PBS alone served as a negative control. The mice were challenged IP two weeks after the second immunization, with 8.5 × 107 virulent H. somni organisms pre-incubated with FCS. This mouse model of H. somni septicemia has previously been used with the bovine transferrin in FCS serving as a source of available iron to increase virulence [29]. All mice survived in groups that were immunized with the culture supernatant or with live organisms in PBS without FCS, both groups being significantly different from the killed cell (commercial vaccine control) group, p=0.024 (Fig 2A). Only one mouse survived of the 5 mice immunized with killed cells and two of 5 in the PBS immunized control group which showed no significant difference (p=0.5), indicative of no protection. Clinical scores of the culture supernatant and live cell immunized groups were zero at all time points after challenge whereas mean scores for the killed cell and PBS groups were close to 3 at 24 h and 3.5 at 36 and 48 h (Fig 2B).
Fig. 2.
Protection of mice by immunization with H. somni culture supernatant, killed cells, or live H. somni (convalescence) against H. somni septicemia. NIH Swiss Webster mice (5/group) were immunized twice with culture supernatant (Sup), 2 × 108 killed cells (Killed), 1 × 108 live organisms in PBS with no FCS (Live), or PBS with Quil A adjuvant. Two weeks after the second immunization, mice were challenged with 8.5 × 107 virulent H. somni organisms mixed with FCS to enhance virulence [29]. Results shown are percent survival (A) and the average clinical score ± standard error at 24 48and 72 h after challenge with H. somni (B).
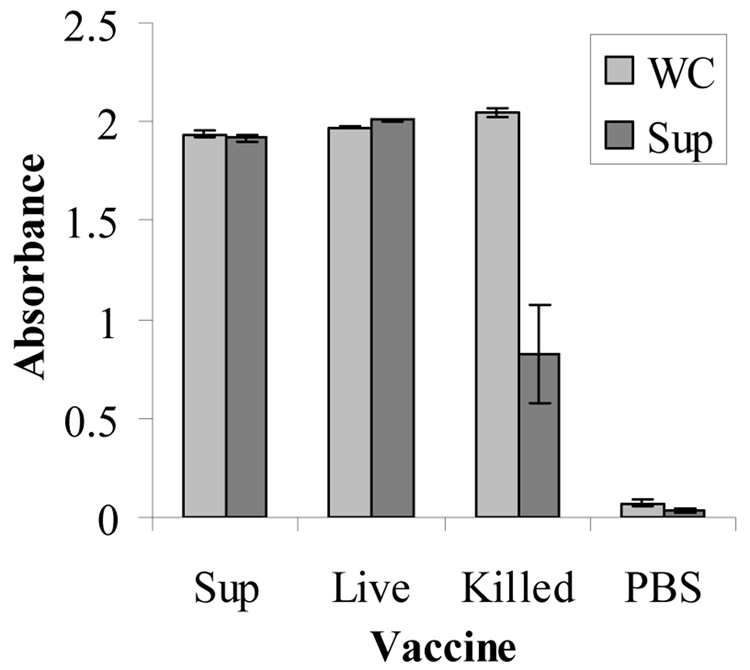
Antibody activity of serum collected at 48 to 72 hours after challenge was tested by ELISA against H. somni whole cell and culture supernatant antigens. All mice that were immunized with H. somni culture supernatant, killed cells or live organisms had very high absorbance values against the whole cell antigens, even at a serum dilution of 1:10,000 (Fig. 3). When the plate was coated with culture supernatant, the mice that were immunized with killed cells had a much lower level of antibody activity than mice from the culture supernatant or live cell groups. Negative controls inoculated with PBS and Quil A adjuvant had baseline antibody values.
Fig. 3.
Antibodies in immunized mouse serum at a dilution of 1:10,000 evaluated by ELISA against H. somni whole cell antigen (WC) or culture supernatant (Sup). Results shown are the means of individual serum antibody values from 3–5 mice ± standard error in groups vaccinated with culture supernatant (Sup), live H. somni (Live), formalin-killed H. somni (Killed) or PBS (negative control).
