Abstract
We have previously demonstrated that the Bcl-2/adenovirus EIB 19-kDa interacting protein 3 (BNIP3), a cell death-related member of the Bcl-2 family, is upregulated in vitro and in vivo in both experimental and clinical settings of redox stress and that nitric oxide (NO) downregulates its expression. In this study we sought to examine the expression and localization of BNIP3 in murine hepatocytes and in a murine model of hemorrhagic shock (HS) and ischemia-reperfusion (I/R). Freshly isolated mouse hepatocytes were exposed to 1% hypoxia for 6 h followed by reoxygenation for 18 h, and protein was isolated for Western blot analysis. Hepatocytes grown on coverslips were fixed for localization studies. Similarly, livers from surgically cannulated C57Bl/6 mice and from mice cannulated and subjected to 1–4 h of HS were processed for protein isolation and Western blot analysis. In hepatocytes, BNIP3 was expressed constitutively but was upregulated under hypoxic conditions, and this upregulation was countered by treatment with a NO donor. Surprisingly, BNIP3 was localized in the nucleus of normoxic hepatocytes, in the cytoplasm following hypoxia, and again in the nucleus following reoxygenation. Upregulation of BNIP3 partially required p38 MAPK activation. BNIP3 contributed to hypoxic injury in hepatocytes, since this injury was diminished by knockdown of BNIP3 mRNA. Hepatic BNIP3 was also upregulated in two different models of liver stress in vivo, suggesting that a multitude of inflammatory stresses can lead to the modulation of BNIP3. In turn, the upregulation of BNIP3 appears to be one mechanism of hepatocyte cell death and liver damage in these settings.
Keywords: redox stress, nitric oxide, hypoxia, cell death, inflammation
the bcl-2/adenovirus eib 19-kDa interacting protein 3 (BNIP3, formerly known as NIP3) was first identified in a yeast two-hybrid screen as a protein capable of interacting with the adenovirus protein E1B 19 kDa, a homolog of Bcl-2 (4). BNIP3 is a membrane-associated protein localized to mitochondria and other cytoplasmic membrane structures and is widely expressed in a large number of mouse and human tissues (8). BNIP3 appears to have an overall resemblance to several BH3-containing Bcl-2 family proteins such as BIK, BID, HRK, and BAD, in which the BH3 domain plays an important role in the induction of apoptosis. However, BNIP3 has been implicated in both apoptotic and necrotic cell death (5). For instance, the expression of BNIP3 (both mRNA and protein) is induced and related to hypoxia-induced apoptosis in a number of human and animal cell lines, including CHO-K1 (Chinese hamster ovary), CV-1 (monkey kidney), Rat-1 (rat fibroblast), PAM212 (human epithelial), HepG2 (human hepatocellular carcinoma), and ECV-304 (human bladder carcinoma) (5). Moreover, BNIP3 and Nix [a BNIP3 homolog sharing both structural and functional similarity (7), also known as BNIP3L (30), BNIP3α (50), and B5 (35)] are the only members of the Bcl-2 family of apoptotic factors induced in response to hypoxia (5). On the other hand, BNIP3 may also mediate a form of necrotic cell death following protein integration into the mitochondrial outer membrane and rapid permeability transition pore opening (48). This mechanism was shown to be independent of caspases and the Apaf-1/cytochrome c mitochondrial pathway, was accompanied by the suppression of the proton electrochemical gradient and increased production of reactive oxygen species, and occurred before the appearance of nuclear damage (48).
The regulation of BNIP3 induction is still under investigation. The hypoxia-inducible factor (HIF)-1α-mediated pathway of BNIP3 induction has been clearly established in cardiac myocytes and various non-tumor and tumor cell lines (24, 45). We have focused our attention on the expression and regulation of BNIP3 in hepatocytes in vitro and in the liver in vivo. We were the first to demonstrate a relationship between nitric oxide (NO), an important regulatory molecule in liver physiology and physiopathology (10, 19, 43), and BNIP3. In the liver, NO can be produced from the inducible nitric oxide synthase (iNOS) (10, 19, 43). We showed that both endogenous (iNOS derived) as well as exogenous NO (supplied via NO donor) suppress the expression of BNIP3 in mouse hepatocytes (52). Yook et al. (51) later found that BNIP3 induced by NO causes cell death in macrophages, whereas Turpaev et al. (47) reported that NO-exposed human monocytic cells differentially express BNIP3. In addition to NO, we showed that treatment with a proapoptotic stimulus (TNF-α in combination with actinomycin D) (20, 26) resulted in increased expression of BNIP3 mRNA (52). More recently, we have shown that NO regulates the intestinal and hepatic expression of BNIP3 in a rat model of intestinal inflammation in vivo (55). Although BNIP3 was initially reported to be localized to mitochondria and other cytoplasmic membrane structures (4), there is recent evidence showing nuclear sequestration of BNIP3 in non-small cell lung cancer (13) and specific localization in the nucleus of hippocampal neurons after transient global brain ischemia in rats (38). The exact localization of BNIP3 in liver cells is not known.
In the present study, we have expanded our understanding of the regulation of BNIP3 in hepatocytes under hypoxic stress. We report that BNIP3 is localized to the nuclei of mouse hepatocytes during normoxic conditions, whereas under hypoxic stress not only are BNIP3 mRNA and protein upregulated, but also the subcellular localization of BNIP3 protein changes dramatically. We show that BNIP3 contributes to hypoxic injury in hepatocytes and that hepatic BNIP3 is upregulated in two different models of liver stress in vivo. Given that BNIP3 is modulated in various settings of cell and tissue stress that are associated with inflammation, we suggest that additional studies of this protein in inflammatory scenarios are warranted.
EXPERIMENTAL PROCEDURES
Materials.
Williams E medium, penicillin, streptomycin, l-glutamine, and HEPES were purchased from Invitrogen (Carlsbad, CA). Insulin (Humulin) was purchased from Eli Lilly (Indianapolis, IN), and calf serum was obtained from HyClone Laboratories (Logan, UT). Tissue culture dishes were obtained from Corning Glass Works (Corning, NY). 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD 98059), 1,9-pyrazoloanthrone, anthrapyrazolone (SP600125), and 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB 203580) were obtained from Sigma-Aldrich (St. Louis, MO). S-Nitroso-N-acetyl-d,l-penicillamine (SNAP) was purchased from Alexis Biochemicals (San Diego, CA). Stock solutions were prepared at 100 mM in DMSO and kept at −20°C until use. Further dilutions were made in culture medium. Unless indicated otherwise, all other chemicals and proteins were purchased from Sigma-Aldrich.
Hepatocyte isolation and culture.
All procedures involving animals were approved by the Animal Care and Use Committee of the University of Pittsburgh. Primary hepatocytes were harvested from male Sprague-Dawley rats (Harlan, Indianapolis, IN), C57BL/6 mice (Charles River Laboratories, Wilmington, MA), or iNOS-null mice (27). Hepatocytes were isolated by collagenase perfusion using the method of Seglen (39) and purified to >98% purity by repeated centrifugation at 50 g, followed by further purification over 30% Percoll. Viability at time of plating was checked using trypan blue exclusion. Highly purified hepatocytes (>98% purity and >95% viability by trypan blue exclusion) were suspended in Williams' E medium supplemented with 10% heat-inactivated calf serum, 15 mM HEPES (pH 7.4), 16 units of insulin, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were plated on collagen-coated cell culture dishes (3 × 106 cells/6-cm dish or 5 × 106 cells/10-cm dish) or plates (250,000 cells/well in 6-well plates) and cultured overnight at 37°C under normoxic conditions (5% CO2). The old medium was then removed, and cells were further incubated with fresh medium containing 5% heat-inactivated calf serum. Hypoxic conditions were obtained by placing the cells into a modular incubator chamber (Billups-Rothenburg, Del Mar, CA) flushed with a hypoxic gas mixture containing 1% O2, 5% CO2, and 94% N2. Duplicate hypoxic cultures were returned for reoxygenation overnight (18 h) in the normoxic incubator. Hepatocytes incubated under normoxic conditions (21% O2) served as controls.
