Abstract
Background
Ovarian cancer is the second most prevalent gynecologic cancer in women. However, it is by far the most lethal. This is generally attributed to the absence of easily detectable markers specific to ovarian cancers that can be used for early diagnosis and specific therapeutic targets.
Methodology/Principal Findings
Using end point PCR we have found that a family of retrogenes, previously thought to be expressed only in the male testis during spermatogenesis in man, are also expressed in normal ovarian tissue and a large percentage of ovarian cancers. In man there are at least eleven such autosomal retrogenes, which are intronless copies of genes on the X chromosome, essential for normal spermatogenesis and expressed specifically in the human testis. We tested for the expression of five of the known retrogenes, UTP14C, PGK2, RPL10L, RPL39L and UBL4B in normal human ovary and ovarian cancers.
Conclusions/Significance
We propose that the activation of the testis specific retrogenes in the ovary and ovarian cancers is of biological significance in humans. Because these retrogenes are specifically expressed in the ovary and ovarian cancers in the female they may prove useful in developing new diagnostic and/or therapeutic targets for ovarian cancer.
Introduction
Although ovarian cancer is the second most prevalent gynecological cancer in women, it is by far the most lethal. It is the fifth leading cause of cancer-related death among women in the United States even though it represents less than 4% of the total cancers diagnosed. In 2008, it is estimated that there will be about 21,650 new cases of ovarian cancer diagnosed in the United States and there will be 15,520 attributed deaths[1]. Approximately 45% of women diagnosed with ovarian cancer will die within 5 years of diagnosis. This compares with 14% of those diagnosed with breast cancer and 30% with cancer of the cervix and uterus[2]. This high level of morbidity is thought to be due to an inability to recognize the presence of early stage ovarian cancer in the clinical setting due to the lack of cancer specific markers that could aid early diagnosis and post operative monitoring for disease recurrence. This situation is clearly illustrated by the fact that when ovarian cancers are diagnosed at stage I and II 82% of victims survive five years after diagnosis whereas those diagnosed at stage III and IV have a five year survival rate of 25%. Only 32% of cases are diagnosed at stage I and II, compared with 68% at stage III and IV[3]. There is therefore a great need to identify patterns of gene expression that are specific to ovarian cancer so that new diagnostic and therapeutic strategies can be developed.
As part of an ongoing program studying the role of genes critical for spermatogenesis, we previously identified a retrogene, UTP14C, associated with male fertility in man and mouse[4]–[6]. Retrogenes arise when fully or partially processed mRNAs are reverse transcribed into double stranded DNA and this DNA copy is inserted into the genomic DNA present in the chromosomes. If the copy is expressed as a messenger RNA and translated into a protein the new gene is known as a functional retrogene. The gene from which the retrogene originated is termed the progenitor gene. In humans UTP14C is located on chromosome 13 and originated as a reverse-transcribed copy of a gene located on the X chromosome, UTP14A. It has recently been established that a disproportionate number of functional autosomal retrogenes have originated from the X chromosome and acquired male germ-line specific function[7]–[9]. Several different hypotheses have been proposed to explain this evolutionary phenomenon[10], nevertheless the most compelling is that of compensation for transcriptional inactivation of the sex chromosomes that occurs in mammals when the sex bodies form during spermatogenesis[11]. During gametogenesis in the male the sex chromosomes pair via a short pseudoautosomal region during early prophase I and condense to form a macrochromatin body known as the sex, or XY, body[11]. Unlike the autosomal chromosomes that form homologous pairs known as synaptonemal complexes and remain transcriptionally active throughout meiosis, the sex chromosomes are transcriptionally silenced (meiotic sex chromosome inactivation) from the time of sex body formation until meiosis is competed and haploid spermatids form[12]. The X chromosome contains a large number of housekeeping genes essential for normal cell function and survival. Thus silencing of the X chromosome during male meiosis may have some metabolic disadvantage for later stages of spermatogenesis. Retrotransposition of X linked housekeeping genes to the autosomes, with subsequent acquisition of testis specific expression, is one mechanism by which such metabolic disadvantage can be corrected during spermatogenesis without disrupting normal function in somatic tissue[13]. In man there are at least eleven such autosomal retrogenes that have retrotransposed off the X chromosome and play an important role in maintaining efficient spermatogenesis[10].
Using endpoint RT-PCR to screen cDNAs prepared from a comprehensive panel of human tissues we found that UTP14C was expressed not only in the male testis but also in the female ovary and no other tissue[6]. The unexpected expression of UTP14C in the human ovary prompted us to explore whether or not UTP14C was also expressed in ovarian cancers. To determine if expression of testis specific retrogenes in normal ovaries and ovarian cancers was a general phenomenon in humans we also tested for the expression of four other retrogenes (PGK2, RPL10L, RPL39L and UBL4B) that were previously thought to be expressed exclusively during spermatogenesis in the male.
