Abstract
The crucial pathology underlying progressive chronic kidney disease in diabetes is tubulointerstitial fibrosis. Central to this process is epithelial-mesenchymal transformation (EMT) of proximal tubular epithelial cells driven by maladaptive transforming growth factor-β1 (TGF-β1) signaling. Novel signaling roles for C-peptide have recently been discovered with evidence emerging that C-peptide may mitigate microvascular complications of diabetes. We studied the potential for C-peptide to interrupt injurious TGF-β1 signaling pathways and thus block development of EMT in HK2 human kidney proximal tubular cells. Cells were incubated with TGF-β1 either alone or with C-peptide in low or high glucose. Changes in cell morphology, TGF-β1 receptor expression, vimentin, E-cadherin, and phosphorylated Smads were assessed. Luciferase reporters were used to assess Smad activity. The cytoskeleton was visualized by TRITC-phalloidin staining. The typical TGF-β1-stimulated, EMT-associated morphological alterations of proximal tubular cells, including increased vimentin expression, decreased E-cadherin expression, and cytoskeletal rearrangements, were prevented by C-peptide treatment. C-peptide also blocked TGF-β1-induced upregulation of expression of both type I and type II TGF-β1 receptors and attenuated TGF-β1-mediated Smad phosphorylation and Smad transcriptional activity. These effects of C-peptide were inhibited by pertussis toxin. The results demonstrate that C-peptide almost completely reversed the morphological changes in PT cells induced by TGF-β1 and suggest a role or C-peptide as a renoprotective agent in diabetic nephropathy.
Keywords: tubulointerstitial fibrosis
following cleavage of proinsulin in the secretory vesicles of pancreatic β cells, C-peptide and insulin are released in equimolar amounts (38). Until recently C-peptide has been regarded as a biologically inert molecule. However, over the past 10 years, increasing evidence has accumulated supporting important cellular and physiological effects of C-peptide (48). Multiple signaling effects of C-peptide have now been described in several cell types, and, based on in vivo studies, a potential role for C-peptide in alleviating the microvascular complications of type 1 diabetes has been proposed (49).
In vitro exposure of kidney proximal tubular cells (PTC) to picomolar/low-nanomolar concentrations of C-peptide elicits activation of extracellular signal-regulated kinase, phosphatidylinositol 3-kinase, protein kinase C, elevations of intracellular calcium, and stimulation of the transcription factors NF-κB and peroxisome proliferator-activated receptor-γ (1, 2). Replacement of C-peptide in animal models of diabetes and patients with type I diabetes ameliorates a number of the structural and functional disturbances associated with uncontrolled hyperglycemia that lead to the development and progression of nephropathy (17, 39, 43). These include abrogation of glomerular hyperfiltration (39), reduced microalbuminuria (24), decreased mesangial expansion (40), and increased endothelial nitric oxide synthase (eNOS) levels (41, 50).
Diabetic nephropathy is a leading worldwide cause of chronic kidney disease (CKD) and end-stage renal disease. The crucial pathology underlying progressive CKD in diabetes is tubulointerstitial fibrosis (11, 34). Important in this process is epithelial-mesenchymal cell transformation (EMT), the transdifferentiation of tubular epithelial cells into myofibroblasts (19, 26). This complex process involves loss of cell integrity and decreased expression of proteins critical to intercellular junctional complex formation with alterations in cell morphology, reorganization of the cell cytoskeleton, and de novo expression of fibroblastic markers (3, 25). EMT of PTC has been clearly documented in diabetic nephropathy (5, 27).
Overwhelming evidence implicates transforming growth factor-β1 (TGF-β1) as the predominant factor mediating PTC phenotypic changes and fibrosis in diabetic nephropathy (22, 42). Production of TGF-β1 by PTC in diabetes is stimulated in part by high glucose and advanced glycation end products (27, 32). In the proximal tubule, TGF-β1 is a key mediator of EMT and modulates the expression of several epithelial cell recognition and organizational proteins, including the cadherins (44), catenins, and the actin cytoskeleton (18).
TGF-β1 signals through cell surface serine-threonine kinase type I and II receptors. Binding of TGF-β1 to its type II receptor (TβRII) results in recruitment of the type I receptor (TβRI) to form a heteromeric complex. The TβRI kinase domain is then phosphorylated by TβRII, and this in turn phosphorylates downstream Smad proteins, which eventually translocate to the nucleus (8). In the nucleus, Smads may regulate the transcription of target genes by either binding directly to DNA and functioning as transcriptional activators (9), or by associating with nuclear transcription factors such as AP-1. In many cell lines, TGF-β1 is capable of positively regulating its own expression (46), where autoinduction of TGF-β1 transcription is mediated through binding of an AP-1 complex to the TGF-β1 promoter. This autoinduction may be responsible for the pathological induction of TGF-β1 that is associated with fibrosis of the kidney.
Blockade of the deleterious consequences of TGF-β1-induced PTC phenotypic changes is a key therapeutic target for the treatment of renal fibrosis in diabetic nephropathy. In mouse podocytes, C-peptide blocks TGF-β1-induced production of collagen and plasminogen-activated inhibitor-1 (24). Stimulated by this finding and our previous observations of multiple effects of C-peptide in kidney PTC, we hypothesized that C-peptide deficiency may contribute to the development of high glucose and TGF-β1-induced kidney PT phenotypic changes in type 1 diabetic nephropathy. Using the human PTC line HK-2, we studied the influence of C-peptide on TGF-β1 signaling elements and their downstream effects on cell phenotypic alterations. The results demonstrate that C-peptide was able to almost completely reverse the adverse morphological changes in PTC induced by TGF-β1.
MATERIALS AND METHODS
Materials.
