Abstract
Small GTP-binding (G) proteins act as molecular switches to regulate a number of cellular processes, including vesicular transport. Emerging evidence indicates that small G proteins regulate a number of steps in the secretion of pancreatic acinar cells. Diverse small G proteins have been localized at discrete compartments along the secretory pathway and particularly on the secretory granule. Rab3D, Rab27B, and Rap1 are present on the granule membrane and play a role in the steps leading up to exocytosis. Whether the function of these G proteins is simply to ensure appropriate targeting or if they are involved as regulatory molecules is discussed. Most evidence suggests that Rab3D and Rab27B play a role in tethering the secretory granule to its target membrane. Other Rabs have been identified on the secretory granule that are associated with different steps in the secretory pathway. The Rho family small G proteins RhoA and Rac1 also regulate secretion through remodeling of the actin cytoskeleton. Possible mechanisms for regulation of these G proteins and their effector molecules are considered.
Keywords: vesicular transport, actin cytoskeleton, secretory granule, Rab, Rho, Rac, Rap
the exocrine pancreas is the major provider of digestive enzymes for the luminal phase of digestion. These enzymes are synthesized and secreted by acinar cells, which make up the bulk of the pancreas. Acinar cells, as is the case for cells found in salivary, lacrimal, and other exocrine glands, are polarized with structures related to their secretory function, such as an apical secretory membrane. Protein secreted at the apical membrane enters the ductular system and is conveyed to the intestinal lumen. In acinar cells, digestive enzymes are synthesized in the rough endoplasmic reticulum (RER), pass through the Golgi apparatus, and are then packaged into secretory granules termed zymogen granules (ZGs). Each granule contains a mixture of ∼20 digestive enzymes and proenzymes. ZGs are ∼1 μm in diameter and, along with granules of other exocrine glands, are significantly larger than most endocrine and neuroendocrine granules, which are from 0.1 to 0.3 μm in diameter.
One aspect of secretion is the vectoral vesicular transport guided by the microtubule cytoskeleton from the basolateral portion of the cell, which is filled with RER, to the apical pole. At the end of this journey, mature granules must pass through a subapical network of actin filaments and then dock and ultimately fuse with the apical plasma membrane. Small GTP-binding (G) proteins are involved as molecular switches in a number of these transport steps where their role is often to ensure compartment fidelity (42, 129). These steps include vesicular transport from the RER to Golgi, interaction of vesicles with microtubules, local remodeling of the actin cytoskeletal network, and docking and fusion with the apical plasma membrane.
Pancreatic Stimulus-Secretion Coupling
The major extracellular regulators of pancreatic acinar cell secretion in response to nutrient ingestion are the neurotransmitter acetylcholine (ACh), which is released from vagal nerve endings, and the gastrointestinal (GI) hormone cholecystokinin (CCK). These regulators act through specific membrane receptors to increase the intracellular concentration of free Ca2+, the primary intracellular signal for secretion. Some stimulation of pancreatic secretion or potentiation of the action of ACh and CCK is provided by the GI hormone secretin and the neuropeptide vasoactive intestinal polypeptide (VIP), which act via cyclic AMP. For detailed reviews of these processes consult references 92 and 125.
How Ca2+ acts as an intracellular messenger to stimulate secretion is still poorly understood. In neurosecretion, Ca2+ binds to synaptotagmin, a Ca2+ receptor on the external surface of the synaptic vesicle (37). The presence of a synaptotagmin on exocrine secretory granules has not been clearly documented. However, there could be yet unidentified Ca2+-binding molecules similar to proteins involved with neuronal secretion, such as CAPS (56). Ca2+ could also activate a kinase or phosphatase. Ca2+-activated kinases investigated in relation to secretion include Ca2+-calmodulin kinase II, which has broad substrate specificity, and more specific kinases such as myosin light-chain kinase. Diacylglycerol can stimulate a modest amount of secretion through activation of protein kinase C (PKC).
Another class of proteins involved directly in secretory granule fusion with the plasma membrane are the SNARE proteins, so named from their function as soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (53, 54). The best-characterized SNARE complex is the synaptic complex made up of synaptobrevin-VAMP on the synaptic vesicle and SNAP-25 and syntaxin-1 on the plasma membrane. Pancreatic acinar cells contain SNARE proteins, although the full complement mediating exocytosis is not fully defined. Recent work suggests that a VAMP-8-SNAP-29-syntaxin-4 complex may mediate regulated secretion, while VAMP-2 may mediate constitutive secretion (124). Other SNARE proteins are involved in the earlier steps of vesicular transport during the secretory pathway (16).
Small G Proteins: General Properties
GTP-binding proteins are molecular switches that use a common enzymatic cycle of GTP binding, hydrolysis, and dissociation to activate and deactivate the protein. The changes in guanine nucleotide binding affect the conformation of the protein, and the GTP bound form interacts with and stimulates effector molecules. The two main classes of G proteins are the heterotrimeric G proteins and the small or monomeric G proteins sometimes also referred to as Ras-like G proteins in reference to this prototypical protein. The six families of small G proteins are the Ras, Rho, Rab, Arf, Ran, and Sar proteins, with the last four of these generally linked to intracellular trafficking whereas Ras is associated with cell growth control and the Rho family with actin cytoskeletal remodeling (109). The Rab family is the largest branch of small G proteins, with over 60 mammalian members, each of which has specific cellular locations and regulate particular steps in exocytotic and endocytotic vesicular transport (24). All of the small G proteins are ∼20–29 kDa in size and share a core GTP-binding domain of ∼200 amino acids. They also contain unique amino- and carboxyl-terminal domains, which target the G protein to a specific intracellular location, and effector domains, which interact with effector proteins (42). Many possess posttranslational lipid modifications including amino-terminal myristic acid groups or carboxyl-terminal isoprenylation or palmitoylation, which allow attachment to cellular membranes. All of the small G proteins identified in pancreatic acinar cells and what is known of their cellular location and function is summarized in Table 1.
Table 1.
Small G Proteins identified in acinar cells
| Family | G Protein | Localization | Function | Refs. |
|---|---|---|---|---|
| Ras | Ras | PM | Growth | 20, 80 |
| Rap1 (A & B) | ZG, membrane | Exocytosis | 15, 96,100 | |
| RalA | ? | ? | 96 | |
| Rab | Rab1 | ER | ER-Golgi transport | 14, 96 |
| Rab2 | ER | ER-Golgi transport | 14, 96 | |
| Rab3D | ZG | Exocytosis | 11, 14, 86 | |
| Rab4 | Terminal web | Exocytosis, endocytosis | 87, 117, 129 | |
| Rab5 (A, B, & C) | ZG, endosomes | ?, Endocytosis | 14, 96 | |
| Rab6A | Golgi | Golgi transport | 15, 96 | |
| Rab7 | ? | ? | 96 | |
| Rab8A | ZG | Granule formation | 15, 29, 96 | |
| Rab10 | ? | ? | 96 | |
| Rab11 (A & B) | Apical pole | ? | 14, 15, 46, 96 | |
| Rab14 | ZG | Exocytosis? | 14, 15, 96 | |
| Rab18 | ? | ? | 96 | |
| Rab26 | ? | ? | 96, 120 | |
| Rab27B | ZG | Exocytosis | 13, 14, 15, 96 | |
| Rab35 | ? | ? | 96 | |
| Rho | RhoA | Cytoplasmic, membrane | Actin cytoskeleton, secretion | 3, 4, 62, 83 |
| RhoG | ? | ? | 96 | |
| Rac1 | Cytoplasmic, membrane | Actin cytoskeleton, secretion | 3, 4, 15, 96 | |
| Cdc42 | ? | ? | 4, 96 | |
| Arf | Arf4 | ? | ? | 96 |
Rab7, Rab10, Rab18, Rab35, Arf4, and RalA have been reported in mass spectrometry studies in which at least two unique peptides were identified. Abreviations: PM, plasma membrane; ZG, zymogen granule; ER, endoplasmic reticulum.