Here, we present the results of our screen for the expression of the testis specific retrogenes in normal ovarian tissue and a panel of ovarian cancers. We propose that a pattern of testis-like transcriptional regulation that results in retrogene expression frequently occurs in ovarian cancers and could contribute to the pathogenesis of this disease. In addition the unique ovarian and ovarian-cancer specific expression of retrogene encoded products may provide new understanding of gene expression in the female and lead to the identification of new diagnostic and/or therapeutic targets for ovarian cancer treatment.
Materials and Methods
Sources of Tissue Samples and RNA
Samples of pre- and post- menopausal ovaries were prospectively collected during clinical-indicated surgeries performed at Baylor College of Medicine affiliated hospitals by gynaecological surgeons. In addition samples of flash-frozen ovarian cancers were obtained from the Multidisciplinary Gynaecologic Tumor Bank at The University of Texas M.D. Anderson Cancer Center (MDACC, Houston, Texas), human total testis and ovarian RNA was purchased from Panomics (Fremont, California, USA. Product #NA2007) and the human RNA panel was purchased from Clontec (Clontech Laboratories Inc., Mountain View California USA. Master Panel II, Product #636643).
All collection was done under the approval from the Institutional Review Board at the Baylor College of Medicine (Protocol H-14372). Permission to use human tissues was obtained from each patient by written consent using a form approved by the Institutional Review Board for Baylor College of Medicine and its affiliated institutions. Written consent was similarly obtained from patients donating tissue samples to the Multidisciplinary Gynecologic Tumor Bank at The University of Texas M.D. Anderson Cancer Center.
RNA extraction and first strand synthesis
Prospectively collected tissue samples were placed into 10 ml of RNAlater (Ambion, Inc. Austin Texas, USA) and stored at −20°C until RNA extraction. RNA was extracted from weight tissue samples by thawing in TRIzol Reagent (Invitrogen Corp. Carlsbad California USA) followed by immediate homogenization using a Tissue-Tearor homogenizer (BioSpec Products, Inc. Bartlesville Oklahoma, USA). RNA was recovered using the TRIzol Reagent protocol. RNA was resuspended in nuclease free water and stored at −80°C. To produce cDNA 5 µg of RNA was treated with DNase (Turbo DNA-free kit, Ambion Inc.), precipitated and re-suspended to a concentration of 1 µg per µl. A RETROscript Kit (Ambion, Inc.) was used for first strand cDNA synthesis from 2 µg of RNA with a combination of oligo(dT)18 and random(dN)15 primers. After synthesis the 20 µl reaction mixtures were diluted to 150 µl with nuclease free water and stored at −20°.
RT-PCR
Genes of interest were detected by end point PCR using gene specific primers (Table 1). Reaction volumes of 20 µl of diluted AccuPrime Super Mix II (Invitrogen, Corp.) containing 1 µl of cDNA and retrogene specific primers were heated to 94°C for 2 minutes then cycled 35 times through 94°C for 20 seconds, 58°C for 20 seconds, 68°C for 30 seconds, and kept at 15°C after the final cycle. PCR products were run out on 1.75% agarose gels containing ethidium bromide and bands visualized by UV illumination. Gels were photographed using a Kodak Gel Logic 200 Imaging System.
Table 1. Primers used for gene expression analysis by endpoint PCR.
| Gene | Primer Name | Primer Sequence |
| UTP 14a | hUTP14x3F | TCCAGCTGCACACTAGAAGAAC |
| hUTP14x3R | CTTTCAGAAACTCCATGGGCT | |
| GT8 | HumJSD5′test | CATTTGCTGGTTTCTGTTGGCCAG |
| UTP14b | HumJSDLend | GCCTTTGCTAAATTGCTGAATAAG |
| HumJSD2R | AGGCTCATGGCTTGGAGAGAG | |
| HumJSD3R | TTGGCCATAATTGCCTTTGAC | |
| PGK1 | PGK1F | CACTGCTCACAGAGCCCACAGC |
| PGK1R | CTGCTTAGCCCGAGTGACAGCCTC | |
| PGK2 | PGK2F | CACTGCACACCGCGCTCATAGT |
| PGK2R | TAGCCTTGCTTGAGCCACAACTTG | |
| RPL10 | RPL10F | AGGGTTCACATTGGCCAAGTT |
| RPL10R | TAAGAGGGGGGCAGCACA | |
| RPL10L | RPL10LF | GGGTCCACATTGGTCAAGTC |
| RPL10LR | CCCAAGGAGACAGTACTGCC | |
| RPL39 | RPL39proF | CCTCCTCTTCCTTTCTCCGCCATC |
| RPL39proR | GTTCATAACAGATTCAGAGAGG | |
| RPL39L | RPL39LF S2 | TGGATTCAGATGAAACCTGGT |
| RPL39LR S2 | ATCCACCCTACTAGCACAGAGC | |
| UBL4A | UBL4AF | CAGCTGACGGTGAAGGCGCTGCA |
| UBL4AR | GTTTGACCACTAGGTTGAGCTTG | |
| UBL4B | UBL4BF | TTCCTCACAGTCAAGCTGCTCCT |
| UBL4BR | GCTGCATGATGACATTGATAGAG |
Results and Discussion