3.2. Immunization with recombinant IbpA subunits and challenge with H. somni
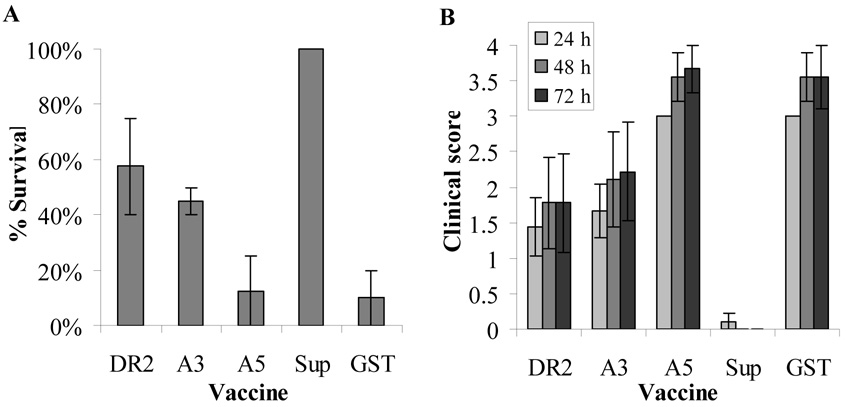
To evaluate the protective value of the recombinant IbpA subunits from H. somni septicemia, mice were immunized twice, three weeks apart with 40 µg of the IbpA DR2, A3 or A5 subunits mixed with Quil A adjuvant in two experiments (Experiment 1 and Experiment 2). Results from the two experiments were combined. In these studies, 56% survival was detected in the DR2 immunized groups (approaches a significant difference from the control group at p=0.066) and 44% with A3 immunization (not significantly different from control (p=0.147) (Fig 4A). Only one mouse survived with A5 vaccine (not significantly different from the control group, p=0.765). All mice immunized with the culture supernatant survived and only one mouse survived in the PBS/GST group (significantly different at p=0.00). Clinical scores of the mice in the DR2 and A3 groups averaged 1.4–2.2 whereas the A5 and GST/PBS vaccinated groups had scores of 3 at 24 h and 3.5 at 48 and 72 h (Fig 4B). Mice in the culture supernatant vaccinated group had scores of zero at all time points after challenge with the exception of one mouse that was assigned a score of 1 at 24 h.
Fig. 4.
Survival and clinical scores after immunization with IbpA subunits and challenge of mice with H. somni. Mice (4–5/group; 2 separate trials) were immunized twice with the recombinant IbpA subunits DR2, A3 or A5, culture supernatant, or GST/PBS (as the negative control). Two weeks after the second immunization, mice were challenged with 5.5 or 8.25 × 107 virulent H. somni organisms mixed with FCS. Results shown are the means of percent survival (A) and clinical scores (B) ± standard error for data from both trials.
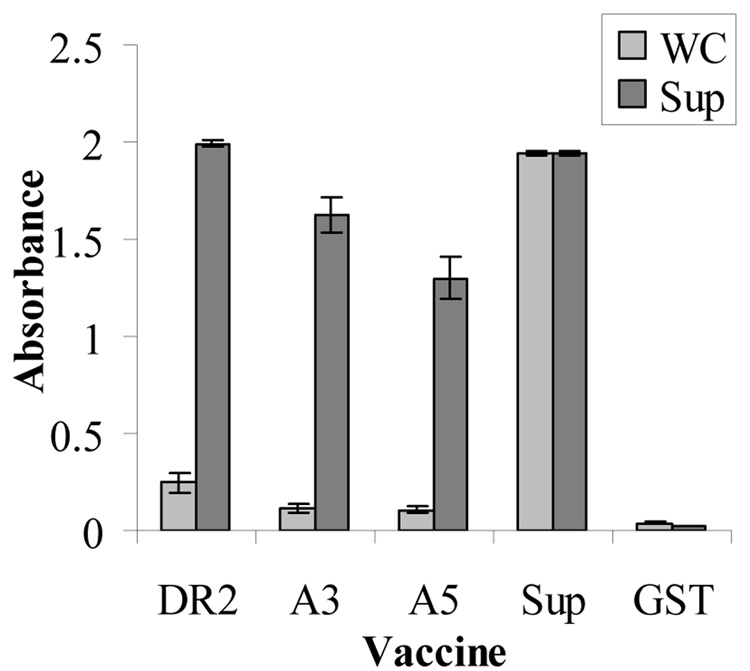
Antibody values against H. somni whole cell and culture supernatant of the different groups of immunized mice was compared by ELISA with serum from each mouse tested separately. Serum from mice in the three IbpA subunit groups had very low absorbance values against the whole cell antigen (Fig. 5). However, DR2 immunized mice had the highest absorbance values when the plate was coated with culture supernatant with the A3 and A5 groups slightly lower. The mice immunized with culture supernatant had very high antibody values to both whole cells and culture supernatant. Baseline antibody values against supernatant or whole cells antigens were seen in the control mice that received PBS or GST.
