Abstract
Cigarette smoke (CS)–induced lung inflammation involves the reduction of histone deacetylase 2 (HDAC2) abundance, which is associated with steroid resistance in patients with chronic obstructive pulmonary disease and in individuals with severe asthma who smoke cigarettes. However, the molecular mechanism of CS-mediated reduction of HDAC2 is not clearly known. We hypothesized that HDAC2 is phosphorylated and subsequently degraded by the proteasome in vitro in macrophages (MonoMac6), human bronchial and primary small airway epithelial cells, and in vivo in mouse lungs in response to chronic CS exposure. Cigarette smoke extract (CSE) exposure in MonoMac6 and in bronchial and airway epithelial cells led to phosphorylation of HDAC2 on serine/threonine residues by a protein kinase CK2-mediated mechanism, decreased HDAC2 activity, and increased ubiquitin-proteasome–dependent HDAC2 degradation. CK2 and proteasome inhibitors reversed CSE-mediated HDAC2 degradation, whereas serine/threonine phosphatase inhibitor, okadaic acid, caused phosphorylation and subsequent ubiquitination of HDAC2. CS-induced HDAC2 phosphorylation was detected in mouse lungs from 2 weeks to 4 months of CS exposure, and mice showed significantly lower lung HDAC2 levels. Thus, CS-mediated down-regulation of HDAC2 in human macrophages and lung epithelial cells in vitro and in mouse lung in vivo involves the induction of serine/threonine phosphorylation and proteasomal degradation, which may have implications for steroid resistance and abnormal inflammation caused by cigarette smoke.
Keywords: COPD, HDAC2, cigarette smoke, lung, inflammation
CLINICAL RELEVANCE
Cigarette smoke down-regulates histone deacetylase 2 by phosphorylation and proteasomal degradation in epithelial cells and macrophages and in mouse lung, which may have implications for steroid resistance in patients with chronic obstructive pulmonary disease and in individuals with severe asthma who smoke cigarettes.
Cigarette smoke (CS), a complex mixture of oxidants/free radicals and different chemical compounds that include reactive aldehydes and semiquinones known to cause oxidative stress in the lungs, is the primary risk factor for the pathogenesis of chronic obstructive pulmonary disease (COPD) (1–3). It is believed that cigarette smoking is the primary cause of steroid resistance observed in patients with COPD and in individuals with asthma who smoke (4). Corticosteroids suppress inflammation by glucocorticoid receptor recruitment of histone deacetylase 2 (HDAC2) specifically to acetylated histones on promoters of proinflammatory genes, such as IL-8 and GM-CSF (5, 6).
Histone deacetylases (HDACs) are a family of cellular enzymes that regulate gene expression by catalyzing the removal of acetyl groups from the lysine tails of core histones (7). HDAC2 is a class I histone deacetylase that resides almost exclusively in the nucleus and is a critical part of co-repressor complexes recruited to proinflammatory gene promoters by associated proteins (7). The inability of corticosteroids to recruit HDAC2 or the presence of post-translationally modified HDAC2 may explain the abnormal inflammatory response and ineffectiveness of corticosteroid therapy in patients with COPD (6, 8–10). In animal inhalation experiments, lungs of rats and different strains of mice exposed to CS exhibit significantly decreased HDAC2 levels and activity similar to that observed in peripheral blood mononuclear cells (PBMCs) of patients with mild to severe asthma, alveolar macrophages of patients with COPD, and individuals with chronic asthma who smoke (8–12). Bronchial biopsies of patients with COPD and of individuals with asthma who smoke exhibit decreased HDAC2 levels, which correlates with disease severity, increased cytokine production, and corticosteroid insensitivity (13, 14). Several studies have suggested that corticosteroid insensitivity is closely related to reduced levels of HDAC2, increased post-translational modifications, and subsequent degradation of HDAC2 (8–11, 15, 16). For example, HDAC2 is modified by nitration of tyrosine and S-nitrosylation on cysteine residues by nitric oxide/reactive nitrogen species (RNS), formation of protein-aldehyde adducts in response to CS, or by reactive oxygen species (ROS)/aldehydes, subsequently leading to loss of HDAC2 activity (9, 10, 17, 18). Despite overwhelming evidence that loss of HDAC2 by ROS/RNS/aldehyde-mediated post-translational modifications is closely linked to corticosteroid insensitivity, the molecular mechanism of CS-induced degradation of HDAC2, particularly relating to kinase signaling in vivo and in vitro, is still unclear.