As part of an ongoing program studying the role of genes crucial for spermatogenesis we previously reported the identification of a retrogene associated with male fertility in man and mouse[4]–[6]. In humans this retrogene, UTP14C, is located on chromosome 13 and originated as an intronless, reverse-transcribed copy of the X-linked gene, UTP14A. Although the function of UTP14A and C and their products is not known, analogy with the product from the yeast gene, Utp14, suggests that the human peptides, UTP14A and C, form part of the Small-Subunit (SSU) processome which is responsible for generating 18S rRNA from its precursor, the U3 snoRNA, in the nucleolus[14], [15]. Using endpoint RT-PCR to screen cDNAs prepared from a comprehensive panel of human tissues we found UTP14C to be expressed not only in the male testis, but also in the female ovary[6]. This unexpected expression of UTP14C in the human ovary prompted us to see if UTP14C was also expressed in ovarian cancers with the hope of identifying tissue specific markers selectively expressed in normal ovaries and ovarian cancers. End point RT-PCR was used to detect transcripts for UTP14A and C in primary ovarian cancers and established ovarian cancer cell lines. Determining the expression of UTP14C by RT-PCR is complicated by the fact that it is located within an actively transcribed host gene, GT8. UTP14C is the result of a retrotransposition event where an intronless DNA copy of the UTP14A coding sequence inserted immediately upstream of the coding region present in the terminal exon of GT8 on chromosome 13. Subsequently UTP14C has acquired a promoter that drives its expression during spermatogenesis. Because the transcripts of UTP14C and GT8 overlap the possibility of a single transcript containing open reading frames from both genes arises. To distinguish between the overlapping transcripts that are produced from the retrogene and its host, primer pairs were designed to specifically amplify either UTP14C or GT8 [6] and detect any transcript that may contain both open reading frames. Fig. 1 shows the expression profiles of UTP14A, UTP14C and GT8 in a representative sample of six different papillary serous ovarian cancers, the most common histological subtype of this malignancy, two clear cell and two endometrioid samples. UTP14A, the X chromosome linked progenitor gene from which UTP14C arose, was expressed in all of the cancer samples (lane A). Similarly, GT8, the host gene for UTP14C, was expressed in all samples (lane C). The spermatogenesis-associated retrogene, UTP14C, was expressed in all but one of the cancer samples (lane D). We also tested our samples using the combination of a 5′-primer specific to GT8 (that is up stream of the UTP14C transcriptional start site) and 3′-primer specific to UTP14C (that is upstream of the GT8 transcript)[6]. The absence of a PCR product using this primer pair confirms that a read-through mRNA, spanning both the GT8 and UTP14C genes, is not produced and establishes the independence of the two overlapping transcripts (lane B). Similar results were obtained for all primer combinations when a panel of established ovarian cancer cell lines were tested under identical conditions (2774, ES-2, TOV112D, OV90, SKOV3, TOV21G, HEY and OVCAR3; Fig. 2). However, it should be noted that several of the cell line samples also showed the presence of RNA transcripts that spanned both the host gene (GT8) and UTP14C suggesting aberrant transcriptional regulation in some of the ovarian cancer cell lines. We have never observed transcription that spanned both genes in human tissue samples.
Figure 1. RT-PCR of cDNA samples from ten Papillary Serous ovarian cancers with primers specific to UTP14A, UTP14C and GT8.
Lane A shows the product specific to UTP14A. Lane B shows the reaction product from a primer pair specific to GT8 (5′ end) and UTP14C (3′ end), these two genes overlap on human chromosome 1 and the absence of a PCR product demonstrates that transcripts from the two gene are exclusive. Lane C shows the GT8 gene specific product and, lane D, the PCR product from UTP14C. Molecular weight marker was run in lane M.
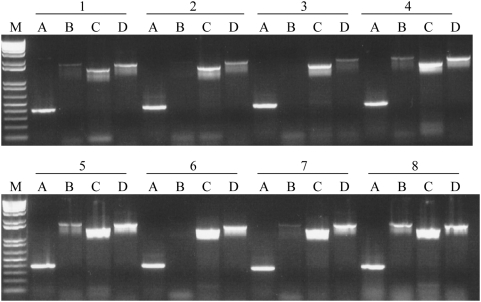
Figure 2. RT-PCR of cDNA from established ovarian cancer cell lines.
Lane A: UTP14A specific product. Lane B: Product from primers common to both UTP14C and GT8. This primer combination fails to give a PCR product in ovarian or ovarian cancer tissue samples. Lane C: GT8 specific RT-PCR product. Lane D: Product specific to UTP14C. Primer combinations are described in the text. Established ovarian cancer cell lines used were 1. 2774, 2. ES-2, 3. TOV112D, 4. OV90, 5. SKOV3, 6. TOV21G, 7. HEY, 8. OVCAR3, and M, molecular size marker.