Fig. 5.
Serum antibodies to whole cell (WC) or supernatant antigens determined by ELISA after immunization with recombinant subunits DR2, A3, A5, Supernatant positive control), or GST/PBS (negative control). Results shown are the means of serum ELISA values of 9 mice per group ± standard error (combined results from two experiments).
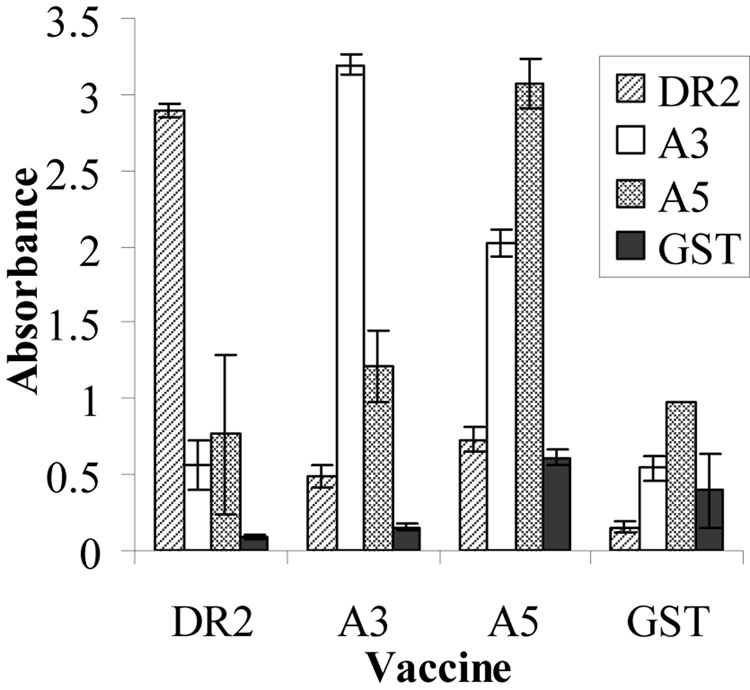
Serum pools from the mice immunized with IbpA subunits, or GST were also tested by ELISA against the IbpA GST fusion protein subunits (A3, A5 and DR2) or the negative control, GST (Fig. 6). There was some cross-reactivity, as would be expected since all immunizing subunits were GST fusion proteins. However, it is clear that serum from mice immunized with GST had very little reactivity with any subunit or GST itself, indicating that GST was not the most immunogenic portion of the subunit fusion proteins. The assay was done twice with similar results.
Fig. 6.
ELISA reactivity of pooled serum from groups of mice immunized with IbpA subunits (Vaccines A3, A5, DR2) or the GST negative controls assayed against A3, A5, DR2 or GST antigens on the solid phase.
3.3. Western blot analysis of the specificity of immunized mouse serum
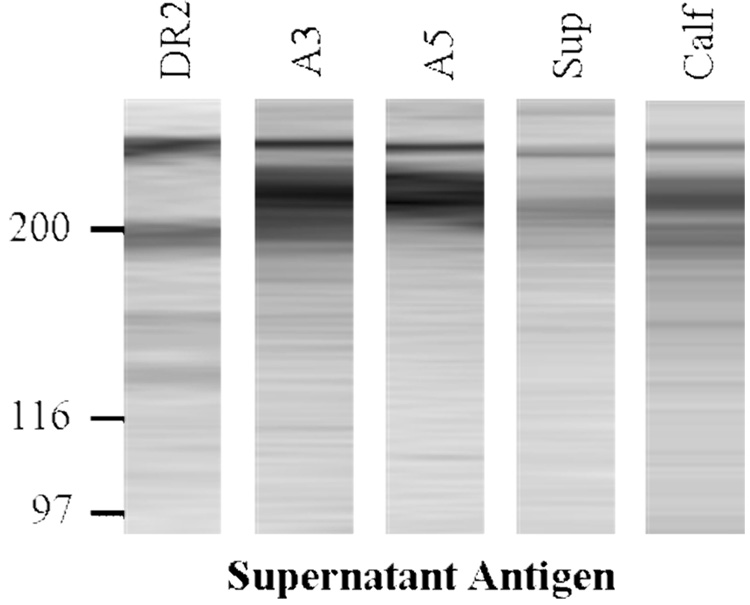
Pooled serum samples from each of the immunized mouse groups were analyzed by Western blotting against H. somni supernatant antigens and compared with the reactivity of convalescent phase bovine serum (Fig. 7). The serum from mice immunized with the IbpA subunits and culture supernatant reacted strongly with the HMW antigens, similar to the convalescent calf serum. The DR2 serum recognized slightly different bands than did the A3 or A5 serum.