Human 31-amino acid C-peptide and a 31-amino acid scrambled C-peptide (ScC-peptide) were provided by Dr. John Wahren (Karolinska Institute, Stockholm, Sweden). Recombinant human TGF-β1 was from Sigma (Poole, UK). Tissue culture media and plastic ware were from Invitrogen Life Technologies (Paisley, UK). Pertussis toxin (PTX) was obtained from Calbiochem (Nottingham, UK). Luciferase assays were performed using the LucLite assay system and LumiCount luminometer (Packard, Pangbourne, UK). β-Galactosidase assay kits were from Promega (Madison, WI). Fugene-6 transfection agent was from Roche Diagnostics. Immobilon P membranes (Millipore, Watford, UK), ECL detection reagents (Amersham Biosciences), anti-fade Citifluor (glycerol/PBS solution: Agar Scientific) were obtained. Antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), R&D Systems (Abingdon, UK), and Affinity Bioreagents (Cambridge, UK). All other laboratory chemicals and reagents were from Sigma.
Cell culture.
HK-2 cells (passages 18-30) were maintained in DMEM/Ham's F12 (DMEM/F12) medium, supplemented with 10% FCS, glutamine (2 mM), penicillin (100 IU/ml), and streptomycin (100 μg/ml). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air. Before treatment, cells were cultured in DMEM/F12 low glucose (5 mM) for 48 h. Basal (5 mM) glucose culture media was generated as described previously (15). We wished to mimic both healthy control and hyperglycaemic diabetic conditions in vitro. Therefore, for both TGF-β1 and C-peptide experiments, cells were cultured in either low (5 mM) or high (25 mM) glucose containing unsupplemented DMEM/F12 for 48 h. Cells were treated with either TGF-β1 (2 ng/ml) with or without C-peptide or ScC-peptide and pretreated with PTX (100 ng/ml) for 18 h. In all experiments, cells were serum starved overnight before agonist stimulation.
Luciferase reporter assay.
Cells were transiently transfected using Lipofectamine with either pMF1 and pARE-luc to measure activation of Smad2 activity (12), or pCAGA-luc to measure Smad3 activity (plasmids kindly provided by Dr. Donald Fraser, University of Cardiff, Cardiff, Wales) (30). In all studies, cells were cotransfected with pSV-βgal to normalize for transfection efficiency. Briefly, 2.5 × 104 cells/well were plated in 24-well plates and were grown to 50% confluence in DMEM/F12 (5 mM glucose). After transfection, the cells were incubated in 1 ml serum-free medium overnight before agonist stimulation in DMEM/F12 containing either low or high glucose. In some experiments, inhibitors were applied to cells 30 min before the addition of agonists, and the cells were subsequently incubated with agonists ± inhibitors for an additional 48 h. The medium was removed, and cells were lysed in a buffer containing 500 mM HEPES, 2% Triton X-100, 1 mM CaCl2, and 1 mM MgCl2 (pH 7.8). Cell lysis was allowed to proceed for 10 min, and luciferase activity in lysates was measured. In all experiments, a 50-μl aliquot of lysate was removed for β-galactosidase assay, and luciferase activity was normalized to β-galactosidase content.
Immunoblotting.
Cytosolic proteins were prepared and separated by gel electrophoresis and electroblotting onto Immobilon P membranes as described previously (4). Membranes were probed with specific polyclonal antibodies against human TβR1, TβRII (Santa Cruz Biotechnology), phospho-Smad3 (p-Smad3), and total Smad3 (AbCam, Cambridge, UK), E-cadherin (R&D Systems), and anti-human vimentin (Affinity Bioreagents, Golden, CO) at dilutions of 1:400, 1:400, 1:800, 1:500, 1:500, and 1:400, respectively. After three 10-min washes in PBS-Tween 20 (0.1%; PBS-T), the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies diluted up to 1:40,000 in PBS-T (0.05% Tween) for 60 min at 25°C followed by three 10-min washes in PBS-T (0.1%). Specific proteins were detected using ECL detection reagents and were visualized after exposure of membranes to X-ray film. All blots were stripped and probed with anti-GAPDH (Biogenesis) diluted at 1:20,000 to control for protein loading in subsequent densitometric analysis of expression levels.
Immunocytochemistry.
Cells at 80% confluence on coverslips were fixed with 4% paraformaldehyde (PFA). Nonspecific binding was blocked for 1 h at 25°C with 10% goat serum in PBS-Triton X-100 (0.01%). Following three 10-min washes with PBS, the nuclear stain 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; 1 mM) was added to each coverslip for 3 min. After three further 10-min washes, cells were incubated with TRITC-conjugated phalloidin diluted at 1:100 in PBS-Triton X-100 (0.01%) for 1 h at 25°C or incubated overnight at 4°C with primary antibody (1:100) diluted in 0.01% PBS-Triton X-100. Following antibody incubation, cells were washed with PBS and then incubated in Alexa 488-conjugated secondary antibody for 1 h at 25°C in the dark. Secondary antibodies were diluted (1:400) in PBS-Triton X-100 (0.01%). Cells were washed for 3 × 10 min, and coverslips were mounted in anti-fade Citifluor (glycerol/PBS solution) on glass slides. Immunofluorescence was visualized using an Axiovert 200 Research Inverted fluorescence microscope (Carl Zeiss, Welwyn Garden City, UK).
Data analysis.
Autoradiographs were quantified by densitometry using TotalLab 2003 (NonLinear Dynamics, Durham, NC). In all experiments where data was quantified, the nonstimulated, low-glucose control condition was normalized to 100%, and data from all other experimental conditions were compared with this. Statistical analysis of data was performed using one-way ANOVA with a Tukey's multiple comparison posttest. Data are expressed as means ± SE, and n denotes the number of experiments. P < 0.05 was taken to signify statistical significance.
RESULTS
TGF-β1 induces morphological changes in HK-2 cells.