The high frequency with which UTP14C was expressed in our initial survey led us to explore the incidence of its expression in a large panel of ovarian cancers with different clinical histology. In aggregate, we found that that transcripts for UTP14C can be readily detected in 41/51 (80%) of papillary serous cancers, the most common form of cancer examined, and most other cancer subtypes. The frequency of UTP14C expression in a variety of ovarian cancer histological subtypes is shown in Table 2.
Table 2. The frequency of retrogene expression in a panel of ovarian cancer samples as well as pre- and post- menopausal ovary samples.
| Cancer Histology | UTP14a | UTP14c | PGK1 | PGK2 | RPL10 | RPL10L | RPL39 | RPL39L | UBL4A | UBL4B |
| Pap Serous | 50/51 (98%) | 41/51 (80%) | 48/51 (94%) | 40/51 (78%) | 45/51 (88%) | 43/51 (84%) | 50/51 (98%) | 50/51 (98%) | 50/51 (98%) | 31/51 (61%) |
| Mixed | 17/17 (100%) | 10/17 (59%) | 17/17 (100%) | 13/17 (76%) | 12/17 (71%) | 13/17 (76%) | 16/17 (94%) | 17/17 (100%) | 17/17 (100%) | 5/17 (29%) |
| Endometrioid | 9/9 (100%) | 1/9 (11%) | 7/9 (78%) | 3/9 (33%) | 7/9 (78%) | 4/9 (78%) | 9/9 (100%) | 9/9 (100%) | 7/9 (78%) | 9/9 (100%) |
| Clear Cell | 7/7 (100%) | 1/7 (14%) | 7/7 (100%) | 4/7 (57%) | 6/7 (86%) | 4/7 (57%) | 7/7 (100%) | 7/7 (100%) | 7/7 (100%) | 2/7 (29%) |
| MMMT | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) |
| AGCT | 7/8 (88%) | 2/8 (25%) | 8/8 (100%) | 4/8 (50%) | 7/8 (88%) | 5/8 (65%) | 7/8 (88%) | 7/8 (88%) | 8/8 (100%) | 6/8 (75%) |
| Sex Cord | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) |
| Transitional | 2/2 (100%) | 1/2 (50%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | 1/2 (50%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) |
| Carcinoid | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) |
| Immature Teratoma | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) |
| Pre-Ovary | 5/5 (100%) | 4/5 (80%) | 5/5 (100%) | 4/5 (80%) | 5/5 (100%) | 5/5 (100%) | 5/5 (100%) | 5/5 (100%) | 5/5 (100%) | 3/5 (60%) |
| Post-Ovary | 9/9 (100%) | 7/9 (78%) | 9/9 (100%) | 6/9 (67%) | 9/9 (100%) | 9/9 (100%) | 9/9 (100%) | 9/9 (100%) | 9/9 (100%) | 4/9 (44%) |
Abbreviations: MMMT, Malignant Mixed Mullerian Tumor. AGCT, Adult Granulosa Cell Tumor. Pre-, Premenopausal. Post-, Postmenopausal.
To determine if activation of testis specific retrogenes in ovaries and ovarian cancers is a general phenomenon the expression of four other retrogenes, previously thought to be testis specific, was studied. The spermatogenesis associated retrogenes tested encoded for a variety of important cell functions: metabolic enzymes (PGK2)[13], ribosomal proteins (RPL10L and RPL39L)[16] and post translational protein modification (UBL4B)[17]. Primer pairs specific to the retrogenes and their X linked progenitors were used for molecular characterization of gene expression in the same panel of ovarian cancer tissues previously described.
In man, phosphoglycerate kinase is encoded by two genes, PGK1 and PGK2. Phosphoglycerate kinase converts 1.3-diphosphoglycerate into 3-phosphoglycerate in the glycolysis pathway. This leads to the production of pyruvate from glucose and fructose[18]. PGK1 is located on the X chromosome and is ubiquitously expressed whereas PGK2, a retrotransposed copy of PGK1, is located on chromosome 6 and shows a testis specific expression pattern[13]. Another retrotransposed copy of PGK1 is present on chromosome 19 but it translates into a truncated protein and is probably inactive as no ESTs (Expressed Sequence Tags) could be found in the human database. Over expression of the PGK1 protein has been linked to paclitaxel resistance[19] and tumor angiogenesis[20]. In addition, PGK1 protein levels are elevated in serum from pancreatic duct adenocarcinoma patients[20], [21]. Thus, it appears that PGK1 over expression may be of biological significance in some cancers. Detection of PGK1 and PGK2 in a representative panel of ovarian cancers, testis and pre and post-menopausal ovaries is shown in Fig. 3. Expression of PGK2 was detected in all ovarian tumor types with the highest frequency in papillary serous (78%) and mixed histology (76%) tumors (Table 2). As expected for a housekeeping gene, PGK1 was expressed in all tumor samples. However, due to the small sample size of the rare ovarian cancer subtypes the true frequency of PGK2 expression in these tumors could not be estimated. It remains to be determined if PGK2 expression is associated with tumor biology.