Small G proteins are activated by guanine nucleotide exchange factors (GEFs), which accelerate the rate-limiting dissociation of GDP, and are inactivated by guanine nucleotidase-activating proteins (GAPs). Other accessory proteins sequester inactive G protein in the cytoplasm or assist in targeting and membrane insertion. The most important of these are the GDP dissociation inhibitors (GDIs) such as RhoGDI and the Rab escort protein. Some of the GEFs or GAPs are highly specific for individual small G proteins, and others are more class specific. Some are activated by intracellular signaling and often translocate within the cell. Individual mechanisms will be discussed in conjunction with the specific G proteins. Information on GEFs and GAPs identified in acinar cells is summarized in Table 2.
Table 2.
Regulators and effectors of small G Proteins in acinar cells
| G Protein | GEF | GAP | Effector |
|---|---|---|---|
| Ras | SOS | * | cRaf1 |
| Rap1 | Epac1 | Rap-GAP | * |
| CalDAG-GEFIII | |||
| Rab3D | Rab3-GEP | Rab3-GAP | Noc2 |
| GRAB | Slp1 | ||
| Myosin Vc | |||
| Rab27B | Rab3-GEP | * | Slp1 |
| Myosin Vc | |||
| RhoA | p115 Rho-GEF | p50 Rho-GAP | Rho kinase |
| LARG | p190 Rho-GAP-B | Src | |
| Rac1 | * | RICS | PAK2 |
GEF, guanine nucleotide exchange factor; GAP, guanine nucleotidase-activating protein.
Regulatory molecules have been identified in other cell types but not evaluated in acinar cells. References are included in the text.
Small G Proteins and Pancreatic Secretion: Rho Family G Proteins and the Regulation of the Cytoskeleton
Mammalian Rho GTPases include a family of about 20 molecules best known for their role in regulating and remodeling the actin cytoskeleton, although by acting through a variety of effector proteins they are now known to regulate diverse cellular processes, including gene expression, the cell cycle, cell polarity, and cell migration (44, 52). Some of these effects are mediated by actin, and others may be actin independent. The most highly conserved and best-studied Rho family prototypes, RhoA, Rac1, and Cdc42, have well-known actions on the actin cytoskeleton of cultured cells (81). Much of this understanding is derived from overexpression studies of dominant negative or constitutively active mutants that lock the G protein into the GDP- or GTP-bound form, respectively. Rho family members are regulated by GEFs, GAPs, and RhoGDI and can also be regulated by phosphorylation.
The study of Rho family G proteins in acinar cell secretion was prompted by understanding of the importance of the actin cytoskeleton in this process. In secretory cells, granules or other secretory organelles must pass through the cortical actin network. Both positive and negative roles have been ascribed to this network, which can serve as a barrier to secretion. It is now believed that local disassembly and rearrangement of this network is a prerequisite for exocytosis. In acinar cells, filamentous actin is concentrated under the apical membrane as the terminal web, which also contains myosin II, tropomyosin, and α-actinin (25). Smaller amounts of actin are found as cortical actin under the other plasma membrane domains. Filamentous actin exists in equilibrium with its globular subunits, and a number of toxins that affect this equilibrium can affect acinar cell secretion, including phalloidin, cytochalasins, latrunculin, and jasplakinolide (125). Pushing the actin equilibrium too strongly in either direction generally inhibits secretion.
The earliest work studying the role of RhoA in pancreatic secretion involved the botulinum C3 exotoxin. This toxin specifically inactivates RhoA through ADP ribosylation at Asp41 (1). Although it is difficult to get this protein into cells, Rosada et al. (98) incubated rat acini for 2 h with exogenous C3 toxin and were able to ADP-ribosylate RhoA and reduce CCK-8-stimulated amylase secretion by 33%. However, a latter study by the same group showed no effect of C3 toxin on muscarinic receptor-stimulated amylase release (97). By use of digitonin-permeabilized rat acini to allow penetration of exogenous proteins, C3 toxin was shown to block the morphological changes of actin rearrangement and basolateral cell blebbing as well as the tyrosine phosphorylation of p125 FAK, another cytoskeletal regulator (62). In another early study implicating Rho in acinar cells, Nozu et al. (83) showed that secretagogue stimulation of rat acini increased the translocation of RhoA to a microsomal membrane fraction.
To more directly study the activity state of Rho family small G proteins in acinar cells, Bi and colleagues utilized pull down assays using a GST-Rhotekin-Rho binding domain, which specifically binds GTP-liganded RhoA, and a GST-PAK-PBD domain, which specifically interacts with GTP-liganded Rac1 and Cdc42 (4). Little active RhoA or Rac1 is present in untreated cells but CCK treatment activates both in a concentration-dependent manner. RhoA activation in acini has also been demonstrated using a SRE.L reporter in response to CCK, carbachol and bombesin (Bi Y, Lim JW, and Williams JA, unpublished data). RhoA and Rac1 both translocate from cytosol to a membrane fraction in response to CCK and carbachol, and immunohistochemistry shows increased localization in the apical pole of the cell (4). RhoA is located mainly in the subapical area around the lumen, whereas Rac1 shows strong punctuate staining in the area where ZGs are concentrated. Inhibiting RhoA with C3 exotoxin or dominant negative RhoN19 and inhibiting Rac1 with dominant negative RacN17 all decrease stimulated amylase release by 25–30% without affecting basal release (4). Simultaneous inhibition of RhoA and Rac1 results in a greater inhibition of CCK-induced amylase secretion and also reduced basal amylase release. By contrast, dominant negative Cdc42N17 does not affect secretion. When constitutively active RhoV14 or RacV12 is expressed in acini, basal amylase release is normal, but CCK-stimulated release is approximately doubled (3). By contrast, active Cdc42V12 is without effect. Introduction of dominant negative RhoA, Rac1, or C3 exotoxin also decreases the secretory response to carbachol but does not affect the increase in intracellular free Ca2+ (4).
In addition to effects on secretion, the Rho family of small G proteins also have effects on the apical actin cytoskeleton and the basolateral blebs, which are induced by supraphysiological concentrations of CCK and to a lesser extent by carbachol (7, 84). Acini with constitutively active RhoA and Rac1, but not Cdc42, show basolateral blebbing, enhanced basolateral actin staining with fluorescent phalloidin, and reduced apical filamentous (F)-actin (3). Dominant negative RhoA, Rac1, and C3 toxin all partially block the actin reorganization and basolateral blebs induced by supramaximal CCK. That these morphological changes are mediated by the actin cytoskeleton was supported by the finding that the actin-polymerizing toxin jasplakinolide induces basolateral blebs, whereas latrunculin, which sequesters actin subunits, prevents the morphological changes induced by CCK (3).