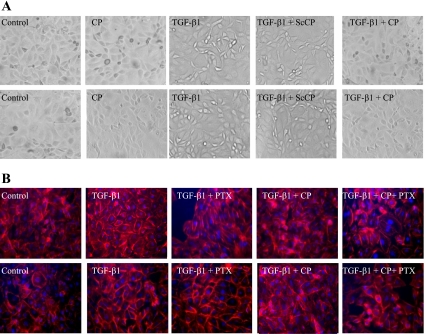
To assess the effect of TGF-β1 on cell morphology, HK-2 cells were serum deprived for 24 h and then treated with TGF-β1 for 2 days at both low and high glucose. Phase-contrast microscopy revealed no differences between cells in low or high glucose alone, but TGF-β1 induced an elongated, fibroblast-like phenotype at both glucose concentrations (Fig. 1A). Coapplication of C-peptide with TGF-β1 prevented these morphological changes, whereas ScC-peptide had no such effect. Addition of C-peptide alone to cells under low- and high-glucose control conditions had no effect on cell morphology.
Fig. 1.
C-peptide reverses transforming growth factor (TGF)-β1-induced morphological and cytoskeletal changes in HK-2 cells. For assessment of HK-2 morphology, cells were examined by phase-contrast microscopy. HK-2 cells were grown under low-glucose conditions or high-glucose conditions. Cells were either unstimulated or stimulated with 5 nm C-peptide (CP) alone, 2 ng/nl TGF-β1 alone, or cotreated with 2 ng/ml TGF-β1 and 5 nM scrambled C-peptide (ScCP), or 2 ng/ml TGF-β1 and 5 nM CP (A). For assessment of the cytoskeleton, cells were stained with TRITC-phalloidin, counterstained with DAPI, and examined by immunofluorescence microscopy (B). Cells were either unstimulated or stimulated with 2 ng/nl TGF-β1 alone or cotreated with 2 ng/ml TGF-β1 and Pertussis toxin (PTX), 2 ng/ml TGF-β1 and 5 nM CP or 2 ng/ml TGF-β1, 5 nM C-peptide, and PTX. Images representative of ≥3 individual experiments are shown (magnification ×20).
Phalloidin staining, which detects filamentous actin, showed loose cytoplasmic organization of actin under control conditions with both low- and high-glucose concentrations. TGF-β1 treatment resulted in a generalized increase in actin stress fibers and an accumulation of actin around the periphery of the cells. This TGF-β1 effect was largely reversed by C-peptide (Fig. 1B). When cells were preincubated with PTX, the protective effect of C-peptide on TGF-β- induced changes in cell morphology was attenuated, although PTX had no effect on the TGF-β1-mediated changes themselves (Fig. 1B).
C-peptide reverses TGF-β1-induced effects on expression of epithelial and mesenchymal markers E-cadherin and vimentin, respectively.
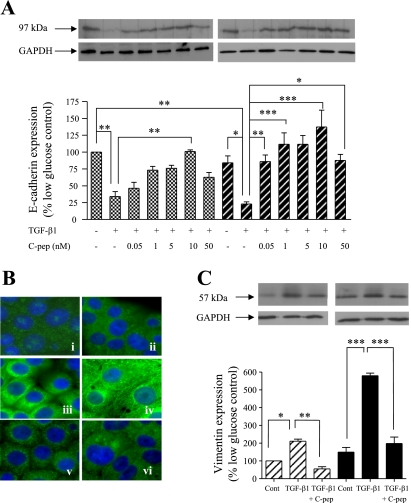
To confirm the transformation to a fibroblast-like phenotype, the expression of E-cadherin as an epithelial marker was examined (Fig. 2A) (47). Exposure of cells to high glucose resulted in a nonstatistically significant reduction in E-cadherin. Under both low- and high-glucose conditions TGF-β1 decreased E-cadherin expression to 34 ± 7 and 23 ± 6% of low-glucose control, respectively. Coapplication of C-peptide concentration dependently attenuated this TGF-β1-induced reduction of E-cadherin expression, maximally at 5–10 nM, when the TGF-β1 effect was completely inhibited.
Fig. 2.
C-peptide reverses TGF-β1-induced changes in E-cadherin and vimentin expression. A: HK-2 cells were grown in low or high glucose alone, or together either with 2 ng/ml TGF-β1 alone or 2 ng/ml TGF-β1 and the indicated concentrations of C-peptide (C-pep), and cell levels of E-cadherin were determined by immunoblotting. Top: representative immunoblots [low glucose (left), high glucose (right)] showing E-cadherin expression (top blots) or the same blots stripped and reprobed for GAPDH as a loading control (bottom blots). Blots were quantified by densitometry, and the nonstimulated low-glucose control condition was normalized to 100% and all other conditions were compared with this. Bottom: results of densitometry of ≥3 blots. Each bar in the histogram represents the same condition in the blots above. Cross-hatched bars, low glucose; hatched bars, high glucose. *P < 0.05, **P < 0.01, ***P < 0.001. B: vimentin localization and expression were studied. HK-2 cells were cultured in low glucose (i, iii, v) or high glucose (ii, iv, vi) and immunostained for vimentin and visualized by fluorescent microscopy. In some experiments, cells were treated with 2 ng/ml TGF-β1 alone (iii, iv) or 2 ng/ml TGF-β1 and 5 nM C-peptide (v, vi). Images representative of ≥3 individual experiments are shown (magnification ×40). C: vimentin expression by immunoblotting. HK-2 cells were grown in low or high glucose alone, or together with either 2 ng/ml TGFβ-1 alone or 2 ng/ml TGFβ-1 and 5 nM C-peptide, and cell levels of vimentin were determined by immunoblotting. Top: representative immunoblots [low glucose (left), high glucose (right)] showing vimentin expression (top blots) or the same blots stripped and reprobed for GAPDH as a loading control (bottom blots). Blots were quantified by densitometry, and the nonstimulated low-glucose control condition was normalized to 100% and all other conditions were compared with this. Bottom: results of densitometry of ≥3 blots. Each bar in the histogram represents the same condition in the blots above. Hatched bars, low glucose; filled bars, high glucose. *P < 0.05, **P < 0.01, ***P < 0.001.