Fig. 7.
Western blot analysis of pooled serum from the different immunized mouse groups or convalescent bovine serum (E5 and E7) [28] against H. somni culture supernatant antigens (enriched for shed IbpA). Strips from the blots were reacted with serum diluted 1:1000. Only the majority of each lane is shown, the edges were trimmed for neatness (no other changes). Relative molecular weight markers in kDa are included on the left side and HMW H. somni IgBPs on the right side.
3.4. Western blot analysis of IbpA subunit antigens
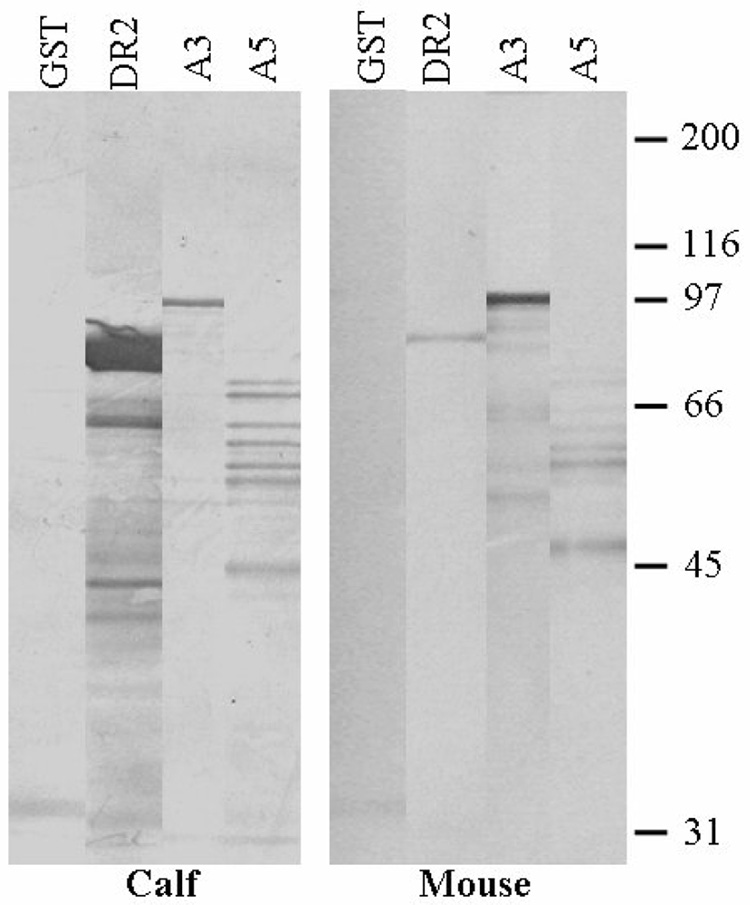
The IbpA subunits also were analyzed by Western blotting, comparing reactivity of antigenic subunits with serum from mice immunized with culture supernatant and from convalescent calves E5 and E7. Both the calf and mouse serum recognized the DR2, A3, and A5 subunits, but with somewhat different patterns (Fig. 8). The serum from mice immunized with culture supernatant reacted most strongly with the A3 subunit antigen and the convalescent phase calf serum most strongly with the DR2 antigen. The calf serum reacted with additional bands of both DR2 and A5 subunit antigens while the mouse serum reacted with additional bands of the A3 subunit. Multiple bands are seen with each subunit, which is characteristic of H. somni IgBPs (IbpA) and IgBPs found in other bacteria.
Fig. 8.
Western blots of the three IbpA GST fusion protein antigens and the GST control reacted with serum from culture supernatant immunized mice or with convalescent bovine serum. Each lane was loaded with 3.0 µg of protein, resolved by SDS-PAGE and transferred to PVDF. The blots were reacted with mouse or bovine serum diluted 1:1000, then incubated with alkaline phosphatase conjugated anti-bovine IgG or anti-mouse IgG & M and developed with NBT/BCIP. (edges of lanes are trimmed for neatness. Relative molecular weight markers in kDa are included on the right side.