Figure 3. Expression of the testis specific retrogenes, and their X linked progenitors, in ovarian cancers and normal ovarian tissue.
a. Retrogene expression in a panel of ovarian cancers was detected by RT-PCR. Samples and cancer histology were: 1–6. Papillary Serous, 7 & 8 Clear Cell, 9 & 10 Endometrioid, 11 pre-menopausal ovary, 12 post-menopausal ovary. Negative control C and marker M. b. Retrogene expression in normal ovarian tissue: 1–4. pre-menopausal ovaries, 5–8. post menopausal ovaries, negative control C and marker M.
Mammalian ribosomes are made up of at least 80 ribosomal proteins[22] of which four are encoded on the X chromosome[23]. Three of these, RPL10, RPL36A and RPL39, have autosomal retrotransposed copies. Two are located on chromosome 14 (RPL10L and RPL36AL) and the third on chromosome 3 (RPL39L)[16]. RPL36AL is ubiquitously expressed whereas RPL10L and RPL39L have previously been reported to be expressed exclusively in the testis[16]. The RPL10 is also a tumor suppressor gene and the protein it encodes is known as the tumor suppression protein QM[24]. Reduced RPL10 expression is associated with increasing grade of prostatic adenocarcinoma[25]. Primers specific to RPL10L and RPL39L were used to screen for expression of these testis specific retrogenes in a panel of ovarian cancers (Table 2; Fig. 3). Transcripts from both retrogenes were detected in papillary serous, clear cell and endometroid samples. RPL10L was expressed in 76% of a large ovarian tumor panel with 84% expression in papillary serous cancers, 76% in tumors with mixed histology and 44% in endometroid tumors (Table 2). RPL39L was expressed at even higher frequency with 98% of papillary serous cancers containing transcript (Table 2). Expression in tumors with other histologies was 88% in AGCT (Adult Granulosa Cell Tumor) and 100% in all other subtypes tested.
Although a direct role for the ribosomal proteins in cancer biology is yet to be established, it should be noted that RPL39 expression is increased in breast cancer[26]. RPL39L expression has been reported in cervical cancer[27] as well as cell lines originating from breast, lung, prostate, colon, pancreatic and ovarian cancers[16], [20] suggesting that the retrogene RPL39L may have an important role in the biology of a large range of cancers. However, it should be noted that a screen for RPL39L expression in a human tissue RNA panel resulted in the detection of this retrogene in a large number of normal tissues suggesting that although RPL39L expression occurs predominantly in the testis it may not be testis specific (Fig. 4). Expression of the retrogenes RPL10L and RPL39L in cancers could possibly enhance ribosome production and/or translational activity. In testes, these retrogenes are expressed during spermatogenesis just before the rapid production of proteins that are required for sperm differentiation and maturation.
Figure 4. Tissue-specific expression patterns of PGK1, PGK2, RPL10, RPL10L, RPL39, RPL39L, UBL4A and UBL4B.
Expression of the X linked progenitor genes PGK1, RPL10, RPL39 and UBL4A is ubiquitous as would be expected for genes encoding essential cellular functions. In contrast expression of the spermatogenesis specific retrogenes, PGK2, RPL10L, RPL39L and UBL4B, is predominantly limited to the testis. Tissue key: AG, Adrenal Gland; BM, Bone Marrow; B, Brain; H, Heart; K, Kidney; L, Liver; Lu, Lung; O, Ovary; Pl, Placenta; Pr, Prostate; SG, Salivary Gland; SM, Skeletal Muscle; SC, Spinal Cord; T, Testis; Ty, Thyamus; Tr, Thyroid; Tch, Trachea; U, Uterus. M is the molecular size marker and C, is the primer only control reaction. The expression pattern for UTP14A and UTP14C has been previously published[5].
Ubiquitin like proteins (UBLs) are post translationally bound to a diverse group of other proteins thereby increasing the functional diversity of the encoded proteome. UBL modified proteins are known to influence the activity of a wide range of cell signaling pathways[28], [29]. UBL4A and UBL4B are recently identified genes that encode small ubiquitin like proteins[17]. In mouse, the X-linked Ubl4A is expressed in all tissue types, whereas the retrogene Ubl4b is reported to be expressed only in elongating spermatids during late stages of spermatogenesis in the testis[17]. Preliminary testing for UBL4A and UBL4B expression in a human RNA panel found that UBL4A was expressed in all tissue types tested. UBL4B, located on chromosome 1, was expressed only in the testis (Fig. 4) although later screening of a larger sample of pre- and post-menopausal ovaries demonstrated that UBL4B was expressed in normal ovarian tissues at low frequency (Table 2). Results of an initial screen for UBL4B activation in ovarian tumor samples is shown in Fig. 3. Table 2 shows the frequency with which UBL4A and UBL4B expression was detected in an extensive panel of ovarian tumor samples. As almost all cellular functions are affected by ubiquitination it is not surprising that alterations in ubiquitin and UBL activity have been implicated in the genesis of different human tumor types[30]. UBL modification by SUMO-1 (small ubiquitin-related modifier, also known as UBL1) has been directly linked to modification of the apoptosis pathway in ovarian tumors[31] and is known to play an important role in spermatogenesis[32].