Small interference RNA treatment and knockdown of BNIP3.
For transient knockdown experiments, hepatocytes were transfected with specific small interference (si)RNA to BNIP3 or control, nonsilencing siRNA (SMARTpool siRNA reagents) from Dharmacon (Chicago, IL). BNIP3 siRNA duplex targeted nucleotides 208–227 of the BNIP3 mRNA sequence (U15174) composed of sense, 5′-UACUGCUGGACGCACAGCAdTdT-3′ and antisense, 5′-UGCAGUGCGUCCAGCAGUAdTdT-3′. Briefly, primary mouse hepatocytes were plated onto 10-cm cell culture dishes. The duplexes were diluted to a final concentration of 1 or 5 nM in Opti-Mem (Invitrogen Life Technologies, San Diego, CA). Lipofectamine 2000 transfection reagent (6 μl/ml; Invitrogen Life Technologies) was added and incubated at room temperature for 20 min. The cells were incubated with the oligonucleotide duplexes in serum-free conditions for 6 h at 37°C. After the cells were washed twice with sterile PBS, the cells were incubated in William's E medium supplemented with 5% calf serum for 24 h, followed by exposure to hypoxia for another 24 h. Additional technical details, reagent preparation, storage, and incubation times were as per the manufacturer's recommendations.
RNA isolation and Northern blot analysis.
Total RNA was isolated from the cultured hepatocytes using an Ultraspec RNA isolation reagent from Biotecx Labs (Houston, TX). Briefly, the cells were lysed directly in a culture dish by adding UltraSpec (1 ml/3.5-cm petri dish) and passing the cell lysate several times through a pipette. After extraction with chloroform (0.2 ml/ml UltraSpec), the total RNA was precipitated from the aqueous phase by addition of isopropanol, washed with 75% ethanol, and solubilized in DEPC-treated water. The RNA (20 μg/lane) was electrophoresed on 0.9% agarose gel containing 12.3 M formaldehyde and transferred to nylon membranes (GeneScreen; NEN Life Science) by vacuum blotting. The membranes were prehybridized for 3–4 h at 43°C and hybridized with [32P]dCTP-labeled probe (106 cpm/ml) at 43°C. Membranes were then washed three times with SSC/SDS at 53°C before exposure. Membranes were then stripped and probed for 18 S RNA to assess RNA loading. Northern blot analysis of BNIP3 mRNA levels in cultured hepatocytes was carried out using a probe as described previously (52). Radioactive membranes were quantified with storage phosphor screens (PhosphorImager; Molecular Dynamics), and the relative amount of mRNA is presented as the ratio of mRNA to 18S RNA.
Protein isolation and Western blot analysis.
Cultured hepatocytes (3 × 106 cells/100-mm petri dish) were washed twice with ice-cold PBS and resuspended in 1 ml of ice-cold lysis buffer (Cell Lysis Buffer 10×; Cell Signaling Technology, Danvers, MA) containing 2 mM Tris·HCl buffer (pH 7.5), 15 mM NaCl, 0.1 mM EDTA and EGTA, 0.1% Triton X-100, 250 μM sodium pyrophosphate, 100 μM β-glycerolphosphate, 100 μM Na3VO4, and the protease inhibitors leupeptin (1 μg/ml) and PMSF (1 mM). After 5 min of incubation on ice, the cells were scraped off the dish and transferred to microcentrifuge tubes. The cells were sonicated two times for 10–15 s each time with 1 min on ice between each sonication. Cell debris was then removed by centrifugation at 10,000 rpm for 10 min, and the supernatant was used as cell lysate and stored at −80°C when necessary. An aliquot was used to determine protein concentration using the BCA protein assay kit from Pierce (Rockford, IL) with bovine serum albumin as standard. Protein samples (75 μg) were separated on 12% SDS-polyacrylamide gels, and the gels were electroblotted onto polyvinylidene difluoride nitrocellulose membranes. Immunodetection of BNIP3 protein was done using the SuperSignal West Dura extended duration substrate (Thermo Scientific, Rockford, IL) and a mouse monoclonal antibody against human BNIP3 (1:1,000 dilution) purchased from Sigma-Aldrich. Instructions for the kit were provided by the supplier. Lysates from MCF-7 cells exposed to hypoxia for 36 h were used as positive control. For immunodetection of ATP synthase, we used a mouse antibody cocktail that contains five monoclonal antibodies, including one against the ATP synthase α-subunit (MitoSciences, catalog no. MS604; generously provided by Dr. Brian Zuckerbraun, University of Pittsburgh).
Scanning laser confocal immunofluorescence and transmission electron microscopy.
Freshly isolated mouse hepatocytes (wild type and iNOS null) plated on coverslips (2 × 105 cells/22-mm glass coverslip; BD Biocoat, Bedford, MA) were cultured under normoxic (control) or hypoxic (1% O2) conditions for 6 h. Parallel hypoxic cultures were returned to the normoxic incubator for 18 h of reoxygenation. The cells were then fixed in 2% paraformaldehyde in PBS for 1 h and processed for imaging as described elsewhere (15). For fluorescence labeling, we used a rabbit polyclonal anti-BNIP3 from Abgent (San Diego, CA), a mouse monoclonal anti-ATP synthase from Affinity Bioreagents (Golden, CO; catalog no. MA1–930), and the high-affinity probe for F-actin rhodamine phalloidin from Invitrogen. For transmission electron microscopy (TEM), cells were fixed in 2.5% glutaraldehyde, processed, and sectioned as previously described (42). Sections were observed on a JEM 1210 electron microscope (JEOL, Peabody, MA).
ATP measurement.
Cellular ATP levels were measured using the CellTiter-Glo luminescent cell viability assay, which measures ATP using a bioluminescent reaction (Promega, Madison, WI). Technical details, reagent preparation, storage, and incubation times were as per the manufacturer's recommendation.
Viability assay.
Cell viability was assessed by measuring the release of the cytoplasmic enzyme lactate dehydrogenase (LDH) from damaged cells into the supernatant using a colorimetric assay cytotoxicity detection kit (LDH assay; Roche Applied Science, Indianapolis, IN).
Mouse models of HS and I/R injury.
Both experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conformed to the NIH guidelines for the care and use of laboratory animals. For HS studies, we used an automated closed-loop hemorrhagic shock model described elsewhere (44). Briefly, male C57Bl/6 mice were either untreated (resting control) or were cannulated and observed for 1, 2, 3, and 4 h (cannulation only) or for 1, 2, 3 and 4 h of surgical cannulation plus HS (bleeding to 25 mmHg). For studies of ischemia-reperfusion (I/R) injury, a nonlethal model of segmental (70%) hepatic warm ischemia was used (46). In this model, all structures in the portal triad (hepatic artery, portal vein, bile duct) to the left and median liver lobes were occluded with a microvascular clamp for 30 or 60 min (ischemia) followed by removal of the clamp (reperfusion). Sham animals underwent anesthesia, laparotomy, and exposure of the portal triad without hepatic ischemia. After euthanasia, livers were isolated and prepared for protein isolation and Western blot analysis as described above.
Statistical analysis.
Experiments were performed at least three times independently, and data are means ± SE. Statistical differences were assessed using Student's t-test or one-way ANOVA followed by a post hoc test, where appropriate, using SigmaStat software (Systat Software, San Jose, CA). Differences were considered significant at the 95% confidence interval (P < 0.05).