Vimentin is an intermediate filament protein that, together with microtubules and actin microfilaments, forms the cytoskeleton. Typical of cells with an epithelial phenotype, nonstimulated HK2 cells under either low (Fig. 2Bi)- or high-glucose conditions (Fig. 2Bii) exhibited diffuse cytoplasmic staining, lacking discrete fibers. In response to TGF-β1, a network of vimentin filaments spanning the breadth of the cell and establishing cell-to-cell contacts with neighboring cells was observed (Fig. 2B, iii and iv). Application of C-peptide completely blocked TGF-β1-induced vimentin fiber formation and restored the localization pattern to that seen under control conditions (Fig. 2B, v and vi).
Vimentin expression in HK-2 cells was assessed and quantified by immunoblotting and densitometry (Fig. 2C). When incubated in high-glucose medium, HK-2 cell vimentin expression was modestly increased compared with cells cultured in low glucose. However, TGF-β1 increased vimentin expression to 211 ± 11.3% of control in low glucose whereas in high glucose an increase of 579 ± 14% compared with low-glucose control was observed. Coapplication of C-peptide with TGF-β1 dramatically reduced expression vimentin to 55.1 ± 12.1% (low glucose) and 198 ± 36.4% (high glucose) of low-glucose control conditions.
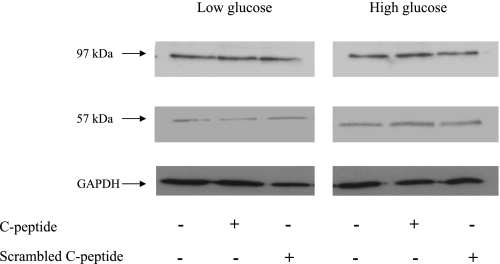
In all of these studies, the C-peptide effect was blocked by PTX and not reproduced by ScC-peptide (data not shown). Furthermore, the addition of C-peptide alone to cells under low- and high-glucose control conditions had no effect on E-cadherin and vimentin expression levels (Fig. 3).
Fig. 3.
No effect of C-peptide or ScC-peptide alone on expression of E-cadherin or vimentin. HK-2 cells were grown in low or high glucose alone, or together with either 5 nM C-peptide or scrambled C-peptide for 48 h, and immunoblotting was used to determine the effects on E-cadherin and vimentin expression [low glucose (left), high glucose (right)]. The same blots were stripped and reprobed for GAPDH as a loading control.
C-peptide reverses TGF-β1-mediated upregulation of TβRII and TβRI.
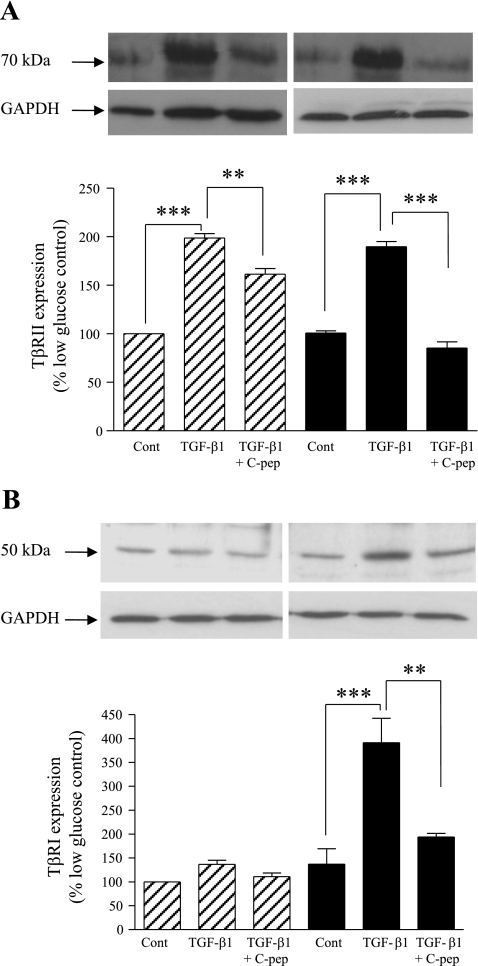
We next examined elements of the TGF-β1 signaling pathway potentially involved in these PTC phenotypic alterations. Binding of TGF-β1 to the TβRII receptor triggers its association with TβRI and enables phosphorylation and activation of the type I receptor. When incubated with TGF-β1 in the presence of either low or high glucose for 48 h, HK-2 cells demonstrate increased expression of TβRII to 198 ± 4 and 189 ± 5%, respectively, compared with the low-glucose control (Fig. 4A). However, coapplication of C-peptide significantly inhibited the TGF-β1-mediated upregulation of TβRII expression to 161 ± 5% under low-glucose conditions and to 85 ± 6% under high-glucose conditions (Fig. 4A).
Fig. 4.