Because physiological secretagogue stimulation does not greatly alter the actin cytoskeleton, it is believed that it is the local actin reorganization, especially at the apical region and not the amount of total F-actin that determines or affects the process of secretion. One recently identified site for actin polymerization is as a coating on ZGs that have fused with the apical membrane (77, 119). Both C3 exotoxin and latrunculin block the ZG coating upon secretagogue stimulation (78).
Only a little is known regarding how the Rho family G proteins are activated or which downstream effector proteins are involved in pancreatic acini (Table 2). Both heterotrimeric G12/13 and Gq proteins are known to activate Rho and Rac-GEFs in various cells (99). RhoA is most often activated by G13. In fibroblasts transfected with CCK receptors, CCK induction of actin stress fibers was used as a readout of Rho activity mediated by G12/13 (66). G12/13 activates specific Rho-GEFs that possess a regulator of G protein signaling (RGS) domain, which binds the α-subunit of G12/13 (35); two of these proteins, p115 Rho-GEF and LARG, are present in acinar cells (Bi Y and Williams JA, unpublished data). Moreover, in the fibroblast model (66) and in acinar cells (Sabbatini ME and Williams JA, unpublished data), the isolated RGS domain from p115 Rho-GEF specifically blocks RhoA activation. Thus, it is likely that G12/13 mediates, at least in part, the activation of RhoA by CCK or ACh. Several downstream effectors of RhoA have been identified, including Rho kinase, protein kinase N, mDIA, rhotekin, and citron (5). Rho kinase is in part responsible for increased phosphorylation of myosin light chain, which leads to stress fiber formation in fibroblasts or contraction in smooth muscle. However, a Rho kinase inhibitor, Y-27632, did not affect stimulated amylase secretion (4, 65). Rho family GTPases are also known to activate phosphatidylinositol 4-phosphate 5-kinase (PIP5K), thereby increasing PIP2 synthesis (123). In other cells, PIP2 is important not only as substrate for phospholipase C (PLC) but as a regulator of the SNARE complex. Although its significance is not clear, RhoA has been shown to immunoprecipitate in a complex with Vav2 and Src from pancreatic acini, with complex formation stimulated by CCK (63).
A number of GEFs can activate Rac1 (99); however, little is known concerning the activation of Rac1 in pancreatic acini. We (68) have recently demonstrated that one downstream effector of Rac1 is PAK2, and that its activation involves an adaptor protein, Beta-PIX. Blockage of the activation of PAK2 inhibited amylase secretion similar to the inhibition by dominant negative Rac1.
Rho family G proteins are inactivated by GAPs and over 70 have been identified in eukaryote cells (112). These differ in their specificity, with some acting on multiple Rho family members and some acting on a single GTPase. Some GAPs show tissue-specific expression and function. To date there have been almost no studies of these proteins in acinar cells, although p50 RhoGAP, p122 RhoGAP, p190 RhoGAP-B, and RICS have been observed by mass spectrometry (96) or PCR (Sabbatini ME and Williams JA, unpublished data).
Small G Proteins on Secretory Granules
A number of small G proteins were initially identified on ZGs by Western blotting or immunohistochemistry. Mapping of GTP-binding proteins on pancreatic ZGs by 2-D gel electrophoresis and blotting with 35S[GTPγS] have revealed that multiple small G proteins are present on ZG membranes (38); however, their identities could not be determined at that time. Recent proteomic analyses of pancreatic ZG membranes have identified a number of small G proteins, including Rab1, Rab2, Rab3D, Rab4, Rab5, Rab6, Rab8, Rab11, Rab14, Rab26, Rab27B, Rac1, and Rap1 (14, 15, 96). All of these small G proteins, when evaluated by protease protection assays, are on the external surface of the ZG.
Rab proteins cosegregating in phylogenetic trees show a pattern of similar cellular localization and/or function and thus can be grouped in “Rab functional groups” (32). These functional groups reflect similarity of sequence and localization and/or function and may also represent shared ancestry. Rab proteins and their effectors play a major role in maintaining specificity during vesicular trafficking through tethering of a vesicle to its correct target organelle (129). Rab-regulated tethering proteins are often multiprotein complexes or large proteins with flexible coiled-coil regions. Among all 60 mammalian Rab proteins, Rab3A, -B, -C, and -D form a cluster, and its nearest neighbor is the Rab27A/B cluster. Both Rab3 and Rab27 have been found to play important roles in regulated secretion in a variety of secretory cells (32). Rab3 is also analogous to the yeast protein Sec4. To date, most biochemical and functional studies of small G proteins on acinar cell secretory granules have been carried out on Rab3D, Rab27B, and the Ras-related small G protein Rap1; so these will be described individually, followed by a summary of other Rabs. Notably, these G proteins participate in the targeting of a mature secretory granule, the ZG, to its target membrane, the apical plasma membrane.
Rab3D.
The Rab3 proteins Rab3A, -B, -C, and -D are associated with secretory vesicles or granules in neurons and neuroendocrine, endocrine, and exocrine cells, and are thought to play an important role in regulated exocytosis (32, 37). While Rab3A and Rab3C are most abundant in neurons and neuroendocrine cells (32, 37), Rab3D was found in pancreatic acinar cells and other exocrine cells, including chief and enterochromaffin-like cells in the stomach, acinar cells in lacrimal and parotid glands, Paneth cells in the intestine, and mast cells (79, 86, 111, 115, 118). While Rab3D is the only Rab3 isoform in acinar cells, islet β-cells in the pancreas contain all four isoforms (94).
In recent years, the functions of Rab3 proteins, especially Rab3A, have been intensively investigated in neurons, chromaffin cells, and PC12 cells (37, 109). Functionally, although it is not clear whether stimulatory or inhibitory, most of the studies point toward a role for Rab3 in the secretion of hormones and neurotransmitters (36, 45). In PC12 cells, Rab3 controls the number of granules docked at the plasma membrane (71). Biochemical data demonstrate that Rab3A is an abundant GTP-binding protein that is localized to synaptic vesicles and dissociates from synaptic vesicles after membrane fusion (31), suggesting an important function for Rab3 in exocytosis. However, mild behavioral deficits from both mice lacking Rab3A (36) and Caenorhabditis elegans Rab3-null mutants (82) indicates that Rab3A is not an essential component of the secretory apparatus but instead plays a regulatory role in neurotransmitter release. Alternatively, redundancy could exist with other small G proteins. Different effects of Rab3A have been observed at the synaptic level, depending on the synapse and process examined, and there are large effects on long-term potentiation (8). More importantly, when all four Rab3 isoforms are genetically deleted, the mice die shortly after birth due to respiratory failure (103).