RESULTS
Effect of hypoxia and hypoxia-reoxygenation on BNIP3 expression in hepatocytes in vitro.
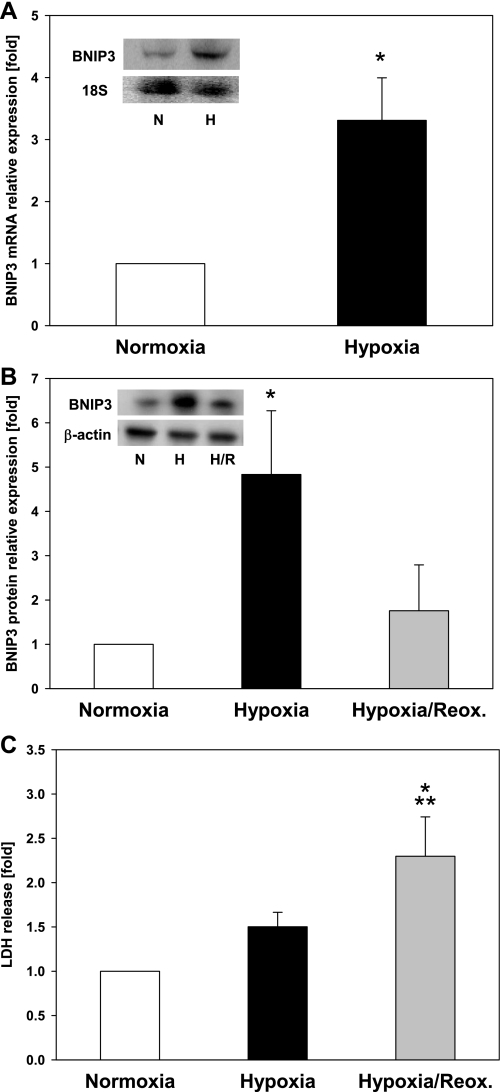
We initially investigated the effect of low oxygen tension, which is relevant to conditions where hypoxia plays an important role (e.g., I/R injury and HS) on BNIP3 expression. To investigate the effects of hypoxia and hypoxia-reoxygenation on endogenous BNIP3 protein in hepatocytes, we used an in vitro culture system as previously reported (15). Freshly isolated hepatocytes were plated and allowed to adhere and equilibrate overnight (18 h). The following day, we cultured them under conditions of 6 h of normoxia (control), 6 h of hypoxia (1% O2), or 6 h of hypoxia followed by overnight reoxygenation. We found that exposure to hypoxia (1% O2) for 6 h led to an increase in BNIP3 mRNA and protein expression in both primary rat (Fig. 1A) and mouse hepatocytes (Fig. 1B). Upon reoxygenation, however, the levels of BNIP3 protein decreased significantly to levels only slightly higher than those seen in control, normoxic hepatocytes (Fig. 1B). Hypoxia did not result in a significant loss of viability after 6 h as demonstrated by LDH release (Fig. 1C), confirming a previous observation by our group (17). After reoxygenation, however, a significant increase in LDH levels was observed indicative of cell death (Fig. 1C).
Fig. 1.
Effect of hypoxia and hypoxia-reoxygenation (H/R) on Bcl-2/adenovirus EIB 19-kDa interacting protein 3 (BNIP3) expression in hepatocytes. Freshly isolated rat and mouse hepatocytes were exposed to hypoxia (H; 1% O2) for 6 h with or without reoxygenation for 18 h, and the mRNA and protein were isolated and analyzed as described in materials and methods. Control samples were hepatocytes cultured under normoxic (i.e., nonischemia) conditions (N). A: Northern blot analysis for BNIP3 mRNA in rat hepatocytes. A representative blot (inset) and the densitometric quantification of 5 independent experiments are shown. *P = 0.01 vs. normoxia. Data were analyzed by Student's t-test. B: Western blot analysis for BNIP3 protein in mouse hepatocytes. A representative blot (inset) and the densitometric quantification of 5 independent experiments are shown. *P < 0.05 vs. normoxia and H/R. Data were analyzed by 1-way ANOVA followed by Student-Newman-Keuls test. Reox, reoxygenation. C: lactate dehydrogenase (LDH) release in mouse hepatocytes exposed to hypoxia or H/R. Results are means ± SE (n = 9–16). *P < 0.001 vs. normoxia. **P < 0.05 vs. hypoxia. Data were analyzed by 1-way ANOVA followed by Tukey's test.
Effect of hypoxia on BNIP3 expression in the presence of NO in hepatocytes in vitro.
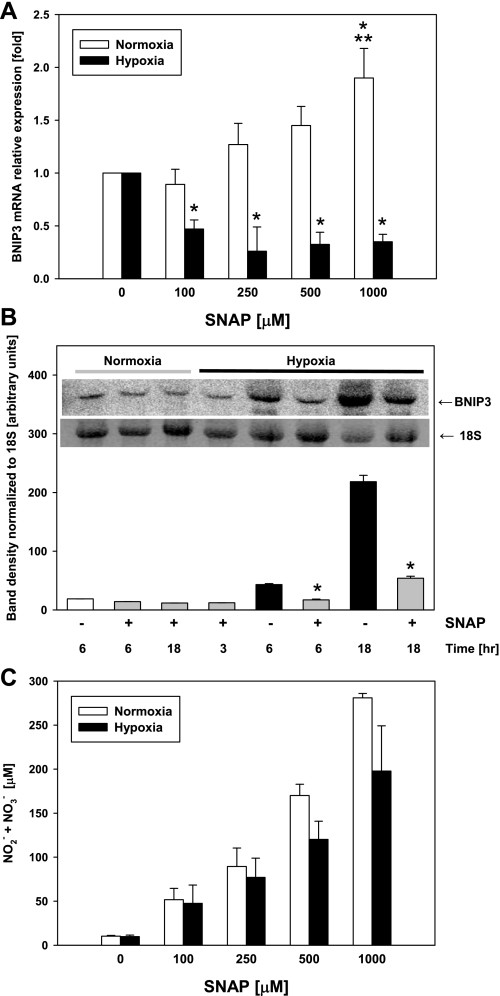
We have previously established that BNIP3 gene expression is regulated by NO in hepatocytes under normoxic conditions (52). Rat hepatocytes exposed to high concentrations (>100 μM) of the NO donor SNAP exhibited a concentration-dependent upregulation of BNIP3 mRNA expression under normoxic conditions. However, exposure to NO under hypoxic conditions significantly inhibited the hypoxia-induced upregulation of BNIP3 mRNA (Fig. 2A). This inhibitory effect under hypoxic conditions was independent of the concentration of the NO donor used. The reduction in the expression of BNIP3 was also demonstrated by confocal immunofluorescence (data not shown). Similarly, when mouse hepatocytes were exposed to hypoxia in the presence of a dose of SNAP (100 μM) that had little or no inhibitory effect under normoxic conditions, the hypoxia-induced upregulation of BNIP3 mRNA expression was significantly inhibited (Fig. 2B). As expected, the decomposition of SNAP under hypoxic conditions produced lower, but not significantly different, levels of nitrite and nitrate in the culture supernatants compared with normoxic conditions (Fig. 2C).
Fig. 2.
