Abstract
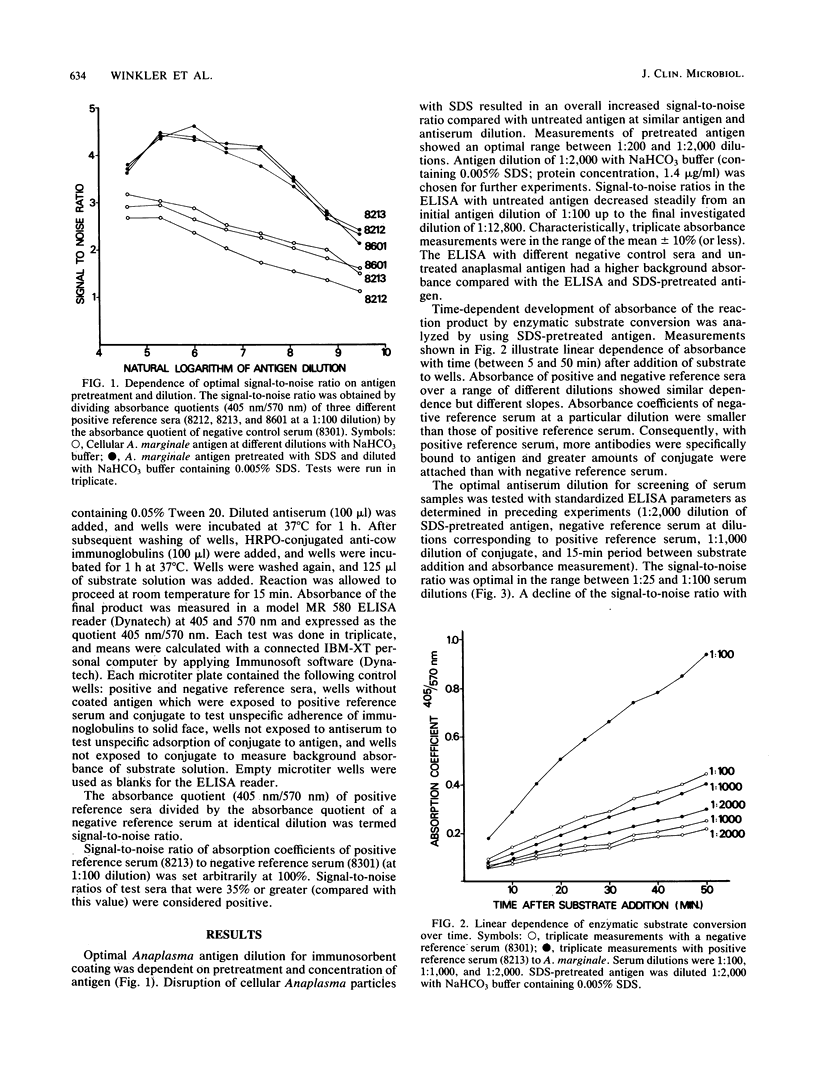
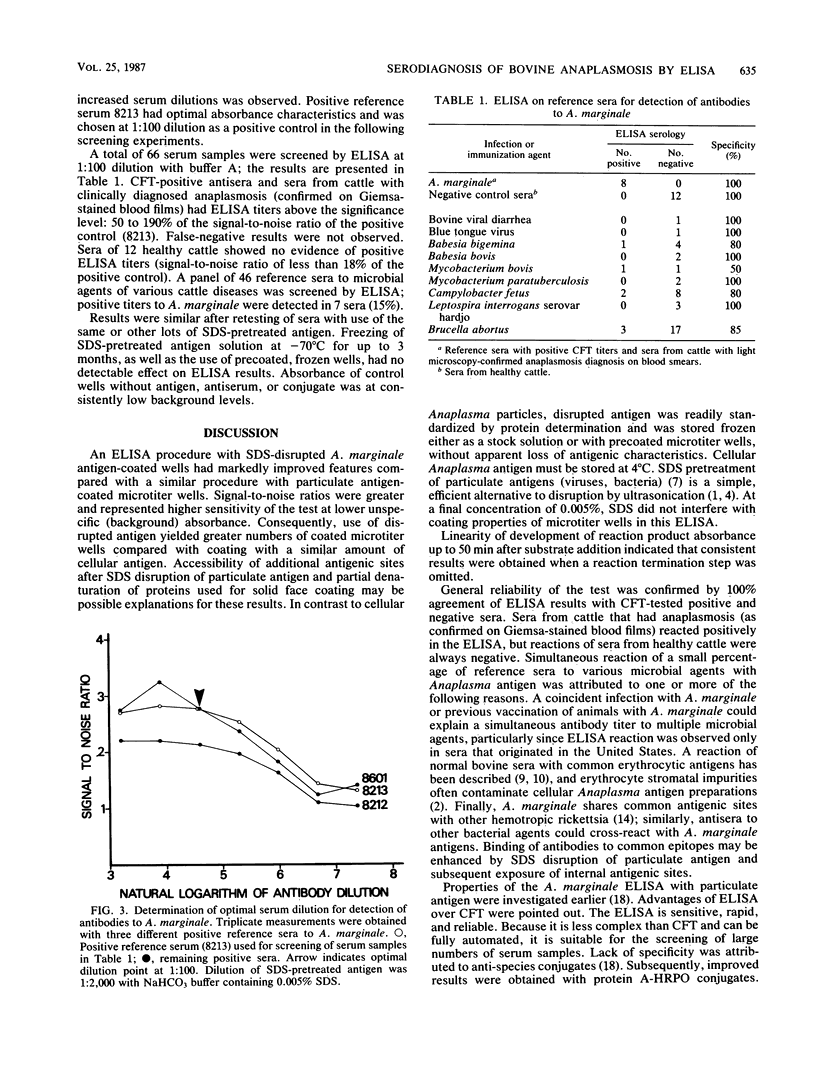
Sensitivities of two enzyme-linked immunosorbent assays (ELISAs) with particulate and sodium dodecyl sulfate (SDS)-disrupted Anaplasma marginale antigen were compared. The quotient of positive reference sera divided by the absorbance quotient of a negative reference serum at identical dilution was termed the signal-to-noise ratio. Optimal signal-to-noise ratios were dependent on both pretreatment of antigen and antigen concentration. SDS disruption of anaplasmal antigen resulted in a markedly improved signal-to-noise ratio of ELISA compared with ELISA with untreated antigen at identical antigen and serum dilutions. This represented higher sensitivity and lower background absorbance of the ELISA with disrupted antigen. SDS-disrupted A. marginale antigen was standardized by protein determination, and antigen, as well as precoated microtiter wells, was stored frozen without apparent loss of antigenic properties. ELISA results were in agreement with results of positive and negative control sera tested by the complement fixation test or by light microscopy Anaplasma diagnosis in Giemsa-stained blood films.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry D. N., Parker R. J., De Vos A. J., Dunster P., Rodwell B. J. A microplate enzyme-linked immunosorbent assay for measuring antibody to Anaplasma marginale in cattle serum. Aust Vet J. 1986 Mar;63(3):76–79. doi: 10.1111/j.1751-0813.1986.tb02934.x. [DOI] [PubMed] [Google Scholar]
- Budden J. R., Dimopoullos G. T. Finite purification of Anaplasma marginale: serologic inactivity of the anaplasma body. Am J Vet Res. 1977 May;38(5):633–636. [PubMed] [Google Scholar]
- Goff W. L., Johnson W. C., Kuttler K. L. Development of an indirect fluorescent antibody test, using microfluorometry as a diagnostic test for bovine anaplasmosis. Am J Vet Res. 1985 May;46(5):1080–1084. [PubMed] [Google Scholar]
- Janitschke K., De Vos A. J., Bigalke R. D. Serodiagnosis of bovine besnoitiosis by ELISA and immunofluorescence tests. Onderstepoort J Vet Res. 1984 Dec;51(4):239–243. [PubMed] [Google Scholar]