Screening for retrogene expression in normal pre- and post- menopausal ovaries revealed that the retrogenes are not expressed in all women. The X linked progenitor gene is expressed in all cases (Table 2; Fig. 3) as would be expected for genes encoding essential gene functions that are ubiquitously expressed in the human body. However, retrogene expression in healthy ovarian tissue may be as high as 100% (RPL10L and RPL39L) of the samples tested or as low as 44% (UBL4B). This suggests that, for at least some of the retrogenes, expression in the ovary is limited to particular individuals within the general population. Most work on the spermatogenesis specific retrogenes has been done on the mouse model where ovarian expression of these genes is absent. Expression of these retrogenes in the human ovary may be a species specific phenomenon and may reflect an important biological difference between man and mouse. The retrogene and progenitor X gene specific primers were tested against a panel of RNAs from different human tissues to confirm the ubiquitous expression of the X linked progenitor and limited expression of the retrogene (Fig. 4). In most cases retrogene expression was limited to the gonads in man. Whether there is any relationship between expression of testis specific retrogenes in the human ovary and increased probability of cancer development is yet to be established.
Of special note is our observation that the X linked progenitor genes are not always expressed in all ovarian cancer samples. When this was the case the complimentary retrogene was always expressed suggesting that the retrogene was compensating for the loss of gene activity on the X chromosome in a manner similar to that which occurs when the X chromosome is transcriptionally silenced during male meiosis. The deletion of segments of the X chromosome in ovarian cancer cells and subsequent loss of heterozygosity is well documented[33]. Such deletions could result in the loss of genes which have retrotransposed copies. The loss of a copy of PGK1 due to deletions within the X chromosome has been previously reported in a variety of ovarian cancer histological subtypes[34]. Such loss could be biologically significant if the deleted copy is from the active X chromosome or if both copies of a gene are transcriptionally active in normal cells.
The high frequency of spermatogenesis specific retrogene activation in ovarian cancers reported in this paper has important theoretical and practical implications for ovarian cancer treatment and biology. As these retrogenes are expressed only in the ovary and/or ovarian tumors and nowhere else in the female body the potential to develop tumor specific markers and therapies presents itself. Precedence for this is established by the expression of cancer/testis (CT) genes and antigens in a broad range of human tumors and is a phenomenon that has been recognized for some time[35], [36]. Expression of CT antigens in cancers was first reported with MAGE-1 in 1991, even though at the time it was thought to be a gene expressed specifically in human melanoma tumors[37] and its expression in the testis was not recognized. Later reports added MAGE-3 and BAGE to the list of CT antigens that are expressed in tumors but not expressed in normal tissues other than the testis[38], [39]. Identification of other CT antigens moved forward rapidly with the application of serological analysis of recombinant expression libraries (SEREX) technology[40]. To date at least forty four different CT antigens have been recognized[41]. The reason why genes, which encode proteins that are essential for spermatogenesis in the male or have a structural role in sperm, are expressed in cancers is not clear. The situation is complicated by the fact that the role of many of the CT genes in spermatogenesis is poorly understood[36]. However, this has not prevented the utilization of the CT antigens as biomarkers for developing novel cancer diagnostics[42] and/or targets for treatments such as immunotherapy[43]. One limiting factor in the practical application of CT antigens has been the low frequency with which the cancer/testis genes are expressed in any given tumor type. This restriction is not present in the case of spermatogenesis specific retrogene expression in ovarian cancers as these genes are expressed in a relatively high percentage of tumors.
An interesting feature of the previously recognized cancer/testis genes and antigens is their disproportionate association with the X chromosome[36]. The X chromosome and the genes it encodes appear to play a pivotal role in tumor genesis and development[44]. However, the relationship between tumor biology and spermatogenesis is less clear. In man it appears that the testis specific retrogenes are also be expressed in the ovary. However, the specific cell types within which expression occurs remains to be determined. Expression of these retrogenes may have a distinct metabolic advantage for ovarian tumor development as it may supplement gene products from the X chromosome, particularly in cases where the progenitor gene activity is down regulated by methylation[45] or lost due to deletion[34]. A link between metabolic events occurring during spermatogenesis and cancer development may be inferred from recent advances in the