Effect of hypoxia on BNIP3 expression in the presence of NO in hepatocytes. Freshly isolated rat and mouse hepatocytes were exposed to hypoxia (1% O2) in the presence or absence of SNAP (100–1,000 μM), and the mRNA was isolated and analyzed as described in materials and methods. Control samples were hepatocytes cultured under normoxic conditions. A: Northern blot analysis for BNIP3 mRNA in rat hepatocytes. Data were normalized using 18S band density as loading control and represent fold change vs. respective nontreated controls. Results are means ± SE (n = 4). *P < 0.01 vs. 0 SNAP. **P < 0.01 vs. 100 μM SNAP. Data were analyzed by 1-way ANOVA followed by the Tukey's test. B: Northern blot analysis (inset) and densitometric quantification for BNIP3 mRNA in mouse hepatocytes cultured under normoxic or hypoxic conditions in the presence or absence of SNAP (100 μM) as indicated. Results are means ± SE (n = 3). *P < 0.001 vs. normoxia. Data were analyzed by Student's t-test. C: levels of nitrite/nitrate (NO2−/NO3−) in supernatants of rat hepatocytes cultured in the presence or absence of SNAP under normoxic or hypoxic conditions as indicated. Results are means ± SE of 4 independent experiments.
BNIP3 localization in mouse hepatocytes: effect of hypoxia and hypoxia-reoxygenation.
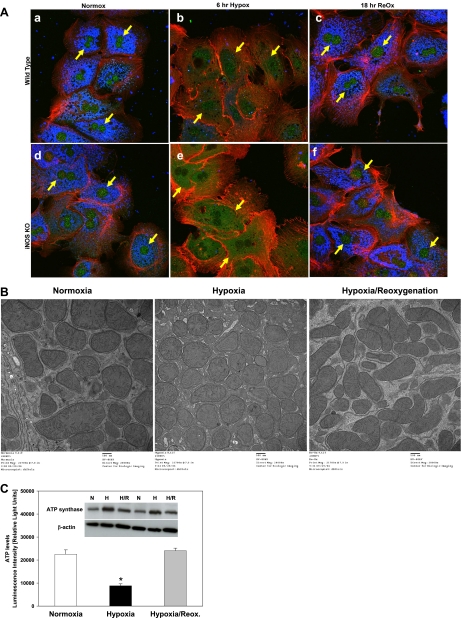
We next sought to examine the effects of hypoxia and hypoxia-reoxygenation on the subcellular localization of BNIP3 protein by using confocal immunofluorescence. BNIP3 was generally localized to the nucleus, with some cytoplasmic staining, in primary mouse hepatocytes under normoxic conditions (Fig. 3 Aa). Hypoxia, however, induced a translocation of BNIP3 protein from the nucleus to the cytoplasm of mouse hepatocytes by 6 h (Fig. 3Ab). Upon reoxygenation, BNIP3 was again localized primarily to the nucleus (Fig. 3Ac). To address the possible role of endogenous, iNOS-derived NO in this process, we repeated these experiments using hepatocytes from iNOS-null mice. In these cells, BNIP3 was generally localized to the nucleus, with some cytoplasmic staining, under normoxic conditions (Fig. 3Ad). Interestingly, the levels of cytoplasmic BNIP3 under normoxia were higher in the iNOS-null hepatocytes compared with wild-type cells. Similar to the effect in wild-type cells, hypoxia not only upregulated BNIP3 protein expression (increased staining) but also induced a translocation of BNIP3 protein from the nucleus to the cytoplasm after 6 h (Fig. 3Ae). Similar to wild-type hepatocytes, iNOS-null hepatocytes that were exposed to hypoxia for 6 h followed by reoxygenation showed only nuclear staining for BNIP3 (Fig. 3Af). Interestingly, no blue staining for ATP synthase was seen in the hypoxic cultures (Fig. 3A, b and e) and in none of the other separate experiments with hepatocytes exposed to hypoxia only (not shown). Since ATP synthase is commonly used as a mitochondrial marker, we then performed TEM to detect whether mitochondria were present in the cells exposed to hypoxia. As shown in Fig. 3B, we observed no significant differences in the number and appearance of mitochondria between control normoxic cells or cells exposed to hypoxia (6 h) or hypoxia followed by reoxygenation (18 h) as detected by TEM, confirming that the lack of immunostaining for ATP synthase in the hypoxic cells was not due to loss of mitochondria. Since hypoxia-induced ATP depletion has also been reported in hepatocytes (25), we assessed intracellular ATP as an index of the stress of exposure of hepatocytes to hypoxia. As shown in Fig. 3C, ATP levels were significantly reduced during hypoxia and completely restored on reoxygenation, confirming that hypoxia depletes cells from ATP as shown previously (25). We next performed Western blotting analysis to determine the levels of ATP synthase protein expression under the same experimental conditions as in Fig. 3A. Since the antibody used for immunocytochemistry is not suitable for Western blotting analysis (manufacturer's recommendation; data not shown), we performed the analysis using a different antibody (see MATERIALS AND METHODS). As shown in Fig. 3C, inset, hypoxia did not affect the protein expression of ATP synthase, suggesting that the lack of immunocytochemical expression of ATP synthase (Fig. 3A) was likely due to a hypoxia-induced modification of the enzyme that cannot be detected by the mouse antibody against the β-subunit used in our immunofluorescence study.
Fig. 3.
Effect of hypoxia and H/R on BNIP3 subcellular localization in wild-type and inducible NO synthase (iNOS)-null mouse hepatocytes. A: freshly isolated hepatocytes (wild type, a–c; iNOS null, d–f) were cultured under normoxic (control; a and d) or hypoxic conditions (1% O2; b and e) for 6 h. Parallel hypoxic cultures were returned to the normoxic incubator for 18 h of reoxygenation (c and f). Cells were then fixed and processed for confocal immunofluorescence imaging as described in materials and methods. Fluorescent labeling: BNIP3, green; ATP synthase, blue; F-actin, red. B: in parallel experiments, cells were fixed and processed for transmission electron microscopy as described in materials and methods. Compared with normoxic control, no loss of mitochondria was observed after exposure to hypoxia (6 h) or H/R for 18 h. C: freshly isolated hepatocytes were cultured under normoxic (control) or hypoxic conditions (1% O2) for 6 h. Parallel hypoxic cultures were returned to the normoxic incubator for 18 h of reoxygenation. ATP levels were measured using the CellTiter-Glo luminescent cell viability assay kit as described in materials and methods. Results are means ± SE (n = 6). *P < 0.001 vs. normoxia and H/R. Data were analyzed by 1-way ANOVA followed by Tukey's test. Inset shows a Western blot for ATP synthase protein expression under the same experimental conditions as described above (n = 2).
Overexpression of BNIP3 is involved in hypoxia-induced cell death of hepatocytes.