In pancreatic acinar cells Rab3D is localized to the outer surface of the ZG membrane (14, 86, 118); moreover, by cell fractionation ∼80% of total Rab3D is in the membrane fraction (12). The secretory granule localization of Rab3D in various exocrine cells implies that it may be involved in regulated exocytosis. Several lines of functional evidence suggest that Rab3D plays a positive role in regulated exocytosis in pancreatic acini. Redistribution of Rab3D from cytosol to membrane is observed during development, concurring with the onset of regulated exocytosis (116), and redistribution of a Rab3 from secretory granule to the Golgi complex is seen during regulated exocytosis (55). Second, a Rab3 effector peptide increases Ca2+-stimulated amylase release from permeabilized pancreatic acini (87, 89) and enhances fusion between isolated granules and plasma membranes (27). Finally, overexpression of Rab3D enhances the initial phase of regulated amylase secretion from pancreatic acini of transgenic mice (88), whereas overexpression of two dominant negative Rab3D mutants by recombinant adenovirus inhibits stimulated amylase release in cultured pancreatic acini, with a stronger inhibition during the early phase (11). To evaluate the potential mechanisms by which the dominant negative Rab3D mutants act to inhibit regulated secretion, an affinity precipitation assay has been used based on the property of the Rab3 effector Rim1 to interact only with GTP-bound Rab3D (12). By use of this assay, it was found that Rab3D is predominantly in the active GTP-bound state on ZG membranes and that the dominant negative Rab3D mutants interfere with endogenous Rab3D function by reducing the GTP-bound Rab3D on ZGs (12). It has also been shown that ZGs engaging in exocytosis become coated with actin before fusion, and formation of this actin coating is associated with the release of Rab3D localized on ZGs (119). This may provide a link between actin cytoskeleton and Rab3D in acinar cells.
Recently, a Rab3D knockout (KO) mouse has been developed (95). Similar to the Rab3A KO (36), these mice do not have a dramatically abnormal phenotype. Both the exocrine pancreas and the parotid gland show normal release kinetics in response to secretagogue stimulation (95). However, the size of secretory granules in both the exocrine pancreas and the parotid gland is significantly increased, with the granule volume being doubled (95). These observations led the authors to conclude that Rab3D is not required in exocytosis but instead exerts its function during granule maturation, possibly by preventing homotypic fusion of secretory granules (95). An alternative possibility for this finding is the presence of additional Rab(s) that regulate secretory granule exocytosis in the exocrine pancreas and overlap in function with Rab3D, therefore compensating the loss of Rab3D in the KO mice. Among the Rabs identified on ZGs, Rab27B is the most closely related Rab protein with Rab3D and likely to have redundant function in regulating acinar secretion. It will be interesting to examine the phenotype in exocrine glands of a Rab3D/Rab27B double KO. In contrast to exocrine glands, Rab3D-deficient mice demonstrate a more dramatic osteosclerotic phenotype (91). Although basal osteoclast number in null animals is normal, the total eroded surface is significantly reduced and ultrastructural analysis reveals that Rab3D−/− osteoclasts exhibit irregular ruffled borders, the site at which secretion of lysosomal enzymes occurs (91).
Two putative effectors for mammalian Rab3s have been identified in neurons: rabphilin (67, 107) and Rim family members (121, 122). All preferentially bind to GTP-bound Rab3A. In exocrine pancreas, however, neither of these two proteins has been detected. Possible Rab3 effectors in nonneuronal cells include Noc2 and granuphilin, although granuphilin appears to be localized in islets in the pancreas. Therefore the complement of endogenous Rab3D effector(s) in acinar cells is incompletely understood. In addition to the above effectors, Rab3A interacts with calmodulin in a GTP-independent fashion (90). Rab3A and calmodulin independently are known to regulate Ca2+-triggered release of neurotransmitters and hormones. Recently, it has been reported that the Rab3A-calmodulin interaction is required for Rab3 inhibition of exocytosis in PC12 cells (17). In contrast to Rab effectors, very little is known about the specific regulators (GEFs and GAPs) for Rab3. To date, two Rab3-GEFs, Rab3-GEP (102) and GRAB (69), and one Rab3 GAP (75) have been identified. Rab3-GEP KO mice die shortly after birth due to respiratory failure. In Rab3-GEP−/− embryos, most of the synaptic vesicles at the neuromuscular junction are located apart from the presynaptic plasma membrane, indicating that they did not readily undergo exocytosis (110).
Rab27B.
Evidence of Rab27 proteins as key regulators in exocytotic pathways has recently emerged. In 2000, mutations of the Rab27A gene were identified as the cause of pigmentary dilution and immunodeficiency in human Griscelli syndrome (73) as well as the melanosome transport defects observed in ashen mice (126). These findings and subsequent characterizations of Rab27A localization and function have established its role in exocytotic pathways of various secretory vesicles and lysosome-related organelles (33, 50, 51).
Rab27B, a closely related gene product, was first identified on ZG membrane in a proteomic analysis aiming at a comprehensive identification of ZG membrane proteins (15). The presence of Rab27B on ZG membranes was confirmed by Western blotting analysis of purified ZG membranes and immunohistochemistry in isolated acini and isolated ZGs (15). The presence of Rab27B in exocrine pancreas has been subsequently reported in other, independent studies (40, 101). Rab27B is also abundantly expressed on secretory granules in rat parotid acinar cells (48). Although Rab27A was detected in some proteomic analyses of purified ZG membrane (15, 96), Rab27A is localized primarily to pancreatic islets by immunocytochemistry (50, 101), suggesting that it is a potential contaminant protein in ZG membrane preparations. It is worth noting that Rab27A has been shown to regulate the exocytosis of insulin-containing dense-core granules in a β-cell line and isolated islets (60, 128).
Although the function of Rab27A in melanosome transport and lytic granule exocytosis has been well established (104, 105), the function of Rab27B is much less well understood. In pancreatic acinar cells, overexpression of a dominant negative mutant of Rab27B using recombinant adenovirus significantly inhibits acinar secretion, whereas a constitutively active mutant enhances it (13). In parotid acinar cells, the introduction of either an isolated Rab27-binding domain or functionally blocking antibodies to Rab27B in vitro strongly inhibits isoproterenol-stimulated amylase release from streptolysin O-permeabilized cells (48). Together these results demonstrate that Rab27 plays a key role in regulating exocytosis in exocrine glands. Very recently, Rab27B KO mice have been generated and studied by two groups (40, 74, 113). It has been shown that Rab27B KO mice exhibit significant hemorrhagic disease, reduced secretion of dense granules in platelet cells, and defects in mast cell degranulation (40, 74, 113). Neither of these studies reports the examination of exocrine functions in the Rab27B KO mice.
Rab proteins are believed to exert their functions in vesicular trafficking through their corresponding effectors. To date, 11 putative Rab27 effectors have been reported, including synaptotagmin-like proteins (Slp1-5), Slacs (a, b, c), rabphilin, Noc2, and Munc13-4 (32, 50). The discovery of the Rab27 effector family has led to new insights into the mechanism of Rab27 functions. It is now well established that Slac2-a/melanophilin acts as a linker between Rab27A and myosin Va, and the formation of a tripartite protein complex is essential for melanosome transport (32, 50, 108). By contrast, much less is known about the mechanism of granule exocytosis mediated by Rab27B. In exocrine glands, several potential Rab27B effector proteins have been identified, including Slp1, Slp4-a, Slac2-c, and Noc2 (15, 34, 48, 72, 96, 101). Slp1 colocalizes with Rab27B on pancreatic ZGs and interacts with Rab27B in vivo by coimmunoprecipitation (101). In Slp1 KO mice, an increased number of ZGs in pancreatic acinar cells compared with wild-type mice is observed in fasted but not in fed animals (101). Slp4-a (granuphilin) has also been identified on ZGs by two independent proteomic studies of pancreatic ZG membrane (15, 96); but as mentioned earlier, this may be a contaminant from islet cells. A Slp4-a/syntaxin-2 complex is found in parotid glands, and introduction of the antibody against Slp4-a linker domain in permeabilized parotid acinar cells severely attenuates stimulated amylase release (34). Noc2 is a candidate Rab27 effector because it binds Rab27A and -B in vitro. Interestingly, in Noc2-deficient mice, amylase secretion in response to stimuli is abolished, and ZGs markedly accumulate in pancreatic acinar cells (72). However, the subcellular localization of Noc2 protein in pancreatic acinar cells has not yet been thoroughly investigated. In parotid acinar cells, Noc2 is detected in secretory granule membrane and is bound to Rab27. Furthermore, anti-Noc2 antibody inhibits stimulated amylase release from permeabilized parotid acinar cells (47).