4. Discussion
This study shows that culture supernatant, enriched for shed IbpA [23], protects mice against H. somni septicemia comparable to convalescent immunity but the formalin-killed H. somni vaccine did not protect. Since the killed cell vaccine group had high serum antibody values against H. somni whole cell antigen but relatively little antibody against supernatant or shed antigens, it can be concluded that the most protective antigens were shed in the 6 h (log phase) culture supernatant. This supernatant is enriched for IbpA, so it constitutes a crude preparation of native IgBPs as a positive control for immunization with recombinant GST fusion IbpA subunits A3, A5 and DR2. Although the supernatant crude source of native IgBPs protected best, IbpA DR2 and A3 also showed some evidence for protection as shown by survival rates and clinical scores. In each case the sera from IbpA subunit immunized mice strongly reacted with log phase culture supernatant antigen in ELISA, but not whole cell antigen, suggesting that the subunit antigens were represented primarily in the shed fraction of IbpA rather than that fraction remaining with the cell pellet [16]. It is not surprising that the serum from mice immunized with supernatant reacted with whole cell antigens in ELISA since H. somni blebs outer membrane antigens into the supernatant as well as releasing IgBP fibrils [16]. ELISAs with the subunit antigens on the solid phase confirmed that serum from mouse groups immunized with each IbpA subunit reacted primarily with that subunit and that the GST in the fusion proteins contributed little to the immune reaction.
Western blots of serum from all the immunized mouse groups showed that protected mice (A3, A5, DR2 and supernatant antigens) reacted with HMW IgBPs (bands above the 116 kDa marker) in the supernatant blot whereas serum from the unprotected groups (GST immunization antigen) did not react with HMW IgBPs. The Western blot strip of supernatant antigen reacted with pooled convalescent phase serum from 2 calves (E5 and E7) also reacted with HMW IgBPs, which was similar to our earlier studies with purified IgBPs (or IbpA - then called FcRs) in Western blot assays [17]. This is significant because this pool of serum from calves E5 and E7 passively protected calves in an experimental H. somni pneumonia study [28]. The Western blots of serum from supernatant immunized mice also reacted with some lower molecular weight antigens in shed outer membrane blebs (data not shown). In the blot of supernatant antigen, sera from mice immunized with IbpA subunits A3 and A5 reacted with both similar and different HMW antigen bands than sera from DR2 immunized mice. SDS-PAGE and Western blots of native IgBPs and recombinant IbpA always appear as a series of bands [13,16,21,22,28]. The fact that anti DR2 serum reacted with different bands when compared with antibodies to A3 and A5 may be related to the fact that DR2 is near the C terminus of IbpA, whereas A3 and A5 are upstream. The IbpA molecule has many ATG initiation codons [20]. Antibodies to all three IbpA subunits reacted with a very high molecular weight band which represents the complete IbpA molecule. However, antibodies to IbpA3 and IbpA5 react with a second broad band (or series of bands) about 250 kDa whereas antibodies to IbpADR2 reacted to 200kDa antigen. This suggests that intitation began closer to the N terminus for the IbpA3 and IbpA5 antigens but closer to the C terminus for IbpADR2. The importance of antibodies to the HMW IgBPs in protection is illustrated in these Western blots. The second set of Western blots consisted of the IbpA subunit and GST antigens (rather than supernatant antigens) against sera from immunized mice. This blot showed that the pool of protective convalescent calf serum reacted predominantly with the DR2 subunit whereas serum from mice immunized with supernatant antigen reacted primarily with the A3 recombinant fusion subunit. Again the pattern of reactivity with multiple bands and the molecular weight of the fusion proteins is similar to that which we reported previously [20]. The difference in reactivity of protective convalescent calf serum and serum from protected supernatant immunized mice may indicate that subunits most protective in mice may differ from those most protective in cattle. However, since HMW IgBPs are clearly important antigens in protective convalescent phase bovine serum and at least two IgBP (or IbpA) recombinant subunits protected mice, these recombinant subunit antigens should be tested in cattle. Furthermore, the log phase supernatant (used as a crude source of native IgBPs) protected as well as convalescent immunity (prior H. somni infection) in mice, thus it may be that the crude antigen with both HMW IgBPs and other shed antigens in outer membrane blebs will be the most protective in calves. In preliminary experiments, IbpA3 did protect against pneumonia in a very small number of calves [20]. Now these 3 subunits should be compared with culture supernatant in bovine protection studies.