C-peptide inhibits TGF-β1-induced TGF-β1 type II receptor (TβRII) and type I receptor (TβRI) expression. A: TβRII expression by immunoblotting. HK-2 cells were grown in low or high glucose alone, or together with either 2 ng/ml TGF-β1 alone or 2 ng/ml TGF-β1 and 5 nM C-peptide, and cell levels of TβRII were determined by immunoblotting. Top: representative immunoblots [low glucose (left), high glucose (right)] showing TβRII expression (top blots) or the same blots stripped and reprobed for GAPDH as a loading control (bottom blots). Blots were quantified by densitometry, and the nonstimulated low-glucose control condition was normalized to 100% and all other conditions were compared with this. Bottom: results of densitometry of ≥3 blots. Each bar in the histogram represents the same condition in the blots above. Hatched bars, low glucose; filled bars, high glucose. **P < 0.01, ***P < 0.001. B: TβRI expression by immunoblotting. HK-2 cells were grown in low or high glucose alone, or together with either 2 ng/ml TGF-β1 alone or 2 ng/ml TGF-β1 and 5 nM C-peptide, and cell levels of TβRI were determined by immunoblotting. Top: representative immunoblots [low glucose (left), high glucose (right)] showing TβRI expression (top blots) or the same blots stripped and reprobed for GAPDH as a loading control (bottom blots). Blots were quantified by densitometry, and the nonstimulated low-glucose control condition was normalized to 100% and all other conditions were compared with this. Bottom: results of densitometry of ≥3 blots. Each bar in the histogram represents the same condition in the blots above. Hatched bars, low glucose; filled bars, high glucose. **P < 0.01, ***P < 0.001.
TβRI expression was also assessed in HK-2 cells following incubation with TGF-β1 for 48 h. While there was only a modest increase in TβRI to 136 ± 9% at low glucose, high glucose appeared to potentiate the TGF-β1-induced increase in TβR1 expression, with levels rising to 391 ± 51% compared with the low-glucose unstimulated control (Fig. 4B). Coapplication of C-peptide with TGF-β1 almost fully reversed TGF-β1-induced upregulation of TβR1 such that under high-glucose culture conditions expression levels of TβR1 were reduced to 194 ± 7% compared with the low-glucose control.
As described above, in these experiments the inhibitory effects of C-peptide in the face of TGF-β1 were consistently blocked by PTX and not reproduced by ScC-peptide (data not shown).
C-peptide attenuates TGF-β1 signaling via Smad2 and 3.
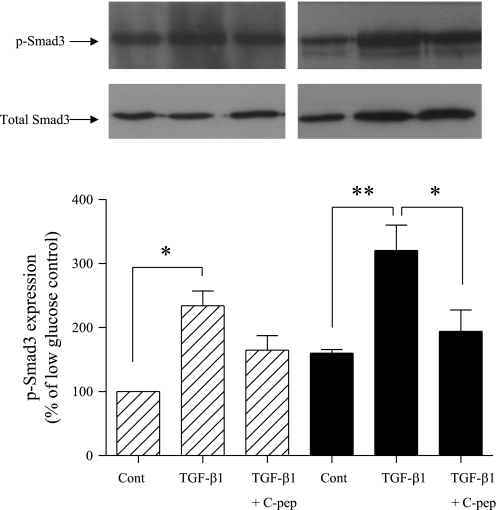
Activation of Smad3 by phosphorylation is central to the fibrotic and EMT response to TGF-β. Exposure of cells to high glucose resulted in significantly increased Smad3 phosphorylation (Fig. 5). TGF-β1-treated HK-2 cells exhibited over a twofold increase in p-Smad3 expression, increasing to 234 ± 23 and 320 ± 39.9% at 5 and 25 mM glucose, respectively, with all values compared with control at low glucose. Coapplication of C-peptide partially inhibited TGF-β1-mediated increases in p-Smad3 expression to only 164.6 ± 22% (low glucose) and 194 ± 33% (high glucose) of control (Fig. 5). All changes in p-Smad3 expression were normalized against expression of total Smad3. All statistical values were compared with control conditions at low (5 mM) glucose.
Fig. 5.
C-peptide attenuates TGF-β1-activated Smad3 phosphorylation. Total Smad3 and pSmad3 expression were determined by immunoblotting in HK-2 cells grown in low or high glucose alone, or together with either 2 ng/ml TGFβ-1 alone or 2 ng/ml TGFβ-1 and 5 nM C-peptide. Top: representative immunoblots [low glucose (left), high glucose (right)] showing pSmad3 expression (top blots) or the same blots stripped and reprobed for total Smad3 as a loading control (bottom blots). Blots were quantified by densitometry, and the nonstimulated low-glucose control condition was normalized to 100% and all other conditions were compared with this. Bottom: results of densitometry of ≥3 blots. Each bar in the histogram represents the same condition in the blots above. Hatched bars, low glucose; filled bars, high glucose. **P < 0.01, *P < 0.05.
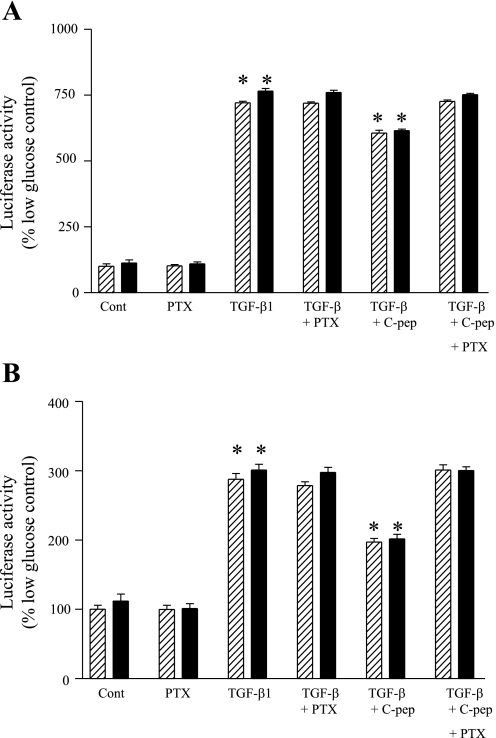
To study Smad3 activation, HK-2 cells were transiently transfected with pCAGA-luc. Culture of cells in high glucose resulted in a significant (P < 0.05) increase in Smad3 activity compared with low-glucose culture. In TGF-β1-treated cells, Smad3 activity increased much further to 721 ± 6 and 765 ± 10.2% compared with controls in low and high glucose, respectively (Fig. 6A). Addition of C-peptide significantly attenuated these increases. Pretreatment of cells with PTX had no effect on TGF-β1-evoked responses but completely blocked the inhibitory effects of C-peptide on TGF-β1 activation of Smad3.
