Abstract
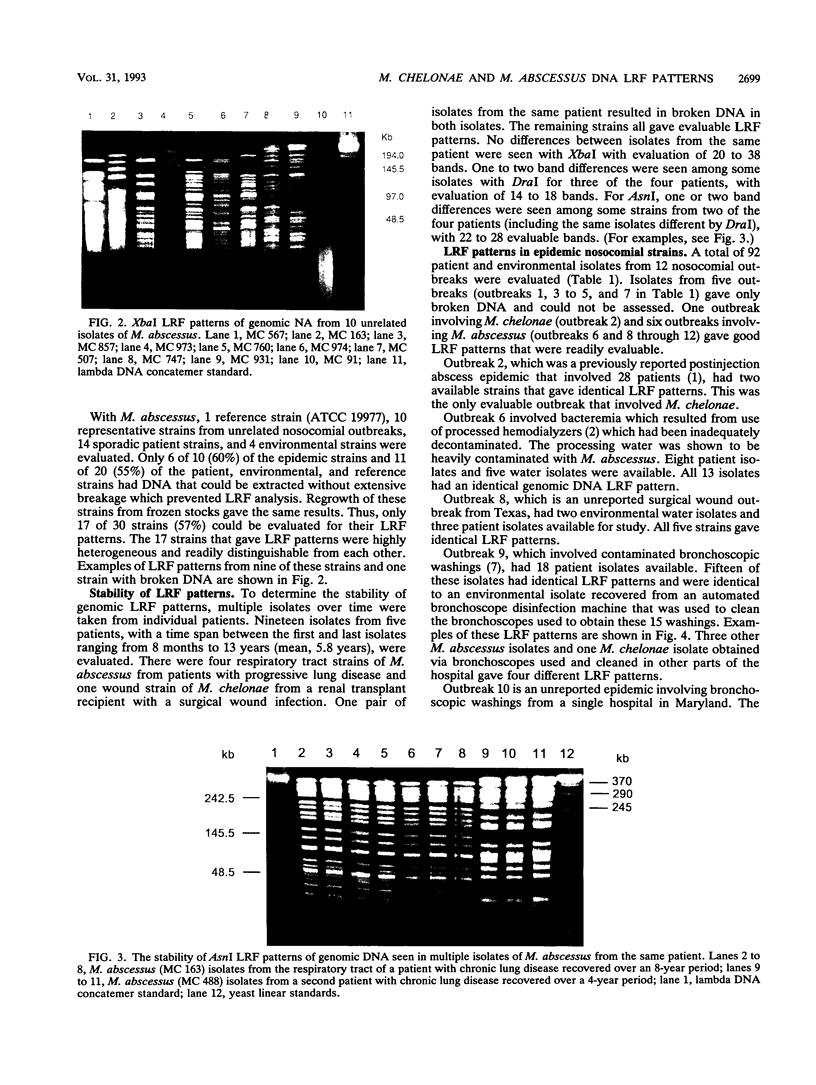
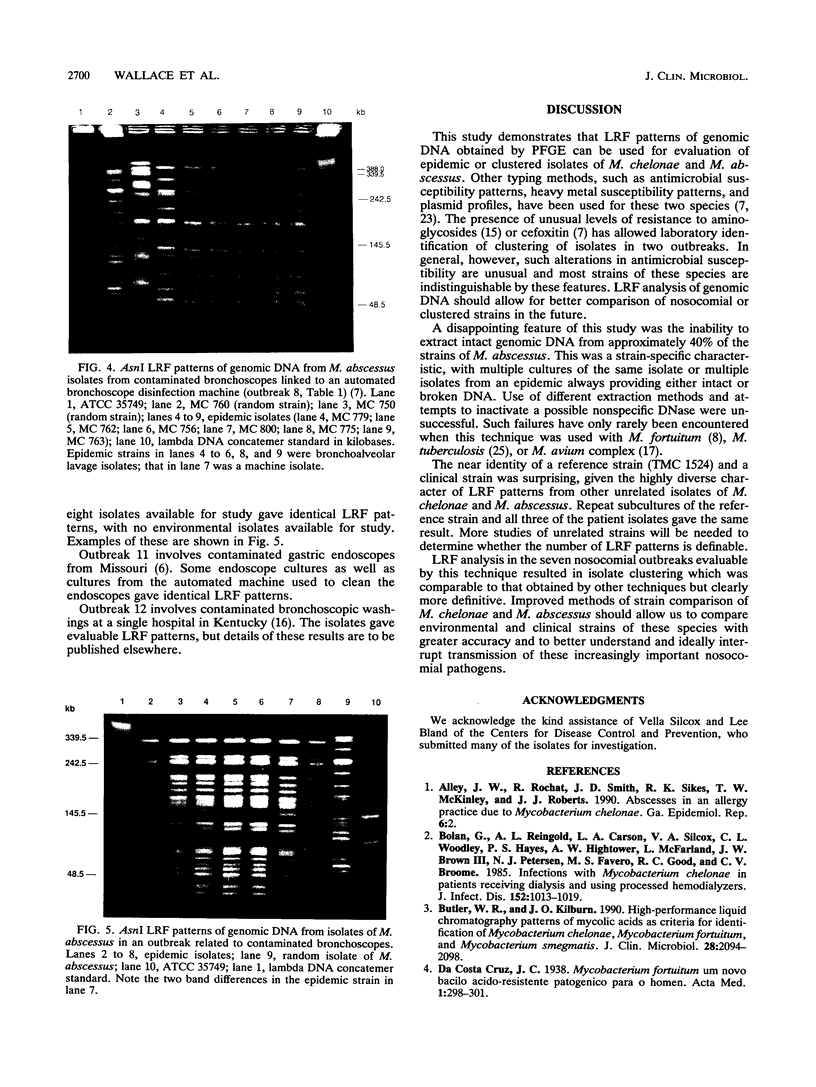
Large restriction fragment (LRF) pattern analysis of genomic DNA using pulsed-field gel electrophoresis was performed on three reference strains, 32 sporadic isolates, and 92 nosocomial isolates from 12 epidemics of Mycobacterium chelonae and Mycobacterium abscessus. Only 17 of 30 (57%) unrelated strains of M. abscessus, compared with 10 of 11 (91%) of M. chelonae strains, gave satisfactory DNA extractions, with the remainder resulting in highly fragmented DNA. DraI, AsnI, XbaI, and SpeI gave satisfactory LRF patterns. Sporadic isolates of the two species had highly variable LRF patterns, except for one reference strain and one sporadic isolate of M. chelonae that differed by only two to five bands. Evaluation of repeat isolates from five patients monitored for 8 months to 13 years (mean, 5.8 years) revealed LRF patterns to be stable, with changes of not more than two bands. LRF analysis of the seven nosocomial outbreaks with evaluable DNA revealed identical patterns in most or all of the patient isolates and in three outbreaks revealed identity with environmental isolates. These outbreaks included endoscope contamination, postinjection abscesses, and surgical wound infections. LRF analysis of genomic DNA is a useful technique for epidemiologic studies of M. abscessus and M. chelonae, although improved technology is needed for the approximately 50% of strains of M. abscessus with unsatisfactory DNA extractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolan G., Reingold A. L., Carson L. A., Silcox V. A., Woodley C. L., Hayes P. S., Hightower A. W., McFarland L., Brown J. W., 3rd, Petersen N. J. Infections with Mycobacterium chelonei in patients receiving dialysis and using processed hemodialyzers. J Infect Dis. 1985 Nov;152(5):1013–1019. doi: 10.1093/infdis/152.5.1013. [DOI] [PubMed] [Google Scholar]
- Butler W. R., Kilburn J. O. High-performance liquid chromatography patterns of mycolic acids as criteria for identification of Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium smegmatis. J Clin Microbiol. 1990 Sep;28(9):2094–2098. doi: 10.1128/jcm.28.9.2094-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foz A., Roy C., Jurado J., Arteaga E., Ruiz J. M., Moragas A. Mycobacterium chelonei iatrogenic infections. J Clin Microbiol. 1978 Mar;7(3):319–321. doi: 10.1128/jcm.7.3.319-321.