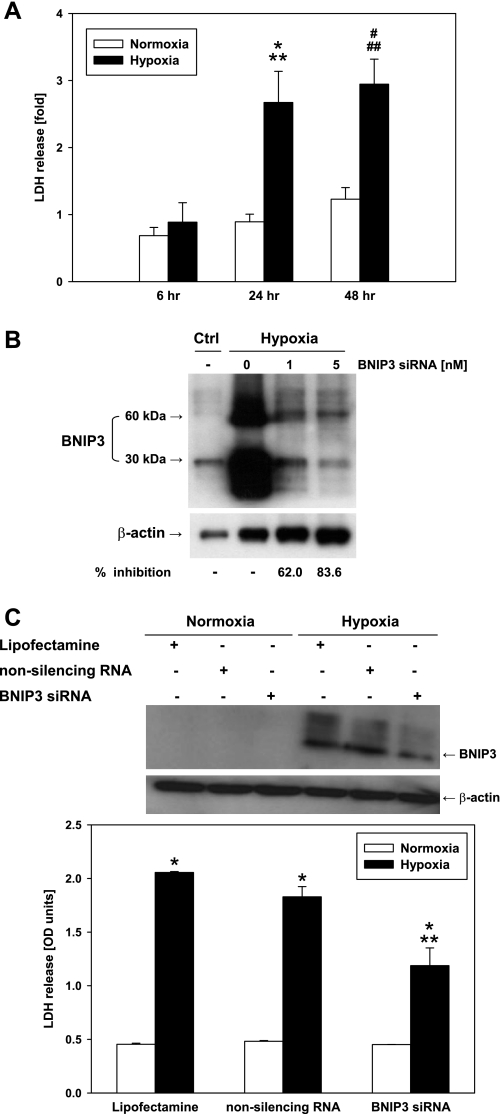
To study whether the expression of BNIP3 is directly involved in hypoxia-induced cell death, we employed siRNA technology to silence the gene. Since exposure to hypoxia for 6 h did not result in significant cell damage (see LDH release, Fig. 1C), we exposed mouse hepatocytes to prolonged periods of hypoxia with or without transient transfection with BNIP3 or control siRNA (1 and 5 nM). Incubation under hypoxia for 24 or 48 h inevitably led to cell death as measured by LDH release (Fig. 4A). Transient transfection with BNIP3 siRNA, but not the nonsilencing siRNA (negative control), led to a significant reduction in the hypoxia-induced overexpression of BNIP3 protein as measured by Western blot analysis (Fig. 4, B and C). BNIP3 siRNA concomitantly resulted in a significant reduction in LDH release compared with hypoxia alone (Fig. 4C). This cytoprotection by BNIP3 siRNA suggests that upregulation of BNIP3 plays a role in the cytotoxic effect induced by hypoxia in hepatocytes.
Fig. 4.
BNIP3 small interference (si)RNA protects hepatocytes from hypoxia-induced cell injury. A: freshly isolated mouse hepatocytes were exposed to 6, 24, and 48 h of hypoxia (1% O2), and viability was assessed by LDH release. Results are means ± SE (n = 9–16). *P < 0.005 vs. normoxia, 24 h. **P < 0.05 vs. hypoxia, 6 h. #P < 0.01 vs. normoxia, 48 h. ##P < 0.005 vs. hypoxia, 6 h. Data were analyzed by 1-way ANOVA followed by Tukey's test. B: after transient transfection with BNIP3 siRNA (1 and 5 nM), primary mouse hepatocytes were exposed to 24 h of hypoxia and the total protein was isolated. A representative Western blot showing the knockdown of BNIP3 gene is shown (n = 3). The inhibition of BNIP3 protein expression was calculated using the densitometric analysis for the 30-kDa band. Ctrl, control. C: freshly isolated mouse hepatocytes were treated with Lipofectamine, nonsilencing (scrambled) RNA, and BNIP3 siRNA (5 nM) for 24 h of hypoxia (1% O2), and viability was assessed by LDH release. OD, optical density. *P < 0.001 vs. normoxia. **P < 0.001 vs. hypoxia (Lipofectamine and nonsilencing RNA). Data were analyzed by 1-way ANOVA followed by Tukey's test. Representative Western blot (top) shows BNIP3 protein expression under the same experimental conditions as described above.
Upregulation of BNIP3 induced by hypoxia is mediated by MAPK signaling.
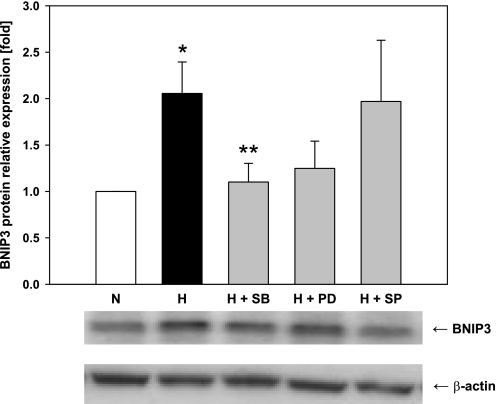
We have previously shown that hypoxia in vitro and HS in vivo induce the mitogen-activated protein kinase (MAPK) pathway (31, 32, 34). Accordingly, we hypothesized that similar pathways may regulate BNIP3 expression. We exposed mouse hepatocytes to 6 h of hypoxia in the presence or absence of 3 μM SB 203580, PD 98059, and SP600125, specific inhibitors of the MAPKs p38, extracellular signal-regulated kinase (ERK1/2), and c-Jun NH2-terminal kinase (JNK1/2), respectively. Western blot analysis revealed that both the p38 MAPK and the ERK inhibitors blocked the hypoxia-mediated upregulation of BNIP3 (Fig. 5). However, although the effect of the former was statistically significant, the effect of the ERK1/2 inhibitor was not statistically different from hypoxia alone. In contrast, the JNK1/2 inhibitor did not affect the BNIP3 protein expression (Fig. 5).
Fig. 5.
Effect of MAPK inhibitors on hypoxia-induced BNIP3 expression in mouse hepatocytes. Freshly isolated rat and mouse hepatocytes were exposed to hypoxia (1% O2) for 6 h in the presence or absence of the specific MAPK inhibitors SB 203580 (SB), PD 98059 (PD), and SP600125 (SP), all at 3 μM. Protein was isolated and analyzed as described in materials and methods. Control samples were hepatocytes cultured under normoxic conditions. A representative Western blot and the densitometric quantification of 8–12 independent experiments are shown. *P < 0.01 vs. normoxia. **P < 0.05 vs. hypoxia. Data were analyzed by 1-way ANOVA followed by Tukey's test.
BNIP3 is upregulated in different settings of liver stress in vivo.
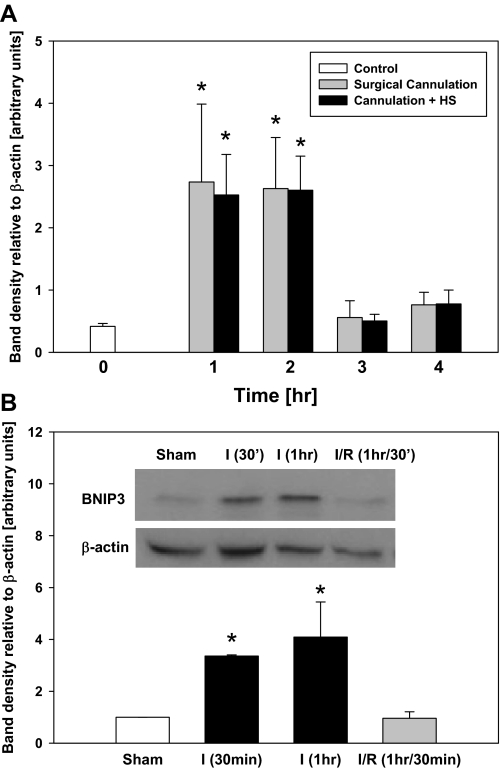
Liver hypoxia is associated with inflammatory conditions such as I/R and HS (31). To study the expression of BNIP3 in the liver in vivo, we subjected C57Bl/6 mice to surgical cannulation or surgical cannulation followed by HS (bleeding to 25 mmHg) for 1, 2, 3, and 4 h. Livers from these animals demonstrated a threefold upregulation of BNIP3 protein (Fig. 6A) compared with resting livers from control animals. We have demonstrated previously that this paradigm of cannulation with or without HS leads to the increased production of various proinflammatory markers (44). Since the upregulation of BNIP3 in both the sham-operated animals and the animals subjected to hemorrhage points to the role of inflammatory stress in general rather than hypoxia in particular alone as the stimulus for increased BNIP3 expression, we performed additional experiments using a mouse model of I/R injury (46) as another form of inflammatory hepatic stress. As shown in Fig. 6B, ischemia led to upregulation of BNIP3 protein expression compared with sham-operated animals, supporting our hypothesis that inflammatory stress can lead to the upregulation of BNIP3 in vivo. Furthermore, similar to what was found in isolated hepatocytes exposed to hypoxia-reoxygenation, livers from mice subjected to I/R had lower protein levels of BNIP3 compared with ischemic liver without reperfusion (Fig. 6B).