understanding of cancer biology. Of special note is the recently identified role of BRCA-1 in the development of breast and ovarian cancers[46]. The BRCA-1 protein has been shown to be associated with the inactive X chromosome in female somatic cells and, during spermatogenesis, is localized to the inactive X chromosome in pachytene spermatocytes during meiosis[47], [48]. This suggests a shared regulatory pathway. Further work is needed to determine the functional role of the spermatogenesis specific retrogene products in both spermatogenesis and cancer. Expression of genes and retrogenes in spermatogenic cells is driven by TATA-independent promoters. Polyadenylation sites are upstream of those utilized in somatic cells and there is a predominance of alternative pre-mRNA splicing[49]. The expression of testis specific genes and retrogenes suggests that cancer cells are able to utilize the transcriptional and RNA processing pathways normally active only during spermatogenesis in the male testis. Any structural identity shared by the mRNAs generated from the CT and other genes expressed in both cancers and testis needs to be determined. If spermatogenesis and tumor genesis share features of gene expression, regulation and RNA processing one could speculate that in long lived mammals cancer is a biological penalty of having separate sexes and the need for spermatogenesis in particular.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this work provided by Saks Fifth Avenue “Key to a Cure” Foundations, the Joann Rosenberg Fund for Ovarian Cancer Research and the Ovarian Cancer Research Program of the Department of Obstetrics and Gynecology, Baylor College of Medicine and an anonymous donation channeled through CLE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.[Anonymous] American Cancer Society 2008 [Google Scholar]
- 2.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131–1135. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 3.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, Berek JS, Osann K. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J, Baltus A, Skaletsky H, Royce-Tolland M, Dewar K, Page DC. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat Genet. 2004;36:872–876. doi: 10.1038/ng1390. [DOI] [PubMed] [Google Scholar]
- 5.Rohozinski J, Bishop CE. The mouse juvenile spermatogonial depletion (jsd) phenotype is due to a mutation in the X-derived retrogene, mUtp14b. Proc Natl Acad Sci U S A. 2004;101:11695–11700. doi: 10.1073/pnas.0401130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohozinski J, Lamb DJ, Bishop CE. UTP14c is a recently acquired retrogene associated with spermatogenesis and fertility in man. Biol Reprod. 2006;74:644–651. doi: 10.1095/biolreprod.105.046698. [DOI] [PubMed] [Google Scholar]
- 7.Betran E, Emerson JJ, Kaessmann H, Long M. Sex chromosomes and male functions: where do new genes go? Cell Cycle. 2004;3:873–875. [PubMed] [Google Scholar]
- 8.Emerson JJ, Kaessmann H, Betran E, Long M. Extensive gene traffic on the mammalian X chromosome. Science. 2004;303:537–540. doi: 10.1126/science.1090042. [DOI] [PubMed] [Google Scholar]
- 9.Shiao MS, Khil P, Camerini-Otero RD, Shiroishi T, Moriwaki K, et al. Origins of new male germ-line functions from X-derived autosomal retrogenes in the mouse. Mol Biol Evol. 2007;24:2242–2253. doi: 10.1093/molbev/msm153. [DOI] [PubMed] [Google Scholar]
- 10.Wang PJ. X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol Metab. 2004;15:79–83. doi: 10.1016/j.tem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Handel MA. The XY body: a specialized meiotic chromatin domain. Exp Cell Res. 2004;296:57–63. doi: 10.1016/j.yexcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Hendriksen PJ, Hoogerbrugge JW, Themmen AP, Koken MH, Hoeijmakers JH, et al. Postmeiotic transcription of X and Y chromosomal genes during spermatogenesis in the mouse. Dev Biol. 1995;170:730–733. doi: 10.1006/dbio.1995.1252. [DOI] [PubMed] [Google Scholar]
- 13.McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- 14.Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature. 2002;417:967–970. doi: 10.1038/nature00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leary DJ, Terns MP, Huang S. Components of U3 snoRNA-containing complexes shuttle between nuclei and the cytoplasm and differentially localize in nucleoli: implications for assembly and function. Mol Biol Cell. 2004;15:281–293. doi: 10.1091/mbc.E03-06-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uechi T, Maeda N, Tanaka T, Kenmochi N. Functional second genes generated by retrotransposition of the X-linked ribosomal protein genes. Nucleic Acids Res. 2002;30:5369–5375. doi: 10.1093/nar/gkf696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F, Skaletsky H, Wang PJ. Ubl4b, an X-derived retrogene, is specifically expressed in post-meiotic germ cells in mammals. Gene Expr Patterns. 2007;7:131–136. doi: 10.1016/j.modgep.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blake CC, Rice DW. Phosphoglycerate kinase. Philos Trans R Soc Lond B Biol Sci. 1981;293:93–104. doi: 10.1098/rstb.1981.0063. [DOI] [PubMed] [Google Scholar]
- 19.Duan Z, Lamendola DE, Yusuf RZ, Penson RT, Preffer FI, et al. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2002;22:1933–1941. [PubMed] [Google Scholar]