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser V. J., Jones M., Murray P. R., Medoff G., Zhang Y., Wallace R. J., Jr Contamination of flexible fiberoptic bronchoscopes with Mycobacterium chelonae linked to an automated bronchoscope disinfection machine. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):853–855. doi: 10.1164/ajrccm/145.4_Pt_1.853. [DOI] [PubMed] [Google Scholar]
- Fraser V. J., Zuckerman G., Clouse R. E., O'Rourke S., Jones M., Klasner J., Murray P. A prospective randomized trial comparing manual and automated endoscope disinfection methods. Infect Control Hosp Epidemiol. 1993 Jul;14(7):383–389. doi: 10.1086/646766. [DOI] [PubMed] [Google Scholar]
- Hector J. S., Pang Y., Mazurek G. H., Zhang Y., Brown B. A., Wallace R. J., Jr Large restriction fragment patterns of genomic Mycobacterium fortuitum DNA as strain-specific markers and their use in epidemiologic investigation of four nosocomial outbreaks. J Clin Microbiol. 1992 May;30(5):1250–1255. doi: 10.1128/jcm.30.5.1250-1255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. C., Fraser D. W., Robicsek F., O'Bar P. R., Mauney C. U. Two outbreaks of sternal wound infection due to organisms of the Mycobacterium fortuitum complex. J Infect Dis. 1981 Apr;143(4):533–542. doi: 10.1093/infdis/143.4.533. [DOI] [PubMed] [Google Scholar]
- Hoy J., Rolston K., Hopfer R. L. Pseudoepidemic of Mycobacterium fortuitum in bone marrow cultures. Am J Infect Control. 1987 Dec;15(6):268–271. doi: 10.1016/0196-6553(87)90121-0. [DOI] [PubMed] [Google Scholar]
- Kuritsky J. N., Bullen M. G., Broome C. V., Silcox V. A., Good R. C., Wallace R. J., Jr Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med. 1983 Jun;98(6):938–939. doi: 10.7326/0003-4819-98-6-938. [DOI] [PubMed] [Google Scholar]
- Kusunoki S., Ezaki T. Proposal of Mycobacterium peregrinum sp. nov., nom. rev., and elevation of Mycobacterium chelonae subsp. abscessus (Kubica et al.) to species status: Mycobacterium abscessus comb. nov. Int J Syst Bacteriol. 1992 Apr;42(2):240–245. doi: 10.1099/00207713-42-2-240. [DOI] [PubMed] [Google Scholar]
- Laskowski L. F., Marr J. J., Spernoga J. F., Frank N. J., Barner H. B., Kaiser G., Tyras D. H. Fastidious mycobacteria grown from porcine prosthetic-heart-valve cultures. N Engl J Med. 1977 Jul 14;297(2):101–102. doi: 10.1056/NEJM197707142970209. [DOI] [PubMed] [Google Scholar]