As another indicator of overlap in function between Rab27 and Rab3 is that the same GEF acts on both Rabs in C. elegans (70) and in melanocytes (30). This suggests that members of related but functionally distinct Rab subfamilies such as Rab27 and Rab3 can be controlled by a common activator. By contrast, Rab27 may have distinct GAPs, and one, EPI64, has been identified in melanocytes (49).
Rap1.
Rap1 is normally included in the Ras family of small G proteins on the basis of its structure and because it was originally identified as an inhibitor of Ras. Rap1 is best known for its role in the control of cell morphology, cell adhesion, and cell cycle (6, 64, 130). Two isoforms of Rap1 exist, Rap1A and Rap1B, which are 95% identical at the amino acid sequence and appear to mediate similar action (109, 130). In addition to being posttranslationally modified by geranylgeranylation, the carboxyl-terminal domain of Rap1 contains a polybasic sequence, which also participates in binding to the membrane (109).
Rap1 is activated within the cell by a variety of second messengers, including Ca2+, DAG, PLCγ, and cAMP, which interact with specific Rap1-GEFs (64, 109, 130). Ca2+ and DAG interact with CalDAG-GEFs (127) whereas cAMP interacts with Epacs (23, 61). Four CalDAG-GEFs exist but only CalDAG-GEFI and -III are able to active Rap1 (127). All CalDAG-GEFs have a CDC25 domain, which is necessary for the GEF activity, and Ca2+- and DAG-binding domains. With respect to Epacs, there are two isoforms, Epac1 and Epac2, which have distinct tissue-specific patterns of expression (23, 61). Both Epac1 and Epac2 can activate Rap1 (2, 28, 106). In addition to the CDC25 domain, Epac1 contains a single cAMP-binding domain, whereas Epac2 contains two cAMP-binding domains, one of lower affinity and another of higher affinity for cAMP (22). Other Rap1-GEFs have been found, such as C3G, which contains a proline-rich domain that interacts with the SH3 domain of members of the Crk adaptor proteins, and PDZ-GEF, which contains PDZ, and Ras association, and Ras-GEF domains, as well as a carboxyl-terminal motif for binding to PDZ domains (109). Unlike PDZ-Rho-GEF, PDZ-GEF lacks the RGS domain (109).
In pancreatic and parotid acini, Rap1 may also be involved in the regulation of enzyme secretion. Rap1 has been identified on secretory granule membranes in mouse and rat pancreatic acini by mass spectrometry and immunocytochemistry (15, 100) as well as in rat parotid glands (18, 57). In addition, two Rap1-GEFs have recently been found in mouse pancreatic acini, CalDAG-GEFIII and Epac1 (100). Unlike Rap1, which attaches to granule membranes through its lipid, Epac1 is most likely associated with ZG membranes through protein-protein interaction (100). Recently, Epac has been shown to be a mediator of cAMP signaling in pancreatic acini. By use of two cAMP analogs that activate the Epac pathway but not the PKA pathway, Epac-specific effects to enhance carbachol-stimulated amylase secretion by rat acini are observed (10). In mouse pancreatic acini, amylase release evoked by cAMP is PKA independent and Epac1 dependent, since a PKA inhibitor does not modify the response to the unselective cAMP analog 8-bromo-cAMP, the Epac-selective cAMP analog 8-(p-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-Me-cAMP) or vasoactive intestinal peptide (VIP) (100). Moreover, the effect of Epac1 on amylase release by acinar cells is not mediated by Ca2+ mobilization (100), though Epac2 is a stimulator of Ca2+-induced Ca2+ release in pancreatic β-cells (58, 59).
Several second messengers and secretagogues activate Rap1 in mouse pancreatic acini. By use of the Rap1-binding domain of RalGDS, a Rap1 effector, as an activation-specific probe for Rap1, it has been found that Rap1 is rapidly activated by Ca2+ and DAG most likely through CalDAG-GEFIII as well as by cAMP acting via Epac1. Stimulation with CCK, carbachol, and VIP as well as the Ca2+ ionophore A-23187, phorbol ester, forskolin, 8-bromo-cAMP, and the Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP all induce an increase in GTP-Rap1 levels (100). Moreover, activation of Rap1 is involved in pancreatic amylase secretion. Overexpression of Rap1GAP to block Rap1 activation not only reduces the effect of 8-bromo-cAMP, 8-pCPT-2′-O-Me-cAMP, and VIP on amylase release by 60% but also reduces CCK- and carbachol-stimulated pancreatic amylase release by 40% (100).
Rap1 is also implicated in parotid secretion. In rat parotid acini, Rap1 translocates from membranes to cytosol upon stimulation with the β-adrenergic agonist isoproterenol, and this event occurs in parallel to an increase in amylase release (19). Unlike in rat parotid acini, Rap1 does not translocate in mouse pancreatic acini upon stimulation (100). This difference could be related to the finding that PKA, which mediates Rap1 translocation in certain cell types (93, 114), is not involved in Rap1 activation in pancreatic acini (100).
There is little information about the mechanism by which Rap1 could exert its effect on enzyme secretion. In the past few years, several potential effector proteins have been shown to interact with the active form of Rap1 and in some way participate in the regulation of actin cytoskeleton (6). Among others, Arap3, Vav2, and Tiam1 are likely Rap1 effectors that link Rap1 to actin dynamics. Another potential target for Rap1 is the light-chain 2 (LC2) of the microtubule-associated protein MAP1A, which not only acts as a linker between Epac1 and microtubules but also enhances activation of Rap1 by Epac1 (43). However, to date, there are no reports about the effectors implicated in the response to Rap1 in digestive exocrine glands.
Another possible mechanism that needs to be considered is that Rap1 acts through Ca2+, an important mediator in parotid and pancreatic protein secretion. In cardiac myocytes, activation of Rap induces Ca2+ mobilization and PLCɛ stimulation mediated by Epac (85). However, in pancreatic acini, this mechanism is unlikely, because preventing Rap1 activation by overexpression of Rap1-GAP in pancreatic acini does not modify either CCK- or carbachol-induced Ca2+ mobilization, although a decrease in both CCK- and carbachol-induced amylase release is observed (100).
Other Rabs on ZGs.
Several other Rab proteins have also been identified on ZG membranes by mass spectrometry or immunodetection including Rab1, Rab2, Rab4, Rab5, Rab8, Rab11, and Rab26 (Fig. 1). Some of these, such as Rab1 and Rab2, which normally function in ER to Golgi transport, may avoid recycling and follow the secretory pathway to the ZG membranes in small amounts. The distribution of Rab6 overlaps Golgi and is present on only a small fraction of ZGs, which in acini are close to the trans-Golgi network (TGN) (15). In other cells, Rab6 is associated with retrograde intra-Golgi and Golgi-ER transport (21). Since Rab6 is not seen on ZGs in the apical pole of the acinar cell, it may well dissociate from ZGs and recycle. Rab6 may also play a role in microtubule dependent movement through its interaction with Rab kinesin-6. Rab 6 also regulates constitutive secretion of small vesicles (41).