Previously, it has been shown that phosphorylation plays an important role in regulating HDAC2 trans-repression ability, enzymatic activity, and levels (19, 20). Furthermore, basal HDAC2 abundance in cells is controlled by the ubiquitin-proteasome pathway (21), but no studies have been conducted to determine whether a phosphorylation-ubiquitination-proteasome pathway is involved in the cellular loss of HDAC2 in response to CS, ROS/RNS, or any environmental stimuli. In the light of this, we sought to determine whether CS mediated HDAC2 degradation by phosphorylation on serine/threonine residues and whether this played a role in regulating the enzymatic activity of HDAC2. Hence, we hypothesized that CS-induced HDAC2 degradation is associated with increased phosphorylation by kinase signaling and ubiquitin-proteasome–dependent degradation of HDAC2 in human cells and mouse lungs.
MATERIALS AND METHODS
Cell Culture
Human monocyte-macrophage cells (MonoMac6) were maintained in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 2 mM L-glutamine, 100 μg/ml penicillin, 100 U/ml streptomycin, 1% nonessential amino acids, 1% sodium pyruvate, 1 μg/ml human holo-transferrin, 1 mM oxaloacetic acid, and 9 μg/ml bovine insulin (10, 22). Cells were cultured at 37°C in humidified atmosphere under 5% CO2.
Human bronchial epithelial cells (H292) were purchased from the American Type Tissue Culture Collection (Manassas, VA) and grown in RPMI 1640 supplemented with 10% FBS (HyClone Laboratories), 2 mM L-glutamine, 100 μg/ml penicillin, and 100 U/ml streptomycin in humidified atmosphere under 5% CO2 at 37°C.
Human primary small airway epithelial cells (SAEC) derived from a single healthy nonsmoker were purchased from Lonza (previously Cambrex, Walkersville, MD) along with growth media (SAGM) bulletKit supplemented with 52 μg bovine pituitary extract, 0.5 ng/ml human recombinant epidermal growth factor (EGF), 0.5 μg/ml epinephrine, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid (RA), 6.5 ng/ml triidothyronine, 50 μg/ml gentamicin/amphotericin B (GA-1000), and 50 μg/ml fatty acid–free bovine serum albumin (BSA). Cells were cultured for no more than seven passages.
Preparation of Aqueous Cigarette Smoke Extract
Research-grade cigarettes (2R4F) with a filter from the Kentucky Tobacco Research and Development Centre at the University of Kentucky (Lexington, KY) were used to prepare cigarette smoke extract (CSE) by slowly bubbling smoke from one cigarette into 10 ml of RPMI 1640 with 0.5% serum at a rate of 1 cigarette/minutes according to the Federal Trade Commission protocol, each puff of 2-second duration and 35-ml volume. pH of cigarette smoke extract was adjusted to 7.4 and sterile-filtered through a 0.45-μm filter (25-mm acrodisc; Pall, Ann Arbor, MI) (10, 23). Extract, defined as 10% CSE as stock solution, was diluted to indicated concentrations and used for all experiments within 15 minutes of preparation. Air was bubbled into 10 ml of RPMI 1640 with 0.5% serum and pH adjusted to 7.4, filtered, and used as control medium.