- Kocan K. M., Barron S. J., Ewing S. A., Hair J. A. Transmission of Anaplasma marginale by adult Dermacentor andersoni during feeding on calves. Am J Vet Res. 1985 Jul;46(7):1565–1567. [PubMed] [Google Scholar]
- Kuttler K. L. Anaplasma infections in wild and domestic ruminants: a review. J Wildl Dis. 1984 Jan;20(1):12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- Lutz H., Pedersen N. C., Durbin R., Theilen G. H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods. 1983 Jan 28;56(2):209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-James S., James M. A., Ristic M. Modified indirect fluorescent antibody test for the serodiagnosis of Anaplasma marginale infections in cattle. Am J Vet Res. 1985 Nov;46(11):2401–2403. [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Kocan K. M., Barron S. J., Hair J. A., Barbet A. F., Davis W. C., McGuire T. C. Presence of common antigens, including major surface protein epitopes, between the cattle (intraerythrocytic) and tick stages of Anaplasma marginale. Infect Immun. 1985 Dec;50(3):881–886. doi: 10.1128/iai.50.3.881-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Saunders G. C., Bartlett M. L. Double-antibody solid-phase enzyme immunoassay for the detection of staphylococcal enterotoxin A. Appl Environ Microbiol. 1977 Nov;34(5):518–522. doi: 10.1128/aem.34.5.518-522.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., Levy M. G., Kuhlenschmidt M. S., Adams J. H., Rzechula D. L., Hardt T. A., Kocan K. M. Isolate of Anaplasma marginale not transmitted by ticks. Am J Vet Res. 1986 Jan;47(1):127–129. [PubMed] [Google Scholar]
- Thoen C. O., Blackburn B., Mills K., Lomme J., Hopkins M. P. Enzyme-linked immunosorbent assay for detecting antibodies in cattle in a herd in which anaplasmosis was diagnosed. J Clin Microbiol. 1980 May;11(5):499–502. doi: 10.1128/jcm.11.5.499-502.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg J. L. Bovine anaplasmosis: transplacental transmission as it relates to stage of gestation. Am J Vet Res. 1985 Mar;46(3):570–572. [PubMed] [Google Scholar]