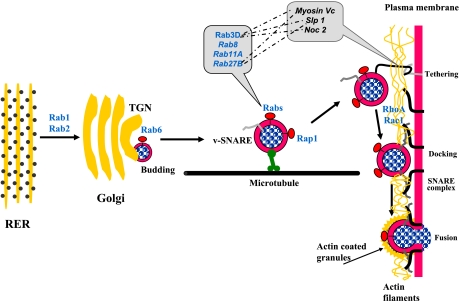
Fig. 1.
Small G proteins as regulators of the steps by which secretory proteins move through the secretory pathway from rough endoplasmic reticulum (RER) to enzyme release by exocytosis. TGN, trans-Golgi network. Small G proteins are listed in blue and shown next to steps where they have been identified. Other proteins identified on zymogen granules (ZGs), which may play a role in secretion, are listed in black. Right: mature ZGs pass through the actin filaments of the terminal web with the aid of RhoA and Rac1, are attached to the membrane by tethering proteins, and then dock and fuse with the apical membrane to release their contents. These terminal steps involve the participation of SNARE proteins. At the time of fusion, the granules also become coated with actin, and at least some of the G proteins are released.
Rab4 and Rab5 are generally associated with endosomal recycling (129) and may have reached the ZG by that route. Alternatively, Rab5 may be delivered to the apical plasma membrane to participate in the subsequent endocytosis of added secretory membrane. In acinar cells, Rab4 is colocalized with the actin terminal web (117). Rab4 has been suggested to be involved in regulated exocytosis in rat pancreatic acini, since both a carboxyl-terminal peptide and an antibody to Rab4 enhance secretion in permeabilized acini (87). These data imply a negative modulation of secretion by Rab4 without specifying its exact localization.
In a recent study, Rab8 was localized to ZGs in acinar cells of the rat pancreas. Furthermore, RNA interference experiments to “knock down” the expression of Rab8 were performed in pancreatic AR42J cells (29). Silencing of Rab8, but not of Rab3, resulted in a decrease in the number of ZGs and in an accumulation of granule marker proteins within the Golgi complex. Those authors concluded that Rab8 is involved in ZG formation (29). Rab8 is also present on melanosomes, where experimental evidence suggests it plays a role in regulating actin dependent movement of melanosomes (9).
It has been reported previously that Rab11 is present in rat isolated pancreatic acini and translocates from cytosol to a membrane fraction upon stimulation with CCK; moreover, immunohistochemistry localizes Rab11 to the apical region of the cell (46). In a study in which a monoclonal antibody developed against recombinant Rab11 was used in rabbit tissues, immunoreactivity is highly enriched in most epithelial cells and punctuate subapical staining is observed in pancreatic acinar cells (39). Similar results are found in isolated rat acini where staining of some but not all isolated rat ZG was also observed (15). Although there are no functional data for acinar cells, in gastric parietal cells a dominant negative Rab11 inhibits gastric acid secretion, which involves tubulovesicles fusing with the apical plasma membrane (26).
Rab26 was originally identified in rat pancreas by homology screening with a Rab3 probe (120), and later the protein was identified on ZG by mass spectrometry (96). It appears to participate in several types of regulated secretion, including amylase release from parotid acinar cells (76).
Additional small G proteins have been identified in acinar cells by mass spectrometry through at least two unique peptides but have not yet been further studied. These include RalA, Rab7, Rab10, Rab14, Rab18, and Rab35.
Conclusions and Future Directions
Small G proteins play important roles in the secretory pathway leading up to exocytosis in pancreatic acinar cells. Distinct Rab proteins are associated with different steps in the secretory pathways. However, it is still unclear whether they exist in the activated state with a primary purpose of securing fidelity of vesicular transport. Several Rabs as well as Rap1 may regulate the terminal steps in secretion whereby granules are tethered, dock, and fuse with the apical membrane as shown in Fig. 1. A major need is to obtain more information about effector proteins with which these G proteins interact and whether this interaction is dependent on the G protein being in the active configuration. More information is needed on how these proteins may bring about granule tethering or the regulation of SNARE protein complexes. Rab proteins may also participate in granule formation and regulate granule size. Rho family G proteins appear to regulate secretion through effects on the actin cytoskeleton. Further information is needed on both their activation and effector mechanisms as well as more high-resolution spatial information on where Rho is being activated.
Much of the limitation in research on the role of small G proteins in exocrine secretion relates to the difficulty in altering protein expression in differentiated exocrine cells. Thus, application of small interfering RNA techniques to knock down specific proteins as well as techniques for tissue-specific transgenic regulation of proteins in vivo need to be developed. This, in conjunction with techniques to study protein-protein interaction with high temporal and spatial resolution, will be necessary to fully understand the role of G proteins as important regulatory molecules in acinar cell secretion.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-41122 to J. A. Williams and by the Michigan Gastrointestinal Peptide Center (P30-DK-34933).
Acknowledgments
We thank the other members of the Williams Laboratory, Matthew Merrins and Edward Stuenkel, for helpful discussion and suggestions on the content of this review.