Treatments
MonoMac6 cells were washed with Ca2+/Mg2+-free PBS and seeded at a density of 5.0 × 106 cells in 100 mm culture plates in a total volume of 7 ml RPMI 1640 containing 0.5% FBS. Cells were treated with CSE (2.5%) or okadaic acid (40 nM and 1 μM) (Alexis Biochemicals, San Diego, CA). For treatments with protein casein kinase (CK2) inhibitor, 4,5,6,7-tetrabromobenzotriazole (TBB), cells were pretreated with 20 μM TBB (Sigma, St. Louis, MO) for 1 or 2 hours. Cells were then washed with sterile PBS before treatment with CSE. For treatments with proteasome inhibitor (N-Acetyl-Leu-Leu-Nle-CHO, ALLN, 1 μM, 5 μM, or 20 μM) (Calbiochem, San Diego, CA), cells were pretreated for 2 hours followed by exposure to CSE. SAEC were grown in 12-well plates containing small airway growth media (SAGM) until confluent and then treated with CSE (0.1%, 0.5%, and 1%) for 4 hours. H292 cells were seeded at a density of 2.5 × 106 cells in 60 mm culture plates in a total volume of 4 ml RPMI 1640 containing 0.5% FBS. Cells were treated with CSE (2.5%), washed in ice-cold sterile Ca2+/Mg2+-free PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris HCl pH 7.4, 150 mM NaCl, 0.25 mM EDTA, 5 mM NaF, 0.1% sodium deoxycholate, 1% Triton X-100); cell extracts and cell culture media were stored at −80°C until analyzed.
Mouse CS Exposure
Male and female A/J mice (Jackson Laboratories, Bar Harbor, ME) at 8 to 10 weeks of age were exposed whole body to either filtered air (FA) or dilute, mainstream CS from 1R3 nonfiltered research grade cigarettes (27.1 mg total particulate matter yield per cigarette; University of Kentucky Tobacco Research and Development Center) for 16 weeks as described (24). For 2-, 4-, and 10-week mouse CS exposures, the 2R4F research (R) grade filtered (F) cigarettes replaced the 1R3 brand, as it was discontinued before the animal exposure period was concluded. The 2R4F brand, unlike the 1R3, is filtered and contains a lower total particulate matter content: 11.7 mg/cigarette, as compared with 27.1 mg/cigarette for 1R3 (this is unusually higher because of the lack of a filter). However, these seemingly apparent differences are eliminated by adjusting mainstream CS smoke and airflow to ensure constant particulate matter exposure of 250 mg/m3. Mice were allowed to acclimatize to CS exposure in the first week by exposing to 100 mg total particulate matter (TPM)/m3 followed by exposure to 250 mg TPM/m3 for 5 days/week, 6 hours/day using either cigarettes as described previously (24).
Lung Tissue Protein Extraction
Frozen lung tissue (100–150 mg) was homogenized in 0.5 ml of buffer A (10 mM HEPES [pH 7.8], 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol, 0.1 M EDTA, 0.2 mM NaF, 0.2 mM Na orthovandate, 1% [vol/vol] Nonidet P-40, 0.4 mM phenylmethyl sulfonyl fluoride, and 1 μg/ml leupeptin) on ice for 30 minutes. Homogenates were centrifuged at 2,000 × g in a bench-top centrifuge for 30 seconds at 4°C to remove cellular debris. Supernatants were then transferred to a 1.7-ml ice-cold Eppendorf tube and centrifuged for 30 seconds at 13,000 × g at 4°C; supernatant was collected as cytoplasmic extract. The pellet was resuspended in 50 μl of buffer C (50 mM HEPES [pH 7.8], 50 mM KCl, 300 mM NaCl, 0.1 M EDTA, 1 mM dithiothreitol, 10% [vol/vol] glycerol, 0.2 mM NaF, 0.2 mM Na orthovanadate, and 0.6 mM phenylmethylsulfonyl fluoride) and placed on rotator in cold room for 30 minutes, centrifuged at 13,000 × g in an Eppendorf tube for 5 minutes, and supernatant collected as the nuclear extract and kept frozen at −80°C. Whole cell lysate was extracted from lung tissue after homogenization in RIPA buffer (12, 25, 26).