Fig. 6.
Hepatic BNIP3 expression in vivo. A: livers from mice subjected to surgical cannulation or surgical cannulation plus hemorrhagic shock (HS; bleeding to 25 mmHg maintained for 1, 2, 3, or 4 h) were collected, and Western blot analysis for BNIP3 protein was performed. Livers from resting untreated animals served as controls. Results are means ± SE (n = 5–9 animals/group). *P < 0.05 vs. control, 3 h of surgical cannulation, and 3 h of cannulation + HS. Data were analyzed by 1-way ANOVA followed by Student-Newman-Keuls test. B: livers from mice subjected to segmental (70%) hepatic warm ischemia for 30 min or 1 h or 1 h of ischemia followed by 30 min of reperfusion (I/R) were collected, and Western blot analysis for BNIP3 protein was performed. Livers from sham animals served as controls. Results are means ± SE (n = 2–3 animals/group). *P < 0.05 vs. sham and I/R. Data were analyzed by 1-way ANOVA followed by Tukey's test.
DISCUSSION
For over 15 years, it has been recognized that NO participates in the regulation of gene expression (12, 28, 29). Confirming our hypothesis that the antiapoptotic effects of NO in hepatocytes (18) may also be mediated by modulation of gene expression, we found induction of the antiapoptotic genes BAG-1 and heat shock protein 70 and reduction of the proapoptotic genes sox-4, calpain, R-ras gene, interleukin-1β-converting enzyme-like cysteine protease (Lice) gene, and RIP gene by iNOS in mouse hepatocytes (54). In a related study, we reported that iNOS increases the expression of cytochrome P-450 2E1 in iNOS-null hepatocytes in the absence of inflammatory stimuli (53). The relationship between NO and BNIP3 was first demonstrated in our study showing that both endogenous (iNOS derived) as well as exogenous NO suppress the expression of BNIP3 in mouse hepatocytes (52), adding BNIP3 to the list of genes that can be regulated by NO. Other groups later found that BNIP3 expression by NO causes cell death in macrophages (2, 51) and that human monocytic cells exposed to NO express BNIP3 differentially (47). We have also shown that NO regulates the intestinal and hepatic expression of BNIP3 in a rat model in vivo (55). Despite these studies and their implication of BNIP3 in cell death, the ultimate significance of the regulation of the cell death inducer BNIP3 by NO still needs to be established.
BNIP3 is overexpressed in human tumors and human tumor cell lines (11, 21, 40, 41) and in primary neonatal rat cardiac myocytes exposed to hypoxia (14, 22). At oxygen tensions used in cell culture experiments (which may or may not reflect the conditions under which these cells would exist in vivo), most tissues have undetectable levels of BNIP3 but activate transcription during hypoxia through a 5′ promoter of HIF-1 binding site (49). Although hypoxia is regarded as the major physiological inducer of BNIP3 expression, much of the data on BNIP3 have been derived from cellular overexpression experiments performed in the absence of any hypoxic stress (33).
Our data show that isolated primary mouse and rat hepatocytes express little or no BNIP3 under normoxic conditions but upregulate BNIP3 expression significantly after 6 h of exposure to hypoxia. We also confirmed that the effect of NO on BNIP3 is not specific to iNOS-null cells (55). Interestingly, whereas exposure to high concentrations (>100 μM) of the NO donor SNAP induced a concentration-dependent upregulation of BNIP3 mRNA expression under normoxic conditions, the presence of NO under hypoxic conditions significantly inhibited the hypoxia-induced upregulation of BNIP3 mRNA expression. This inhibitory effect was independent of the concentration of the NO donor used. Our findings also suggest that the downregulatory effect of NO on BNIP3 can overcome the hypoxia-inducing effect on this gene. In previous work, we found that the hepatocyte cell death induced by SNAP under hypoxic conditions, which increased the production of reactive oxygen species, was accompanied by a necrotic morphology with a concomitant early decrease in ATP levels. This suggests hypoxia-induced oxidative stress subsequent to ATP depletion can switch NO from a hepatoprotective (under resting conditions) to a hepatotoxic agent (17). Whether NO protects or injures is probably determined by the type of insult, the abundance of reactive oxygen species, the source and amount of NO production, the cellular redox status of the liver (9), and, as this present study points out, the effect of NO on regulation of cell death-related genes such as BNIP3.
BNIP3 migrates on SDS-PAGE as a major band of 60 kDa and a minor band of 30 kDa when transiently expressed. However, as reported in one of our prior studies (55), we routinely find that the two bands are not always expressed to the same extent. This finding is consistent with those of other groups, who have reported similar observations in certain epithelial tumors in which the extent of upregulation of the 60-kDa band does not parallel that of the 30-kDa protein (41). More recently, however, Kubli et al. (23) found that most of endogenous BNIP3 does not exist as a DTT-sensitive homodimer under normal conditions in the heart. They further reported that the homodimer increases in hearts subjected to I/R as well as in isolated cardiac myocytes exposed to simulated I/R or hydrogen peroxide treatment (23). We note that we cannot exclude other factors such as differences in sample processing, time of sample collection, and differences between the commercially available antibodies that may explain this issue.
BNIP3 was recently found to be primarily localized to the nucleus of glial cells of the normal human brain, as well as in the malignant glioma cell line U251. Upon exposure of U251 cells to hypoxia, BNIP3 expression in the cytoplasm increased and localized with the mitochondria, contributing to induction of cell death (6). The same study showed that when BNIP3 is forcibly overexpressed in the nucleus, it fails to induce cell death. Another study also showed that prolonged intranuclear BNIP3 immunoreactivity in striatal and cortical neurons after transient focal ischemia in rats was associated with delayed neuronal death. These findings raise the possibility that BNIP3 enters the nucleus and could interact with other proteins involved in DNA structure, transcription, or mRNA splicing after focal brain ischemia (1). The mechanism through which BNIP3 localizes to the nucleus is as yet undetermined but may be selected for if it confers survival benefits under hypoxia (33). BNIP3 protein does not contain any of the well-known nuclear localization signals. To our knowledge, only one study has suggested the presence of a cryptic nuclear localization signal, a degenerate sequence that may be involved in the nuclear targeting of BNIP3 (studied in this case in rat brain) (38). These studies in various cell types, as well as ours in hepatocytes, led us to hypothesize that not only the differential expression of BNIP3 during cellular stress but also the exact localization and trafficking of BNIP3 dictate the role of this protein in hepatocyte cell death in vitro and liver damage in vivo. In this respect, we found that in normoxic conditions, BNIP3 was mostly localized to the nucleus with some cytoplasmic staining in primary mouse hepatocytes, both wild type and iNOS null. Hypoxia, however, not only upregulated the mRNA and protein expression but also induced a translocation of BNIP3 protein from the nucleus to the cytoplasm (lack of nuclear staining), pointing to an altered permeability of the nuclear membrane after ischemia, which could further result in abnormal regulation of gene expression that in turn may contribute to cell death. Upon reoxygenation, however, protein levels decreased significantly, probably due to the rapid protein turnover and degradation (8, 55), and BNIP3 was found again in the nucleus with almost no cytoplasmic staining. As expected, hypoxia treatment did not result in a significant loss of viability after 6 h, as demonstrated by LDH measurement, a soluble cytosolic enzyme that is released into the culture medium following loss of membrane integrity, and therefore can be used as an indicator of cell injury. After reoxygenation, however, a significant increase in LDH activity was observed, indicative of cell death. Lack of cytoplasmic BNIP3 staining in hypoxia-reoxygenation indicates that BNIP3 upregulation and translocation occurs during the hypoxia treatment and that those changes precede damage as shown by the LDH release. These findings support our hypothesis that hypoxia-induced translocation of BNIP3 from the nucleus to cytoplasmic organelles, e.g., mitochondria, renders cells more susceptible to a secondary stimuli or other dangerous signals, especially those related to the mitochondrial cell death signaling. We attempted to prevent the translocation of BNIP3 from the nucleus by using ethacrynic acid and dl-sulforaphane, two compounds shown to inhibit relocalization of nuclear HMGB1 into the cytoplasm of LPS-stimulated RAW 264.7 cells (16), but did not see any change by immunofluorescence (data not shown).