Not only do these studies provide insight into the most protective motifs in IbpA but they may be indirectly relevant to the role of these subunits in pathogenicity. IbpA is a large surface/exoprotein with much homology to Bordetella pertussis [20,31,32]. The IbpA3 subunit has a carbohydrate recognition domain [17,33] and an RGD motif [20] implicated in attachment to mammalian cells. This subunit also has a predicted coiled coil region with homology to streptococcal IgBPs and this subunit bound bovine IgG2 by the Fc portion [20]. Therefore antibodies to IbpA3 may inhibit cell attachment or immunoglobulin Fc binding. The IbpA5 subunit has tandem 200 bp repeats and 22 amino acid repeats which may contribute to the fibrillar structure of IbpA, as in FHA [34]. Lastly, DR2 of IbpA has insertion-sequence-like structure and codon usage different from other H. somni genes sequenced at the time this was reported [21]. Thus, this could be a virulence motif acquired from another organism. Neutralization of these domains or motifs may be related to the protection seen in mice.
Acknowledgements
We thank Dr Robert Corbeil for statistical analysis and Jason Henry for technical assistance. This work was supported in part by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2005-35204-16257 (LBC) and NIH RO1 AI 60662 (JED).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angen O, Ahrens P, Kuhnert P, Christensen H, Mutters R. Proposal of Histophilus somnus gen. nov., sp. nov. for the three species incertae sedis ‘Haemophilus somnus’, ‘Haemophilus agni’ and ‘Histophilus ovis’. Int J Syst Evol Microbiol. 2003;53(Pt 5):1449–1456. doi: 10.1099/ijs.0.02637-0. [DOI] [PubMed] [Google Scholar]
- 2.Gogolewski RP, Leathers CW, Liggitt HD, Corbeil LB. Experimental Haemophilus somni pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol. 1987;24(3):250–256. doi: 10.1177/030098588702400309. [DOI] [PubMed] [Google Scholar]
- 3.Groom SC, Little PB, Rosendal S. Virulence differences among three strains of Haemophilus somnus following intratracheal inoculation of calves. Can J Vet Res. 1988;52(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens LR, Little PB, Wilkie BN, Barnum DA. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981;178(4):378–384. [PubMed] [Google Scholar]
- 5.Humphrey J, Stephens L. Haemophilus somnus: a review. Vet Bull. 1983;53:987–1004. [Google Scholar]
- 6.Miller RB, Lein DH, McEntee KE, Hall CE, Shaw S. Haemophilus somnus infection of the reproductive tract of cattle: a review. J Am Vet Med Assoc. 1983;182(12):1390–1392. [PubMed] [Google Scholar]
- 7.Widders PR, Paisley LG, Gogolewski RP, Evermann JF, Smith JW, Corbeil LB. Experimental abortion and the systemic immune response to "Haemophilus somnus" in cattle. Infect Immun. 1986;54(2):555–560. doi: 10.1128/iai.54.2.555-560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris FW, Janzen ED. The Haemophilus somnus disease complex (hemophilosis): a review. Can Vet J. 1989;30(10):816–822. [PMC free article] [PubMed] [Google Scholar]
- 9.Kwiecien JM, Little PB. Haemophilus somnus and reproductive disease in the cow. Can Vet J. 1991;32(10):595–601. [PMC free article] [PubMed] [Google Scholar]
- 10.Corbeil LB, Gogolewski RP, Stephens LR, Inzana TJ. Haemophilus somnus: antigen analysis and immune responses. In: Donachie W, editor. Haemophilus, Actinobacillus, and Pasteurella. New York: Plenum Press; 1995. pp. 63–73. [Google Scholar]
- 11.Thompson KG, Little PB. Effect of Haemophilus somnus on bovine endothelial cells in organ culture. Am J Vet Res. 1981;42(5):748–754. [PubMed] [Google Scholar]