REFERENCES
- 1.Aktories K, Braun U, Rosener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun 158: 209–213, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Amano R, Lee J, Goto N, Harayama H. Evidence for existence of cAMP-Epac signaling in the heads of mouse epididymal spermatozoa. J Reprod Dev 53: 127–133, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am J Physiol Gastrointest Liver Physiol 289: G561–G570, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bi Y, Williams JA. A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am J Physiol Cell Physiol 289: C22–C32, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348: 241–255, 2000. [PMC free article] [PubMed] [Google Scholar]
- 6.Bos JL Linking Rap to cell adhesion. Curr Opin Cell Biol 17: 123–128, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Burnham DB, Williams JA. Effects of high concentrations of secretagogues on the morphology and secretory activity of the pancreas: a role for microfilaments. Cell Tissue Res 222: 201–212, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388: 590–593, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Chabrillat ML, Wilhelm C, Wasmeier C, Sviderskaya EV, Louvard D, Coudrier E. Rab8 regulates the actin-based movement of melanosomes. Mol Biol Cell 16: 1640–1650, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 292: G1403–G1410, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Edwards JA, Logsdon CD, Ernst SA, Williams JA. Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J Biol Chem 277: 18002–18009, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ernst SA, Williams JA. Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J Biol Chem 278: 50053–50060, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun 323: 1157–1162, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Ulintz PJ, Simon ES, Williams JA, Andrews PC. Global topology analysis of pancreatic zymogen granule membrane proteins. Mol Cell Proteomics 7: 2323–2336, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics 5: 306–312, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Coppola T, Perret-Menoud V, Luthi S, Farnsworth CC, Glomset JA, Regazzi R. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J 18: 5885–5891, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Silva NJ, DiJulio DH, Belton CM, Jacobson KL, Watson EL. Immunolocalization of rap1 in the rat parotid gland: detection on secretory granule membranes. J Histochem Cytochem 45: 965–973, 1997. [DOI] [PubMed] [Google Scholar]
- 19.D'Silva NJ, Jacobson KL, Ott SM, Watson EL. β-Adrenergic-induced cytosolic redistribution of Rap1 in rat parotid acini: role in secretion. Am J Physiol Cell Physiol 274: C1667–C1673, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowski A, Groblewski GE, Schafer C, Guan KL, Williams JA. Cholecystokinin and EGF activate a MAPK cascade by different mechanisms in rat pancreatic acinar cells. Am J Physiol Cell Physiol 273: C1472–C1479, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Darchen F, Goud B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie 82: 375–384, 2000. [DOI] [PubMed] [Google Scholar]
- 22.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275: 20829–20836, 2000. [DOI] [PubMed] [Google Scholar]
- 23.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Deneka M, Neeft M, van der Sluijs P. Regulation of membrane transport by rab GTPases. Crit Rev Biochem Mol Biol 38: 121–142, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Drenckhahn D, Mannherz HG. Distribution of actin and the actin-associated proteins myosin, tropomyosin, alpha-actinin, vinculin, and villin in rat and bovine exocrine glands. Eur J Cell Biol 30: 167–176, 1983. [PubMed] [Google Scholar]
- 26.Duman JG, Tyagarajan K, Kolsi MS, Moore HP, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am J Physiol Cell Physiol 277: C361–C372, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Edwardson JM, MacLean CM, Law GJ. Synthetic peptides of the rab3 effector domain stimulate a membrane fusion event involved in regulated exocytosis. FEBS Lett 320: 52–56, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Tasken K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem 279: 44889–44896, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Faust F, Gomez-Lazaro M, Borta H, Agricola B, Schrader M. Rab8 is involved in zymogen granule formation in pancreatic acinar AR42J cells. Traffic 9: 964–979, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem 283: 23209–23216, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer von Mollard G, Sudhof TC, Jahn RA. Small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature 349: 79–81, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda M Membrane traffic in the secretory pathway : Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 65: 2801–2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda M Rab27 and its effectors in secretory granule exocytosis: a novel docking machinery composed of a Rab27.effector complex. Biochem Soc Trans 34: 691–695, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda M, Imai A, Nashida T, Shimomura H. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18–2-dependent manner. J Biol Chem 280: 39175–39184, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20: 1661–1668, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature 369: 493–497, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Geppert M, Sudhof TC. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci 21: 75–95, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Goke B, Williams JA, Wishart MJ, De Lisle RC. Low molecular mass GTP-binding proteins in subcellular fractions of the pancreas: regulated phosphoryl G proteins. Am J Physiol Cell Physiol 262: C493–C500, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol Gastrointest Liver Physiol 270: G515–G525, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Gomi H, Mori K, Itohara S, Izumi T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell 18: 4377–4386, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 13: 305–314, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta M, Yarwood SJ. MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance Rap1 GTPase activity and cell adhesion. J Biol Chem 280: 8109–8116, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem 269: 10229–10234, 1994. [PubMed] [Google Scholar]
- 46.Hori Y, Takeyama Y, Hiroyoshi M, Ueda T, Maeda A, Ohyanagi H, Saitoh Y, Kaibuchi K, Takai Y. Possible involvement of Rab11 p24, a Ras-like small GTP-binding protein, in intracellular vesicular transport of isolated pancreatic acini. Dig Dis Sci 41: 133–138, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. Functional involvement of Noc2, a Rab27 effector, in rat parotid acinar cells. Arch Biochem Biophys 455: 127–135, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci 117: 1945–1953, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Itoh T, Fukuda M. Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J Biol Chem 281: 31823–31831, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Izumi T Physiological roles of Rab27 effectors in regulated exocytosis. Endocr J 54: 649–657, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Izumi T, Gomi H, Kasai K, Mizutani S, Torii S. The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct Funct 28: 465–474, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell 112: 519–533, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Jena BP, Gumkowski FD, Konieczko EM, von Mollard GF, Jahn R, Jamieson JD. Redistribution of a rab3-like GTP-binding protein from secretory granules to the Golgi complex in pancreatic acinar cells during regulated exocytosis. J Cell Biol 124: 43–53, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131: 796–808, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Kameyama Y, Nagata K, Mizuno-Kamiya M, Yokota Y, Fujita A, Nozawa Y. Localization of a low Mr GTP-binding protein, rap1 protein, in plasma membranes and secretory granule membranes of rat parotid gland. Life Sci 55: 213–219, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol 566: 173–188, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278: 8279–8285, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest 115: 388–396, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Kiehne K, Herzig KH, Folsch UR. CCK-stimulated changes in pancreatic acinar morphology are mediated by rho. Digestion 65: 47–55, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Kim M, Nozu F, Kusama K, Imawari M. Cholecystokinin stimulates the recruitment of the Src-RhoA-phosphoinositide 3-kinase pathway by Vav-2 downstream of G(alpha13) in pancreatic acini. Biochem Biophys Res Commun 339: 271–276, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Kometani K, Ishida D, Hattori M, Minato N. Rap1 and SPA-1 in hematologic malignancy. Trends Mol Med 10: 401–408, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Kusama K, Nozu F, Awai T, Tanaka S, Honma I, Tsunoda Y, Mitamura K. Deactivation of ROCK-II by Y-27632 enhances basolateral pancreatic enzyme secretion and acute pancreatitis induced by CCK analogues. Biochem Biophys Res Commun 305: 339–344, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Le Page SL, Bi Y, Williams JA. CCK-A receptor activates RhoA through Gα12/13 in NIH3T3 cells. Am J Physiol Cell Physiol 285: C1197–C1206, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Li C, Takei K, Geppert M, Daniell L, Stenius K, Chapman ER, Jahn R, De Camilli P, Sudhof TC. Synaptic targeting of rabphilin-3A, a synaptic vesicle Ca2+/phospholipid-binding protein, depends on rab3A/3C. Neuron 13: 885–898, 1994. [DOI] [PubMed] [Google Scholar]