Hypoxia increases the expression of BNIP3 through the transcription factor HIF-1, but despite a considerable number of investigations, it has proven difficult to establish a clear role for BNIP3 in the cellular hypoxic response (33). Our studies employing siRNA technology to reduce the expression of BNIP3 resulted in a significant reduction in LDH release. These studies provide evidence for a role of BNIP3 in chronic, hypoxia-induced hepatocyte injury, most likely a necrotic type of cell death. Although hypoxia-induced autophagy has also been associated with BNIP3 expression (45)(3), a more recent publication has shown that hypoxia signals autophagy in tumor cells independently of BNIP3 (36). Certainly, whether BNIP3 is involved in hypoxia-induced autophagy in hepatocytes warrants further investigation, as does the possibility that nonhypoxic but proinflammatory stresses that result in autophagy also involve BNIP3.
Under low-oxygen conditions, HIF-1 is the major mediator of increased BNIP3 expression (33). The BNIP3 promoter contains a functional HIF-1-responsive element (HRE) and is potently activated by both hypoxia and forced expression of HIF-1α (5). To our surprise, in a recent study we were unable to detect nuclear induction of HIF-1α protein in hypoxic primary rat hepatocytes, suggesting that although hepatocytes do respond to hypoxia, the contribution of HIF-1α to this adaptation may be minor or transient at best, probably due to its translocation to peroxisomes rather than to the nucleus in hypoxia (15). On the other hand, we did observe a nuclear induction of HIF-2α in hepatocytes, but this was only following reoxygenation experiments (15). Interestingly, and in contrast to HIF-1α, the HIF-2α isoforms have been reported to negatively regulate BNIP3 expression (33), raising the intriguing possibility that hypoxia-reoxygenation-induced nuclear HIF-2α also contributes to the dramatic decrease of BNIP3 levels following reoxygenation. In addition, we have previously observed basal levels of HIF-3α in the nuclei of normoxic hepatocytes that shifted out of the nucleus in hypoxia and, unlike HIF-2α, did colocalize with the peroxisomal enzyme catalase, suggesting a similar targeting mechanism as HIF-1α (15). Whether HIF-3α is involved in the regulation of BNIP3 in hepatocytes or in any other cell type has not yet been reported, and it represents another interesting element in the complex relationship between BNIP3 and the HIF family of proteins.
The involvement (or lack thereof) of MAPK signaling in the regulation of BNIP3 expression has not been completely elucidated. An et al. (2) have previously shown that whereas U0126, a specific MEK inhibitor, completely abolished BNIP3 expression and the stimulation of promoter activity by NO and Ras in macrophages, the specific inhibitor of p38 MAPK SB 203580 had no effect. In contrast, the same selective p38 MAPK inhibitor blocked the activation of both the p38 MAPK and the HRE promoter necessary for nuclear accumulation of HIF-1α and BNIP3 gene transcription following exposure to cyanide in an immortalized dopaminergic cell line (56). Our results showing that the p38 MAPK inhibitor SB 203580 blocked the hypoxia-mediated upregulation of BNIP3 suggest that activation of the p38 MAPK pathway, but not the JNK pathway, is necessary for BNIP3 protein upregulation under hypoxic conditions. These findings support our previous observations of a role for MAPKs in hypoxia in vitro and HS in vivo (31, 32, 34).
The response to trauma/hemorrhagic shock, in which the liver is a primary organ affected, is complex and multifaceted (37). The upregulation of BNIP3 in the liver of mice subjected to HS suggests that this protein may participate in the hepatic damage associated with inflammatory conditions in which hypoxia plays a significant role. In the liver I/R injury model, ischemia leads to upregulation of BNIP3 protein expression in the liver compared with sham-operated animals, supporting our hypothesis that hypoxia is partly responsible for the upregulation of BNIP3 in the liver in vivo. However, it is likely that the BNIP3 response also occurs in settings of nonhypoxic, proinflammatory stress. In support of that alternative hypothesis, we cite our present results showing an elevation of BNIP3 in surgically cannulated but nonhemorrhaged animals, as well as unpublished data from our group showing a 3.5-fold upregulation of BNIP3 mRNA in livers from C57BL/6 mice subjected to a more severe stress (bone fracture ± 1.5 h of HS) vs. nonmanipulated animals (B Edmonds, G Tseng, C Lagoa, Y Vodovotz, R Zamora, TR Billiar, unpublished observations).
In summary, our study shows that BNIP3 is not only a mitochondrial protein but is present in the nucleus during normoxic conditions. Under hypoxic stress, however, not only are BNIP3 mRNA and protein expression upregulated, but also the subcellular localization of BNIP3 protein changes dramatically. We also showed that upregulation of BNIP3 requires p38 MAPK activation and that it contributes to hypoxic injury in hepatocytes. Hepatic BNIP3 is also upregulated in two different models of inflammatory liver stress in vivo, and therefore we suggest that BNIP3 is part of a general response to proinflammatory stress that warrants further study.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant P50-GM-53789-09 (to T. R. Billiar).
