Abstract
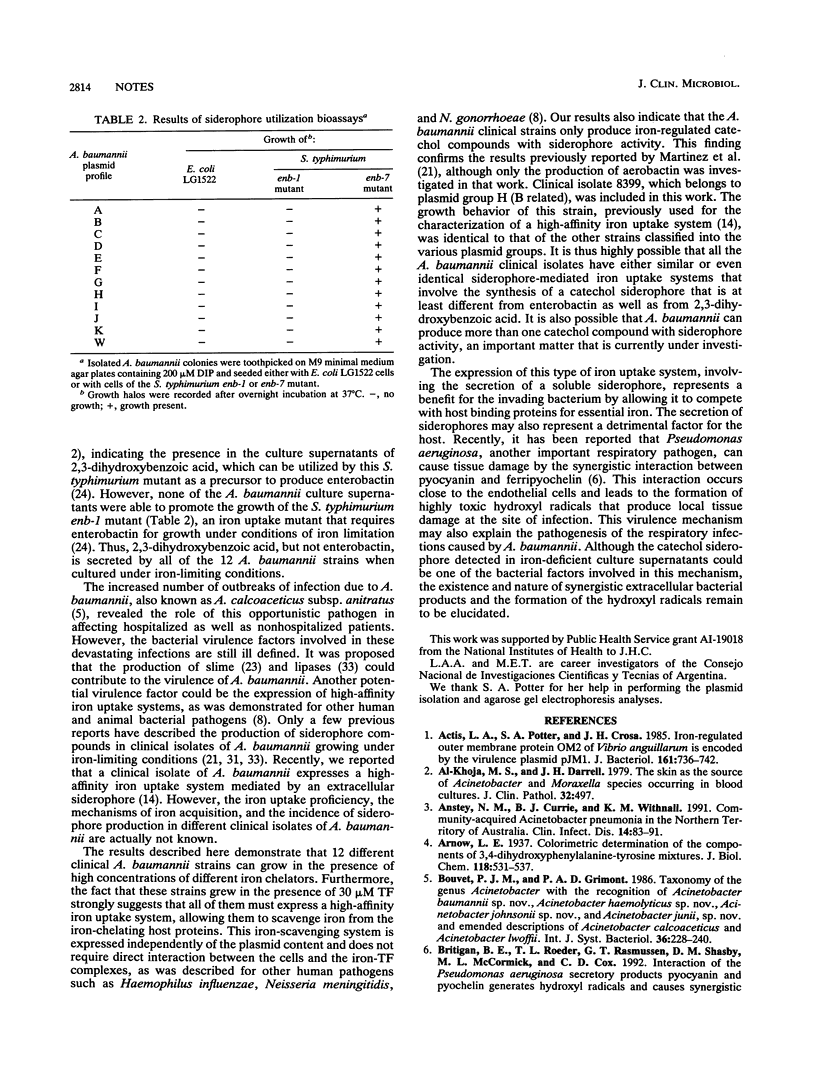
Different clinical isolates of Acinetobacter baumannii, typed by plasmid profile, were able to grow in iron-chelated medium by secreting iron-regulated siderophores. This iron-scavenging phenotype was associated with the production of iron-repressible catechol. Siderophore utilization bioassays showed the presence of 2,3-dihydroxybenzoic acid in the growth medium, and neither enterobactin nor aerobactin was detected in culture supernatants obtained under iron-deficient conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khoja M. S., Darrell J. H. The skin as the source of Acinetobacter and Moraxella species occurring in blood cultures. J Clin Pathol. 1979 May;32(5):497–499. doi: 10.1136/jcp.32.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey N. M., Currie B. J., Withnall K. M. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin Infect Dis. 1992 Jan;14(1):83–91. doi: 10.1093/clinids/14.1.83. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Roeder T. L., Rasmussen G. T., Shasby D. M., McCormick M. L., Cox C. D. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J Clin Invest. 1992 Dec;90(6):2187–2196. doi: 10.1172/JCI116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton A. E., Anderson R. L., Werdegar D., Atlas E. Nosocomial respiratory tract infection and colonization with Acinetobacter calcoaceticus. Epidemiologic characteristics. Am J Med. 1978 Sep;65(3):507–513. doi: 10.1016/0002-9343(78)90777-5. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa L. M., Wolf M. K., Actis L. A., Sanders-Loehr J., Crosa J. H. New aerobactin-mediated iron uptake system in a septicemia-causing strain of Enterobacter cloacae. J Bacteriol. 1988 Dec;170(12):5539–5544. doi: 10.1128/jb.170.12.5539-5544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE TORREGROSA M. V., ORTIZ A. Severe infections in children due to rare gram-negative bacilli (Mima polymorpha and Bacillus anitratum). J Pediatr. 1961 Jul;59:35–41. doi: 10.1016/s0022-3476(61)80206-0. [DOI] [PubMed] [Google Scholar]
- Echenique J. R., Arienti H., Tolmasky M. E., Read R. R., Staneloni R. J., Crosa J. H., Actis L. A. Characterization of a high-affinity iron transport system in Acinetobacter baumannii. J Bacteriol. 1992 Dec;174(23):7670–7679. doi: 10.1128/jb.174.23.7670-7679.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr, Kunz L. J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977 Mar;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Hartstein A. I., Rashad A. L., Liebler J. M., Actis L. A., Freeman J., Rourke J. W., Jr, Stibolt T. B., Tolmasky M. E., Ellis G. R., Crosa J. H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am J Med. 1988 Nov;85(5):624–631. doi: 10.1016/s0002-9343(88)80233-x. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. A decade of nosocomial Acinetobacter. Am J Infect Control. 1984 Feb;12(1):14–18. doi: 10.1016/0196-6553(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Lemos M. L., Salinas P., Toranzo A. E., Barja J. L., Crosa J. H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988 Apr;170(4):1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Morgan M. E., Hart C. A. Acinetobacter meningitis: acquired infection in a neonatal intensive care unit. Arch Dis Child. 1982 Jul;57(7):557–559. doi: 10.1136/adc.57.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obana Y. Pathogenic significance of Acinetobacter calcoaceticus: analysis of experimental infection in mice. Microbiol Immunol. 1986;30(7):645–657. doi: 10.1111/j.1348-0421.1986.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retailliau H. F., Hightower A. W., Dixon R. E., Allen J. R. Acinetobacter calcoaceticus: a nosocomial pathogen with an unusual seasonal pattern. J Infect Dis. 1979 Mar;139(3):371–375. doi: 10.1093/infdis/139.3.371. [DOI] [PubMed] [Google Scholar]
- Rosenthal S., Tager I. B. Prevalence of gram-negative rods in the normal pharyngeal flora. Ann Intern Med. 1975 Sep;83(3):355–357. doi: 10.7326/0003-4819-83-3-355. [DOI] [PubMed] [Google Scholar]
- Rudin M. L., Michael J. R., Huxley E. J. Community-acquired acinetobacter pneumonia. Am J Med. 1979 Jul;67(1):39–43. doi: 10.1016/0002-9343(79)90071-8. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Smith A. W., Freeman S., Minett W. G., Lambert P. A. Characterisation of a siderophore from Acinetobacter calcoaceticus. FEMS Microbiol Lett. 1990 Jun 15;58(1):29–32. doi: 10.1016/0378-1097(90)90097-a. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