- 68.Lim J, Williams JA. Activation of PAK2 by CCK in rat pancreatic acinar cells (Abstract). Gastroenterology 130: T1785, 2006. [Google Scholar]
- 69.Luo HR, Saiardi A, Nagata E, Ye K, Yu H, Jung TS, Luo X, Jain S, Sawa A, Snyder SH. GRAB: a physiologic guanine nucleotide exchange factor for Rab3A, which interacts with inositol hexakisphosphate kinase. Neuron 31: 439–451, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, Wang ZW, Fukuda M, Nonet ML. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell 17: 2617–2625, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martelli AM, Baldini G, Tabellini G, Koticha D, Bareggi R. Rab3A and Rab3D control the total granule number and the fraction of granules docked at the plasma membrane in PC12 cells. Traffic 1: 976–986, 2000. [PubMed] [Google Scholar]
- 72.Matsumoto M, Miki T, Shibasaki T, Kawaguchi M, Shinozaki H, Nio J, Saraya A, Koseki H, Miyazaki M, Iwanaga T, Seino S. Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc Natl Acad Sci USA 101: 8313–8318, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25: 173–176, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Mizuno K, Tolmachova T, Ushakov DS, Romao M, Abrink M, Ferenczi MA, Raposo G, Seabra MC. Rab27b regulates mast cell granule dynamics and secretion. Traffic 8: 883–892, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagano F, Sasaki T, Takai Y. Purification and properties of Rab3 GTPase-activating protein. Methods Enzymol 329: 67–75, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Nashida T, Imai A, Shimomura H. Relation of Rab26 to the amylase release from rat parotid acinar cells. Arch Oral Biol 51: 89–95, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol 3: 253–258, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem 279: 37544–37550, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen D, Jones A, Ojakian GK, Raffaniello RD. Rab3D redistribution and function in rat parotid acini. J Cell Physiol 197: 400–408, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Nicke B, Tseng MJ, Fenrich M, Logsdon CD. Adenovirus-mediated gene transfer of RasN17 inhibits specific CCK actions on pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 276: G499–G506, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62, 1995. [DOI] [PubMed] [Google Scholar]
- 82.Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci 17: 8061–8073, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nozu F, Tsunoda Y, Ibitayo AI, Bitar KN, Owyang C. Involvement of RhoA and its interaction with protein kinase C and Src in CCK-stimulated pancreatic acini. Am J Physiol Gastrointest Liver Physiol 276: G915–G923, 1999. [DOI] [PubMed] [Google Scholar]
- 84.O'Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest 86: 1649–1657, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem 282: 5488–5495, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Ohnishi H, Ernst SA, Wys N, McNiven M, Williams JA. Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am J Physiol Gastrointest Liver Physiol 271: G531–G538, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Ohnishi H, Mine T, Shibata H, Ueda N, Tsuchida T, Fujita T. Involvement of Rab4 in regulated exocytosis of rat pancreatic acini. Gastroenterology 116: 943–952, 1999. [DOI] [PubMed] [Google Scholar]
- 88.Ohnishi H, Samuelson LC, Yule DI, Ernst SA, Williams JA. Overexpression of Rab3D enhances regulated amylase secretion from pancreatic acini of transgenic mice. J Clin Invest 100: 3044–3052, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Padfield PJ, Balch WE, Jamieson JD. A synthetic peptide of the rab3a effector domain stimulates amylase release from permeabilized pancreatic acini. Proc Natl Acad Sci USA 89: 1656–1660, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park JB, Farnsworth CC, Glomset JA. Ca2+/calmodulin causes Rab3A to dissociate from synaptic membranes. J Biol Chem 272: 20857–20865, 1997. [DOI] [PubMed] [Google Scholar]
- 91.Pavlos NJ, Xu J, Riedel D, Yeoh JS, Teitelbaum SL, Papadimitriou JM, Jahn R, Ross FP, Zheng MH. Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol Cell Biol 25: 5253–5269, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299, 2008. [DOI] [PubMed] [Google Scholar]
- 93.Quilliam LA, Mueller H, Bohl BP, Prossnitz V, Sklar LA, Der CJ, Bokoch GM. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J Immunol 147: 1628–1635, 1991. [PubMed] [Google Scholar]
- 94.Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y, Wollheim CB. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci 109: 2265–2273, 1996. [DOI] [PubMed] [Google Scholar]
- 95.Riedel D, Antonin W, Fernandez-Chacon R, Alvarez de Toledo G, Jo T, Geppert M, Valentijn JA, Valentijn K, Jamieson JD, Sudhof TC, Jahn R. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol 22: 6487–6497, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rindler MJ, Xu CF, Gumper I, Smith NN, Neubert TA. Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J Proteome Res 6: 2978–2992, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosado JA, Salido GM, Garcia LJ. Activation of m3 muscarinic receptors induces rapid tyrosine phosphorylation of p125(FAK), p130(cas), and paxillin in rat pancreatic acini. Arch Biochem Biophys 377: 85–94, 2000. [DOI] [PubMed] [Google Scholar]
- 98.Rosado JA, Salido GM, Jensen RT, Garcia LJ. Are tyrosine phosphorylation of p125(FAK) and paxillin or the small GTP binding protein, rho, needed for CCK-stimulated pancreatic amylase secretion? Biochim Biophys Acta 1404: 412–426, 1998. [DOI] [PubMed] [Google Scholar]
- 99.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem 283: 23884–23894, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saegusa C, Kanno E, Itohara S, Fukuda M. Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Arch Biochem Biophys 475: 87–92, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Sakisaka T, Takai Y. Purification and properties of Rab3 GEP (DENN/MADD). Methods Enzymol 403: 254–261, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci 24: 6629–6637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic 5: 393–399, 2004. [DOI] [PubMed] [Google Scholar]
- 105.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med 8: 23–30, 2002. [DOI] [PubMed] [Google Scholar]
- 106.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104: 19333–19338, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol 13: 2061–2068, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem 277: 25423–25430, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153–208, 2001. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka M, Miyoshi J, Ishizaki H, Togawa A, Ohnishi K, Endo K, Matsubara K, Mizoguchi A, Nagano T, Sato M, Sasaki T, Takai Y. Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol Biol Cell 12: 1421–1430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang LH, Gumkowski FD, Sengupta D, Modlin IM, Jamieson JD. rab3D protein is a specific marker for zymogen granules in gastric chief cells of rats and rabbits. Gastroenterology 110: 809–820, 1996. [DOI] [PubMed] [Google Scholar]
- 112.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell 99: 67–86, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA 104: 5872–5877, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol 21: 1921–1929, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tuvim MJ, Adachi R, Chocano JF, Moore RH, Lampert RM, Zera E, Romero E, Knoll BJ, Dickey BF. Rab3D, a small GTPase, is localized on mast cell secretory granules and translocates to the plasma membrane upon exocytosis. Am J Respir Cell Mol Biol 20: 79–89, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Valentijn JA, Gumkowski FD, Jamieson JD. The expression pattern of rab3D in the developing rat exocrine pancreas coincides with the acquisition of regulated exocytosis. Eur J Cell Biol 71: 129–136, 1996. [PubMed] [Google Scholar]
- 117.Valentijn JA, LaCivita DQ, Gumkowski FD, Jamieson JD. Rab4 associates with the actin terminal web in developing rat pancreatic acinar cells. Eur J Cell Biol 72: 1–8, 1997. [PubMed] [Google Scholar]
- 118.Valentijn JA, Sengupta D, Gumkowski FD, Tang LH, Konieczko EM, Jamieson JD. Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur J Cell Biol 70: 33–41, 1996. [PubMed] [Google Scholar]
- 119.Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci USA 97: 1091–1095, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wagner AC, Strowski MZ, Goke B, Williams JA. Molecular cloning of a new member of the Rab protein family, Rab 26, from rat pancreas. Biochem Biophys Res Commun 207: 950–956, 1995. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 388: 593–598, 1997. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem 275: 20033–20044, 2000. [DOI] [PubMed] [Google Scholar]
- 123.Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem 279: 7840–7849, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J Biol Chem 282: 9635–9645, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Williams JA, Yule DI. Stimulus-secretion coupling in pancreatic acinar cells. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by LR Johnson. Burlington, MA: Academic, 2006, p. 1337–1369.
- 126.Wilson SM, Yip R, Swing DA, O'Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA 97: 7933–7938, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamashita S, Mochizuki N, Ohba Y, Tobiume M, Okada Y, Sawa H, Nagashima K, Matsuda M. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J Biol Chem 275: 25488–25493, 2000. [DOI] [PubMed] [Google Scholar]
- 128.Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol 22: 1858–1867, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Zwartkruis FJ, Bos JL. Ras and Rap1: two highly related small GTPases with distinct function. Exp Cell Res 253: 157–165, 1999. [DOI] [PubMed] [Google Scholar]