Fig. 6.
Attenuation of TGF-β1-mediated phosphorylation of Smad3 and Smad2 by C-peptide. A: HK-2 cells transiently transfected with GAGA-luc and pSV-βgal for Smad3 were either untreated (control) or treated for 48 h with TGF-β1 or a combination of TGF-β1 and C-peptide in low (hatched bars) or high glucose (filled bars). Cells were lysed, and luciferase activity in lysates was determined and normalized for transfection efficiency using β-galactidose activity. Normalized luciferase activity is expressed as a percentage compared with control (nonstimulated) cells. Values are means ± SE; n = 4 experiments. *P < 0.05. B: HK-2 cells transiently transfected with ARE-luc, MF1, and pSV-βgal for Smad2 were either untreated (control) or treated for 48 h with TGF-β1 or a combination of TGF-β1 and C-peptide in low (hatched bars) or high (filled bars) glucose. Cells were lysed, and luciferase activity in lysates was determined and normalized for transfection efficiency using β-galactidose activity. Normalized luciferase activity is expressed as a percentage compared with control in nonstimulated cells. Values are means ± SE; n = 4 experiments. *P < 0.05.
To assess the impact of C-peptide on TGF-β1-mediated Smad2 activation, HK-2 cells were transiently transfected with the Smad2-responsive reporters pMF1 and pARE-luc. High-glucose culture alone resulted in significantly increased Smad2 activity (P < 0.05) compared with low-glucose culture. In TGF-β-treated cells, Smad2 activity increased to equivalent levels, 287 ± 5 and 297 ± 7% compared with nontreated low-glucose controls, under low- and high-glucose conditions, respectively. C-peptide significantly negated this increase, with activity levels decreasing to 197 ± 5% (low glucose) and 201 ± 6% (high glucose) of control (Fig. 6B). The inhibitory effect of C-peptide on Smad2 activation was itself abolished by pretreatment of cells with PTX, but PTX had no effect on TGF-β1-evoked Smad activation. No effect of ScC-peptide on luciferase activity was seen in any experiments (data not shown).
DISCUSSION
Maladaptive TGF-β1 signaling contributes to a wide variety of disorders, including autoimmune diseases, malignancies, and chronic renal disease (37). In diabetic nephropathy, the profibrotic actions of TGF-β1 are key to the development of proximal tubular atrophy, matrix production, and the replacement of the healthy renal tubulointerstitium by fibrous scarring (53). EMT occurs as a result of PT cell plasticity in the face of various noxious stimuli and represents the process by which these epithelial cells transform to exhibit a mesenchymal phenotype (21, 45). In diabetic nephropathy, EMT occurs in response to TGF-β1 under high prevailing glucose concentrations (40) and contributes to the reciprocal loss of tubular epithelial cells and accumulation of interstitial fibroblasts that correlate closely with declining renal excretory function (33, 52). Characteristic epithelial cell phenotypic changes associated with EMT include morphological alterations with reorganization of the actin cytoskeleton, de novo acquisition of mesenchymal cytoskeletal markers such as α-smooth muscle actin and vimentin, and the downregulation of epithelial adhesion molecules such as E-cadherin and zona occludens protein (ZO-1) (25, 52).
Recent observations of potentially advantageous activation of cell signaling events by C-peptide in kidney cells (1, 2) and protection against deleterious early kidney structural and adaptive changes following C-peptide administration in diabetic nephropathy (17, 39, 43) led us to question whether C-peptide may also protect against EMT in renal PTC. Accordingly, we now demonstrate that C-peptide strikingly inhibits TGF-β1-induced morphological and phenotypic changes in PTC and negatively impinges on TGF-β1 signaling at several levels.
Controversy exists regarding the best markers for the study of EMT. We chose to examine several well-established events typical of EMT in renal epithelial cells, using a PTC line that has been widely used in the study of TGF-β1-induced EMT (28, 31). We elected to use a single 2 ng/ml concentration of TGF-β1 that has been widely used by others in the study of its phenotypic effects, yet lies at the lower end of the pathophysiological range (3, 16, 25). Confirmation that TGF-β1 evoked EMT in HK-2 cells was obtained by assessment of morphological changes, reorganization of the actin cytoskeleton, increased expression of the intermediate filament protein vimentin, and downregulation of the epithelial marker E-cadherin (44). Several of these changes were produced by culture under high-glucose conditions, most likely due to the autoinduction of TGF-β1 production by this PTC line (5, 10, 14, 23). More importantly, however, we present novel findings that C-peptide is able to almost totally inhibit these classic EMT-associated phenotypic and morphological changes in response to elevated levels of TGF-β1 in human cells derived from the proximal tubule. The inhibition of E-cadherin downregulation seen in the current studies was maximal, with a 5- to 10-nM concentration of C-peptide. This dose-response is absolutely in keeping with previous studies of C-peptide (1, 2, 48), and therefore this concentration was used in all other experiments.
TGF-β1 signaling is elicited after binding to the receptors TβRII and TβRI. Both are serine/threonine kinases that form heteromeric complexes after ligand binding to TβRII. Phosphorylation and activation of the TβRI kinase enable recognition and phosphorylation of the receptor-regulated Smads (R-Smads), including Smad2 and Smad3 for TGF-β1. The assembly of a heteromeric complex between the phospho-R-Smad and the co-Smad Smad4 is then followed by nuclear translocation of the heteromeric Smad complex and activation or repression of target genes (6). Abnormalities in TGF-β1 have been discovered in a wide variety of disorders, including autoimmune diseases, malignancies, and chronic renal disease (6). Increased receptor expression has been described in experimental renal disease models including membranous nephropathy, obstructive nephropathy, and diabetic nephropathy (6).