Acknowledgments
We thank Binnie Betten, Derek Barclay, and Hemamalini Vedagiri for technical assistance with Northern and Western blotting techniques, Carol Meiers for isolation and preparation of primary hepatocyte cultures, and Dr. Xiaoying Zhang for helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Althaus J, Bernaudin M, Petit E, Toutain J, Touzani O, Rami A. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int 48: 687–695, 2006. [DOI] [PubMed] [Google Scholar]
- 2.An HJ, Maeng O, Kang KH, Lee JO, Kim YS, Paik SG, Lee H. Activation of Ras up-regulates pro-apoptotic BNIP3 in nitric oxide-induced cell death. J Biol Chem 281: 33939–33948, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Azad MB, Chen Y, Henson ES, Cizeau J, Millan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4: 195–204, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, Elangovan B, D'Sa-Eipper C, Chinnadurai G. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell 79: 341–351, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Bruick RK Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97: 9082–9087, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton TR, Henson ES, Baijal P, Eisenstat DD, Gibson SB. The pro-cell death Bcl-2 family member, BNIP3, is localized to the nucleus of human glial cells: implications for glioblastoma multiforme tumor cell survival under hypoxia. Int J Cancer 118: 1660–1669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Cizeau J, Vande VC, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem 274: 7–10, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Ray R, Dubik D, Shi L, Cizeau J, Bleackley RC, Saxena S, Gietz RD, Greenberg AH. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med 186: 1975–1983, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Curr Mol Med 3: 519–526, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun 282: 1075–1079, 2001. [DOI] [PubMed] [Google Scholar]
- 11.De Angelis PM, Fjell B, Kravik KL, Haug T, Tunheim SH, Reichelt W, Beigi M, Clausen OP, Galteland E, Stokke T. Molecular characterizations of derivatives of HCT116 colorectal cancer cells that are resistant to the chemotherapeutic agent 5-fluorouracil. Int J Oncol 24: 1279–1288, 2004. [PubMed] [Google Scholar]
- 12.Demple B Genetic responses against nitric oxide toxicity. Braz J Med Biol Res 32: 1417–1427, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, Harris AL. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res 10: 5566–5571, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M, Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ 8: 367–376, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Khan Z, Michalopoulos GK, Stolz DB. Peroxisomal localization of hypoxia-inducible factors and hypoxia-inducible factor regulatory hydroxylases in primary rat hepatocytes exposed to hypoxia-reoxygenation. Am J Pathol 169: 1251–1269, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killeen ME, Englert JA, Stolz DB, Song M, Han Y, Delude RL, Kellum JA, Fink MP. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J Pharmacol Exp Ther 316: 1070–1079, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kim PK, Vallabhaneni R, Zuckerbraun BS, McCloskey C, Vodovotz Y, Billiar TR. Hypoxia renders hepatocytes susceptible to cell death by nitric oxide. Cell Mol Biol (Noisy -le-grand) 51: 329–335, 2005. [PubMed] [Google Scholar]
- 18.Kim PK, Zamora R, Petrosko P, Billiar TR. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol 1: 1421–1441, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kim PKM, Zuckerbraun BS, Otterbein LE, Vodovotz Y, Billiar TR. 'Til death do us part: nitric oxide and mechanisms of hepatotoxicity. Biol Chem 365: 11–15, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272: 31138–31148, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kothari S, Cizeau J, McMillan-Ward E, Israels SJ, Bailes M, Ens K, Kirshenbaum LA, Gibson SB. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene 22: 4734–4744, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA 99: 12825–12830, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 295: H2025–H2031, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Paik SG. Regulation of BNIP3 in normal and cancer cells. Mol Cells 21: 1–6, 2006. [PubMed] [Google Scholar]
- 25.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Bombeck CA, Yang S, Kim YM, Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem 274: 17325–17333, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Macmicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81: 641–650, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood 80: 1880–1884, 1992. [PubMed] [Google Scholar]
- 29.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J 14: 1889–1900, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Matsushima M, Fujiwara T, Takahashi E, Minaguchi T, Eguchi Y, Tsujimoto Y, Suzumori K, Nakamura Y. Isolation, mapping, and functional analysis of a novel human cDNA (BNIP3L) encoding a protein homologous to human NIP3. Genes Chromosomes Cancer 21: 230–235, 1998. [PubMed] [Google Scholar]
- 31.McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR. Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock 22: 380–386, 2004. [DOI] [PubMed] [Google Scholar]
- 32.McCloskey CA, Zuckerbraun BS, Gallo DJ, Vodovotz Y, Billiar TR. A role for angiotensin II in the activation of extracellular signal-regulated kinases in the liver during hemorrhagic shock. Shock 20: 316–319, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Mellor HR, Harris AL. The role of the hypoxia-inducible BH3-only proteins BNIP3 and BNIP3L in cancer. Cancer Metastasis Rev 26: 553–566, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mollen KP, McCloskey CA, Tanaka H, Prince JM, Levy RM, Zuckerbraun BS, Billiar TR. Hypoxia activates c-Jun N-terminal kinase via Rac1-dependent reactive oxygen species production in hepatocytes. Shock 28: 270–277, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Ohi N, Tokunaga A, Tsunoda H, Nakano K, Haraguchi K, Oda K, Motoyama N, Nakajima T. A novel adenovirus E1B19K-binding protein B5 inhibits apoptosis induced by Nip3 by forming a heterodimer through the C-terminal hydrophobic region. Cell Death Differ 6: 314–325, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ 15: 1572–1581, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg 32: 925–1002, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Kastner R, Guirre-Chen C, Kietzmann T, Saul I, Busto R, Ginsberg MD. Nuclear localization of the hypoxia-regulated pro-apoptotic protein BNIP3 after global brain ischemia in the rat hippocampus. Brain Res 1001: 133–142, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Seglen PO Preparation of isolated rat liver cells. Methods Cell Biol 13: 29–83, 1976. [DOI] [PubMed] [Google Scholar]
- 40.Sowter HM, Ferguson M, Pym C, Watson P, Fox SB, Han C, Harris AL. Expression of the cell death genes BNip3 and NIX in ductal carcinoma in situ of the breast; correlation of BNip3 levels with necrosis and grade. J Pathol 201: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61: 6669–6673, 2001. [PubMed] [Google Scholar]
- 42.Stolz DB, Zamora R, Vodovotz Y, Loughran PA, Billiar TR, Kim YM, Simmons RL, Watkins SC. Peroxisomal localization of inducible nitric oxide synthase in hepatocytes. Hepatology 36: 81–93, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Taylor BS, Billiar TR, Geller DA. Regulation and function of nitric oxide in the liver. In: Recent Advances in Nitric Oxide Research. Tokyo, Japan: Springer, 1999, p. 109–137.
- 44.Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, Namas R, Constantine G, Zamora R, Puyana JC, Vodovotz Y. Mathematical modeling of post-hemorrhage inflammation in mice: studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock. In press. [DOI] [PubMed]
- 45.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27: 6229–6242, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201: 1135–1143, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turpaev K, Bouton C, Diet A, Glatigny A, Drapier JC. Analysis of differentially expressed genes in nitric oxide-exposed human monocytic cells. Free Radic Biol Med 38: 1392–1400, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Vande VC, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 20: 5454–5468, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster KA, Graham RM, Bishopric NH. BNip3 and signal-specific programmed death in the heart. J Mol Cell Cardiol 38: 35–45, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Yasuda M, Han JW, Dionne CA, Boyd JM, Chinnadurai G. BNIP3alpha: a human homolog of mitochondrial proapoptotic protein BNIP3. Cancer Res 59: 533–537, 1999. [PubMed] [Google Scholar]
- 51.Yook YH, Kang KH, Maeng O, Kim TR, Lee JO, Kang KI, Kim YS, Paik SG, Lee H. Nitric oxide induces BNIP3 expression that causes cell death in macrophages. Biochem Biophys Res Commun 321: 298–305, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Zamora R, Alarcon L, Vodovotz Y, Betten B, Kim PK, Gibson KF, Billiar TR. Nitric oxide suppresses the expression of Bcl-2 binding protein BNIP3 in hepatocytes. J Biol Chem 276: 46887–46895, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Zamora R, Vodovotz Y, Alarcon L, Betten B, Loughran PA, Aulak KS, Stuehr DJ, Gibson KF, Billiar TR. Nitric oxide from the inducible nitric oxide synthase (iNOS) increases the expression of cytochrome P450 2E1 in iNOS-null hepatocytes in the absence of inflammatory stimuli. Arch Biochem Biophys 390: 287–294, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Zamora R, Vodovotz Y, Aulak KS, Kim PK, Kane JM, III, Alarcon L, Stuehr DJ, Billiar TR. A DNA microarray study of nitric oxide-induced genes in mouse hepatocytes: implications for hepatic heme oxygenase-1 expression in ischemia/reperfusion. Nitric Oxide 7: 165–186, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Zamora R, Vodovotz Y, Betten B, Wong C, Zuckerbraun B, Gibson KF, Ford HR. Intestinal and hepatic expression of BNIP3 in necrotizing enterocolitis: regulation by nitric oxide and peroxynitrite. Am J Physiol Gastrointest Liver Physiol 289: G822–G830, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Li L, Liu H, Prabhakaran K, Zhang X, Borowitz JL, Isom GE. HIF-1alpha activation by a redox-sensitive pathway mediates cyanide-induced BNIP3 upregulation and mitochondrial-dependent cell death. Free Radic Biol Med 43: 117–127, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]