- 12.Widders PR, Dorrance LA, Yarnall M, Corbeil LB. Immunoglobulin-binding activity among pathogenic and carrier isolates of Haemophilus somnus. Infect Immun. 1989;57(2):639–642. doi: 10.1128/iai.57.2.639-642.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SP, Guiney DG, Corbeil LB. Two linked genes for outer membrane proteins absent in four non-disease strains of Haemophilus somnus. Mol Microbiol. 1992;6(14):1895–1902. doi: 10.1111/j.1365-2958.1992.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 14.Yarnall M, Widders PR, Corbeil LB. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol. 1988;28(2):129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 15.Corbeil LB, Blau K, Prieur DJ, Ward ACS. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbeil LB, Bastida-Corcuera FD, Beveridge TJ. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect Immun. 1997;65(10):4250–4257. doi: 10.1128/iai.65.10.4250-4257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarnall M, Corbeil LB. Antibody response to an Haemophilus somnus Fc receptor. J Clin Microbiol. 1989;27(1):111–117. doi: 10.1128/jcm.27.1.111-117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Donkersgoed J, Janzen ED, Harland RJ. Epidemiological features of calf mortality due to hemophilosis in a large feedlot. Can Vet J. 1990;31(12):821–825. [PMC free article] [PubMed] [Google Scholar]
- 19.Corbeil LB, Chikami G, Yarnall M, Smith J, Guiney DG. Cloning and expression of genes encoding Haemophilus somnus antigens. Infect Immun. 1988;56(10):2736–2742. doi: 10.1128/iai.56.10.2736-2742.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagawa Y, Sanders JD, Uchida I, Bastida-Corcuera FD, Kwashima K, Corbeil LB. Genetic and functional analysis of Haemophilus somnus high molecular weight-immunoglobulin binding proteins. Microb Pathog. 2005;39(5–6):159–170. doi: 10.1016/j.micpath.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Cole SP, Guiney DG, Corbeil LB. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J Gen Microbiol. 1993;139(9):2135–2143. doi: 10.1099/00221287-139-9-2135. [DOI] [PubMed] [Google Scholar]
- 22.Sanders JD, Bastida-Corcuera FD, Arnold KF, Wunderlich AC, Corbeil LB. Genetic manipulation of immunoglobulin binding proteins of Haemophilus somnus. Microb Pathog. 2003;34(3):131–139. doi: 10.1016/s0882-4010(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 23.Yarnall M, Gogolewski RP, Corbeil LB. Characterization of two Haemophilus somnus Fc receptors. J Gen Microbiol. 1988;134(7):1993–1999. doi: 10.1099/00221287-134-7-1993. [DOI] [PubMed] [Google Scholar]
- 24.Hall RF, Williams JM, Smith GL. Field evaluation of Haemophilus somnus bacterin. Vet Med Small Anim Clin. 1977;72(8):1368–1370. [PubMed] [Google Scholar]
- 25.Stephens LR, Little PB, Humphrey JD, Wilkie BN, Barnum DA. Vaccination of cattle against experimentally induced thromboembolic meningoencephalitis with a Haemophilus somnus bacterin. Am J Vet Res. 1982;43(8):1339–1342. [PubMed] [Google Scholar]
- 26.Ribble CS, Jim GK, Janzen ED. Efficacy of immunization of feedlot calves with a commercial Haemophilus somnus bacterin. Can J Vet Res. 1988;52(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis JA, Yong C. Systemic adverse reactions in young Simmental calves following administration of a combined vaccine. Can Vet J. 1997;38(1):45–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Gogolewski RP, Kania SA, Inzana TJ, Widders PR, Liggitt HD, Corbeil LB. Protective ability and specificity of convalescent serum from Haemophilus somnus pneumonia. Infect Immun. 1987;55(6):1403–1411. doi: 10.1128/iai.55.6.1403-1411.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geertsema RS, Kimball RA, Corbeil LB. Bovine plasma proteins increase virulence of Haemophilus somnus in mice. Microb Pathog. 2007;42(1):22–28. doi: 10.1016/j.micpath.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Inzana TJ, Corbeil LB. Development of a defined medium for Haemophilus somnus isolated from cattle. Am J Vet Res. 1987;48(3):366–369. [PubMed] [Google Scholar]
- 31.Domenighini M, Relman D, Capiau C, Falkow S, Prugnola A, Scarlato V, et al. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990;4(5):787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 32.Relman DA, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad SM, Yin Y, Rodzinski E, Tuomanen EI, Masure HR. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61(7):2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajava AV, Cheng N, Cleaver R, Kessel M, Simon MN, Willery E, et al. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol. 2001;42(2):279–292. doi: 10.1046/j.1365-2958.2001.02598.x. [DOI] [PubMed] [Google Scholar]