- 20.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 21.Hwang TL, Liang Y, Chien KY, Yu JS. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259–2272. doi: 10.1002/pmic.200500345. [DOI] [PubMed] [Google Scholar]
- 22.Dresios J, Panopoulos P, Synetos D. Eukaryotic ribosomal proteins lacking a eubacterial counterpart: important players in ribosomal function. Mol Microbiol. 2006;59:1651–1663. doi: 10.1111/j.1365-2958.2006.05054.x. [DOI] [PubMed] [Google Scholar]
- 23.Uechi T, Tanaka T, Kenmochi N. A complete map of the human ribosomal protein genes: assignment of 80 genes to the cytogenetic map and implications for human disorders. Genomics. 2001;72:223–230. doi: 10.1006/geno.2000.6470. [DOI] [PubMed] [Google Scholar]
- 24.Stalberg P, Grimfjard P, Santesson M, Zhou Y, Lindberg D, et al. Transfection of the multiple endocrine neoplasia type 1 gene to a human endocrine pancreatic tumor cell line inhibits cell growth and affects expression of JunD, delta-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1. J Clin Endocrinol Metab. 2004;89:2326–2337. doi: 10.1210/jc.2003-031228. [DOI] [PubMed] [Google Scholar]
- 25.Altinok G, Powell IJ, Che M, Hormont K, Sarkar FH, et al. Reduction of QM protein expression correlates with tumor grade in prostatic adenocarcinoma. Prostate Cancer Prostatic Dis. 2006;9:77–82. doi: 10.1038/sj.pcan.4500848. [DOI] [PubMed] [Google Scholar]
- 26.Thakur A, Xu H, Wang Y, Bollig A, Biliran H, et al. The role of X-linked genes in breast cancer. Breast Cancer Res Treat. 2005;93:135–143. doi: 10.1007/s10549-005-4516-0. [DOI] [PubMed] [Google Scholar]
- 27.Wong YF, Cheung TH, Tsao GS, Lo KW, Yim SF, et al. Genome-wide gene expression profiling of cervical cancer in Hong Kong women by oligonucleotide microarray. Int J Cancer. 2006;118:2461–2469. doi: 10.1002/ijc.21660. [DOI] [PubMed] [Google Scholar]
- 28.Kirkin V, Dikic I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr Opin Cell Biol. 2007;19:199–205. doi: 10.1016/j.ceb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 30.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6:776–788. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 31.Mo YY, Yu Y, Theodosiou E, Rachel Ee PL, Beck WT. A role for Ubc9 in tumorigenesis. Oncogene. 2005;24:2677–2683. doi: 10.1038/sj.onc.1208210. [DOI] [PubMed] [Google Scholar]
- 32.Vigodner M, Ishikawa T, Schlegel PN, Morris PL. SUMO-1, human male germ cell development, and the androgen receptor in the testis of men with normal and abnormal spermatogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1022–E1033. doi: 10.1152/ajpendo.00527.2005. [DOI] [PubMed] [Google Scholar]
- 33.Hogdall EV, Ryan A, Kjaer SK, Blaakaer J, Christensen L, et al. Loss of heterozygosity on the X chromosome is an independent prognostic factor in ovarian carcinoma: from the Danish “MALOVA” Ovarian Carcinoma Study. Cancer. 2004;100:2387–2395. doi: 10.1002/cncr.20213. [DOI] [PubMed] [Google Scholar]
- 34.Edelson MI, Lau CC, Colitti CV, Welch WR, Bell DA, et al. A one centimorgan deletion unit on chromosome Xq12 is commonly lost in borderline and invasive epithelial ovarian tumors. Oncogene. 1998;16:197–202. doi: 10.1038/sj.onc.1201479. [DOI] [PubMed] [Google Scholar]
- 35.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 36.Zendman AJ, Ruiter DJ, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]
- 37.van der BP, Traversari C, Chomez P, Lurquin C, De PE, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 38.Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, et al. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 39.Gaugler B, Van den EB, van der BP, Romero P, Gaforio JJ, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 42.Grizzi F, Franceschini B, Hamrick C, Frezza EE, Cobos E, et al. Usefulness of cancer-testis antigens as biomarkers for the diagnosis and treatment of hepatocellular carcinoma. J Transl Med. 2007;5:3. doi: 10.1186/1479-5876-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suri A. Cancer testis antigens–their importance in immunotherapy and in the early detection of cancer. Expert Opin Biol Ther. 2006;6:379–389. doi: 10.1517/14712598.6.4.379. [DOI] [PubMed] [Google Scholar]
- 44.Spatz A, Borg C, Feunteun J. X-chromosome genetics and human cancer. Nat Rev Cancer. 2004;4:617–629. doi: 10.1038/nrc1413. [DOI] [PubMed] [Google Scholar]
- 45.Liao DJ, Du QQ, Yu BW, Grignon D, Sarkar FH. Novel perspective: focusing on the X chromosome in reproductive cancers. Cancer Invest. 2003;21:641–658. doi: 10.1081/cnv-120022385. [DOI] [PubMed] [Google Scholar]
- 46.Palma M, Ristori E, Ricevuto E, Giannini G, Gulino A. BRCA1 and BRCA2: the genetic testing and the current management options for mutation carriers. Crit Rev Oncol Hematol. 2006;57:1–23. doi: 10.1016/j.critrevonc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Ganesan S, Silver DP, Greenberg RA, Avni D, Drapkin R, et al. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell. 2002;111:393–405. doi: 10.1016/s0092-8674(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 48.Scully R, Chen J, Plug A, Xiao Y, Weaver D, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 49.Kleene KC. A possible meiotic function of the peculiar patterns of gene expression in mammalian spermatogenic cells. Mech Dev. 2001;106:3–23. doi: 10.1016/s0925-4773(01)00413-0. [DOI] [PubMed] [Google Scholar]