Having demonstrated that C-peptide blocked TGF-β1-evoked changes in the PTC phenotype, we examined the ability of C-peptide to interfere with these TGF-β1 signaling elements. As such, TGF-β1 caused upregulation of TβRI and TβRII expression, phosphorylation of Smad3, and transcriptional activation of Smad2 and 3. Some of these events were evoked by high glucose alone, presumed due to autoinduction of HK-2 TGF-β1 production, but crucially all were blocked to a greater or lesser extent by C-peptide, but not a scrambled C-peptide. That C-peptide appears to block EMT via inhibition of TGF-β1-induced Smad 2 and Smad3 activation is consistent with previous work demonstrating the involvement of these Smads in TGF-β1-mediated fibrosis. Ju et al. (20) demonstrated a requirement for Smad3 but not Smad2 in TGF-β-induced EMT in primary mouse cells, whereas other workers observed that a Smad3-dependent reduction in E-cadherin in human PTC in response to TGF-β stimulation was paralleled by a Smad2-dependent induction of metallomatrix proteinase 2 (29). Studies by Maezawa et al. (24) previously demonstrated a protective effect of C-peptide on early diabetic glomerular changes in response to TGF-β1 in the streptozotocin diabetic mouse and an inhibitory effect of C-peptide on TGF-β1-induced gene expression in a mouse podocyte cell line. These studies further support our novel findings in HK-2 cells and clearly identify C-peptide as an agent with therapeutic potential in diabetic nephropathy.
While the observed effects of C-peptide on TGF-β1 receptors, p-Smad levels, and Smad activation suggest that the salutary effects on cell phenotypes result from interruption of the TGF-β1 signaling cascade at a proximal point, this remains to be precisely identified. However, the PTX sensitivity of these effects indicates that C-peptide is most likely acting via a G protein-coupled receptor (GPCR) and that a consequence of this receptor interaction is inhibitory cross talk with TGF-β1 signaling pathways. High-affinity, specific binding of fluorophore-labeled C-peptide to various cell membranes has been observed using fluorescence correlation microscopy (35). The maximal number of binding sites was found on renal tubular cells. These binding sites were 50% occupied by 0.3 nM C-peptide and fully saturated by 0.9 nM C-peptide. Furthermore, preincubation with PTX significantly inhibited C-peptide binding (35). The bulk of the available data from previous work therefore strongly supports the hypothesis of a GPCR for C-peptide, but its exact identity remains elusive. The PTX sensitivity of the current C-peptide effects in HK-2 cells further corroborates these findings.
Clinical studies of C-peptide have been performed exclusively in patients with type 1 diabetes, and it is easy to envisage how C-peptide replacement may elicit biological effects in the setting of complete deficiency. The situation in type 2 diabetes, developing with insulin resistance and elevated circulating insulin and C-peptide concentrations, is less clear. Certainly, many type 2 diabetic patients develop complications in the face of increased circulating C-peptide levels. However, with the experimentally derived pharmacology of C-peptide-membrane binding in mind, the high ambient levels of C-peptide in type 2 diabetes will likely fully occupy and potentially downregulate any cognate C-peptide receptor. Also, in the majority of individuals with type 1 diabetes, chronic hyperglycemia and its consequences are the only evident causes of nephropathy, whereas in type 2 diabetes nephropathy more typically reflects a combination of heterogeneous renal insults caused by a mixture of mechanisms that may alter and overcome renal responses to hyperglycemia and the features of pure diabetic nephropathy (36, 51). Consequently, commentators suggest that in terms of responses to treatment, type 1 and type 2 diabetic subjects should be considered independently (36, 51). More study of C-peptide (patho)physiology in type 2 diabetes is required to clarify these issues.
Anti-TGF-β1 strategies using neutralizing antibodies have been shown to protect against the development of nephropathy in the db/db mouse and possibly even to some extent reverse established diabetic nephropathy (54). This novel prophylactic or therapeutic approach to diabetic nephropathy by inhibiting TGF-β1 is highly attractive, and the current results suggest that this may be achievable in type 1 diabetes by the administration of C-peptide.