- Laussucq S., Baltch A. L., Smith R. P., Smithwick R. W., Davis B. J., Desjardin E. K., Silcox V. A., Spellacy A. B., Zeimis R. T., Gruft H. M. Nosocomial Mycobacterium fortuitum colonization from a contaminated ice machine. Am Rev Respir Dis. 1988 Oct;138(4):891–894. doi: 10.1164/ajrccm/138.4.891. [DOI] [PubMed] [Google Scholar]
- Lowry P. W., Jarvis W. R., Oberle A. D., Bland L. A., Silberman R., Bocchini J. A., Jr, Dean H. D., Swenson J. M., Wallace R. J., Jr Mycobacterium chelonae causing otitis media in an ear-nose-and-throat practice. N Engl J Med. 1988 Oct 13;319(15):978–982. doi: 10.1056/NEJM198810133191504. [DOI] [PubMed] [Google Scholar]
- Mazurek G. H., Hartman S., Zhang Y., Brown B. A., Hector J. S., Murphy D., Wallace R. J., Jr Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993 Feb;31(2):390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safranek T. J., Jarvis W. R., Carson L. A., Cusick L. B., Bland L. A., Swenson J. M., Silcox V. A. Mycobacterium chelonae wound infections after plastic surgery employing contaminated gentian violet skin-marking solution. N Engl J Med. 1987 Jul 23;317(4):197–201. doi: 10.1056/NEJM198707233170403. [DOI] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Wallace R. J., Jr, Silcox V. A., Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985 Dec;28(6):807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Dalovisio J. R., Pankey G. A. Disk diffusion testing of susceptibility of Mycobacterium fortuitum and Mycobacterium chelonei to antibacterial agents. Antimicrob Agents Chemother. 1979 Nov;16(5):611–614. doi: 10.1128/aac.16.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musser J. M., Hull S. I., Silcox V. A., Steele L. C., Forrester G. D., Labidi A., Selander R. K. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989 Apr;159(4):708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- Wenger J. D., Spika J. S., Smithwick R. W., Pryor V., Dodson D. W., Carden G. A., Klontz K. C. Outbreak of Mycobacterium chelonae infection associated with use of jet injectors. JAMA. 1990 Jul 18;264(3):373–376. [PubMed] [Google Scholar]
- Zhang Y., Mazurek G. H., Cave M. D., Eisenach K. D., Pang Y., Murphy D. T., Wallace R. J., Jr DNA polymorphisms in strains of Mycobacterium tuberculosis analyzed by pulsed-field gel electrophoresis: a tool for epidemiology. J Clin Microbiol. 1992 Jun;30(6):1551–1556. doi: 10.1128/jcm.30.6.1551-1556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]