GRANTS
This work was supported by a grant from the Medical Research Council of Great Britain.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-gamma by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. J Biol Chem 279: 49747–49754, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3–1α-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology 140: 2027–2034, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 7: 2484–2494, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Hong SW, Iglesias-de la Cruz MC, Isono M, Casaretto A, Ziyadeh FN. The key role of the transforming growth factor-beta system in the pathogenesis of diabetic nephropathy. Ren Fail 23: 471–481, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MP, Sharma K, Guo J, Eltayeb BO, Ziyadeh FN. The renal TGF-β system in the db/db mouse model of diabetic nephropathy. Exp Nephrol 6: 226–233, 1998. [DOI] [PubMed] [Google Scholar]
- 8.De Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst 92: 1388–1402, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor. EMBO J 17: 3091–3100, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Paolo S, Gesualdo L, Ranieri E, Grandaliano G, Schena FP. High glucose concentration induces overexpression of a transforming growth factor-β through the activation of a platelet derived growth factor loop in human mesangial cells. Am J Pathol 149: 2095–2106, 1996. [PMC free article] [PubMed] [Google Scholar]
- 11.Eddy AA Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 12: 2495–2508, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Felici A, Wurthner JU, Parks WT, Giam LRY, Reiss M, Karpova TS. TLP: a novel modulator of TGF-beta signaling, has opposite effects on Smad2- and Smad3-dependent signaling. EMBO J 22: 4465–4477, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanders KC Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85: 47–64, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser D, Brunskill N, Ito T, Phillips A. Long-term exposure of proximal tubular epithelial cells to glucose induces transforming growth factor-beta 1 synthesis via an autocrine PDGF loop. Am J Pathol 63: 2565–2574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hills CE, Bland R, Wheelans DC, Bennett J, Ronco PM, Squires PE. Glucose evoked alterations in connexin-43-mediated cell-to-cell communication in human collecting duct: a possible role in diabetic nephropathy. Am J Physiol Renal Physiol 291: F1045–F1051, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hills CE, Bland R, Bennett J, Ronco PM, Squires PE. High glucose upregulates ENaC and SGK1 expression in HCD-cells. Cell Physiol Biochem 18: 337–346, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose response study. Naunyn Schmiedebergs Arch Pharmacol 365: 67–73, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Humes HD, Nakamura T, Cieslinski DA, Miller D, Emmons RV, Border WA. Role of proteoglycans and cytoskeleton in the effects of TGF-beta 1 on renal proximal tubule cells. Kidney Int 43: 575–584, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju W, Ogawa A, Heyer J, Nierhof D, Yu L, Kucherlapati R, Shafritz DA, Bottinger EP. Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol Cell Biol 26: 654–67, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med 233: 4–11, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Lindschau C, Quass P, Menne J, Güler F, Fiebeler A, Leitges M, Luft FC, Haller H. Glucose-induced TGF-beta1 and TGF-beta receptor-1 expression in vascular smooth muscle cells is mediated by protein kinase C-alpha. Hypertension 42: 335–341, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Maezawa Y, Yokote K, Sonezaki K, Fujimoto M, Kobayashi K, Kawamura H, Tokuyama T, Takemoto M, Ueda S, Kuwaki T, Mori S, Wahren J, Saito Y. Influence of C-peptide on early glomerular changes in diabetic mice. Diabetes Metab Res Rev 22: 313–322, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol 165: 1955–1967, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol 273: F563–F574, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 108: 1853–1863, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S, Mason RM, Suzuki J, Imaizumi A, Kamimura T, Zhang Z. Inhibitory effect of statins on renal epithelial-to-mesenchymal transition. Am J Nephrol 26: 381–387, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells Biochem J 15: 601–607, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 12: 4557–4668, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Pollack V, Sarközi R, Banki Z, Feifel E, Wehn S, Gstraunthaler G, Stoiber H, Mayer G, Montesano R, Strutz F, Schramek H. Oncostatin M-induced effects on EMT in human proximal tubular cells: differential role of ERK signaling. Am J Physiol Renal Physiol 293: F1714–F1726, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Qi W, Chen X, Zhang Y, Waltham M, Schache M, Kelly DJ, Pollock CA. High glucose induces macrophage inflammatory protein-3 alpha in renal proximal tubule cells via a transforming growth factor-beta 1 dependent mechanism. Nephrol Dial Transplant 11: 3147–3153, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Müller GA, Colasanti G, D'Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62: 137–146, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Rigler R, Pramanik A, Jonasson P. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci USA 96: 13318–13323, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritz E, Tarng DC. Renal disease in type 2 diabetes. Nephrol Dialysis Transplant 16: 11–18, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Roberts AB Molecular and cell biology of TGF-beta. Miner Electrolyte Metab 24: 111–119, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein AH, Melani F, Pilkis S, Steiner DF. Proinsulin: Secretion, metabolism, immunological and biological properties. Postgrad Med J 45 Suppl: 476–481, 1969. [PubMed] [Google Scholar]
- 39.Samnegård B, Jacobson S, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int 60: 1258–1265, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Samnegård B, Jacobson S, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjoquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant 20: 532–538, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Scalia R, Coyle K, Levine B, Booth G, Lefer A. C peptide inhibits leukocyte-endothelium interaction in the microcirculation during endothelial dysfunction. FASEB J 14: 2357–2364, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sharma K, Ziyadeh FN. Hyperglycaemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Sjoquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int 54: 758–764, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Tian YC, Fraser D, Attisano L, Philips AO. TGF-β1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol Renal Physiol 285: F130–F142, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Thiery JP Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15: 740–746, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Van Ibberghen-Schilling E, Roche NS, Flanders KC, SpornMB, Roberts AB. Transforming growth factor β-1 positively regulates its own expression in normal and transformed cells. J Biol Chem 263: 7741–7746, 1988. [PubMed] [Google Scholar]
- 47.Vogelmann R, Nguyen-Tat MD, Giehl K, Adler G, Wedliach D, Menke A. TGF-beta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci 118: 4901–4912, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Wahren J, Ekberg K, Johansson J, Henriksson M, Pramanik A, Johansson BL, Rigler R, Jörnvall H. Role of C-peptide in human physiology. Am J Physiol Endocrinol Metab 278: E759–E768, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Wahren J C-peptide: new findings and therapeutic implications in diabetes. Clin Physiol Funct Imaging 24: 180–189, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wallerath T, Kunt T, Forst T, Closs EI, Lehmann R, Flohr T, Gabriel M, Schäfer D, Göpfert A, Pfützner A, Beyer J, Förstermann U. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 9: 95–102, 2003. [DOI] [PubMed] [Google Scholar]
- 51.White KE, Marshall SM, Bilous RW. Are glomerular volume differences between type 1 and type 2 diabetic patients pathologically significant? Diabetologia 50: 906–912, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Ziyadeh FN The extracellular matrix in diabetic nephropathy. Am J Kidney Dis 22: 736–744, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Ziyadeh FN Mediators of diabetic renal disease: the case for TGF-beta as the major mediator. J Am Soc Nephrol 15: S55–S57, 2004. [DOI] [PubMed] [Google Scholar]