Western Blotting
Cells were lysed in 100 μl RIPA buffer supplemented with protease inhibitor cocktail (leupeptin, aprotinin, pepstatin, and PMSF) and stored at −80°C. Protein estimation was performed by the bicinchoninic (BCA) acid method as described by the manufacturer (Pierce, Rockford, IL). Whole cell lysates (10–20 μg) were electrophoresed on 7.5% SDS-polyacrylamide gels, electroblotted on PVDF membranes (Millipore, Burlington, MA), blocked with 5% BSA in Tris-buffered saline (TBS) with 0.1% Tween-20 for 1 hour. Membranes were incubated with rabbit polyclonal anti-HDAC2 (sc-7899), goat polyclonal anti-HDAC1 (sc-6298), and anti-HDAC3 (sc-8138) (Santa Cruz, Santa Cruz, CA) with a 1:1,000 dilution in 5% BSA in TBS. After extensive washing, primary antibodies were detected with secondary antibodies linked to horseradish peroxidase (Dako, Carpinteria, CA) and bound complex detected with enhanced chemiluminescence (PerkinElmer, Waltham, MA).
Immunoprecipitation
Cell lysate (200 μg protein) was incubated overnight with anti-HDAC2 (2 μg) at 4°C, then immunoprecipitated with Protein G–agarose beads for 1 hour with constant agitation. Beads were washed at least four times with RIPA buffer, boiled for 10 minutes in 35 μl 2× sample buffer and resolved on 7.5% SDS-polyacrylamide gel. Cell lysate controls were used. Blots were probed with p-serine (1:1,000) and p-threonine (1:1,000) (Cell Signaling, Boston, MA) monoclonal antibodies for studies on the post-translational modification of HDAC2.
Immunocytochemistry
MonoMac6 cells treated with or without CSE were spun unto cytospin slides, washed with ice-cold PBS, and fixed with 4% paraformaldehyde in PBS. To detect HDAC2 localization, cells were permeabilized with 0.3% Triton X-100, blocked with 10% normal goat serum in immunocytochemistry buffer (1 ml PBS, 50 μl normal goat serum, 5 μg BSA) for 1 hour at room temperature to reduce nonspecific binding, and then incubated with HDAC2 rabbit polyclonal antibody (1:100) overnight at 4°C.
Cells were then incubated in goat anti-rabbit Alexa Fluor 594 (1:200) secondary antibody (Invitrogen, Carlsbad, CA) in immunocytochemistry buffer for 1 hour at room temperature, washed in PBS, and mounted with vectashield mounting media (Vector Laboratories, Burlingame, CA). Hoechst 33342 staining (1:1,000) was used to delineate the nucleus.
HDAC Activity Assay
HDAC2 was immunoprecipitated from cell lysates or lung homogenates (500 μg protein) as described above. Beads were washed and incubated with 150 μl of 1 mM Color de Lys substrate (Biomol, Plymouth Meeting, PA) for 80 minutes with rocking at 37°C. Thirty-microliter aliquots from each sample were placed in 96-well plates, and 20 μl HDAC specific buffer was added. Fifty microliters of Color de Lys developer containing 2 mM trichostatin A (TSA) was then added and incubated for a further 20 minutes with rocking at 37°C. Color development was monitored at 405 nm. HDAC2 activity was expressed relative to standard curve generated from 0–500 μM Color de Lys deacetylated standard.
Statistical Analysis
Data expressed as mean ± SEM of triplicate experiments. Statistical significance was calculated using one-way ANOVA with STATVIEW software (SAS Institute, Cary, NC). NIH ImageJ software was used for densitometry analysis (the P < 0.05 is considered to be statistically significant).
RESULTS
Class I HDACs Are Degraded in Response to CSE Treatment in MonoMac6 Cells
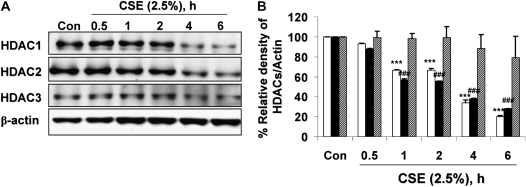
MonoMac6 cells were exposed to CSE (2.5%) for 0.5, 1, 2, 4, and 6 hours. CSE decreased cellular HDAC1 and HDAC2 levels by 44% relative to controls as early as 1 hour after CSE treatment and by 73% at 6 hours (Figures 1A and 1B). Intriguingly, cellular HDAC3 was less sensitive to CSE treatment than HDAC1 and HDAC2. To determine whether class I HDAC degradation was due to decreased CSE-induced cell viability, lactate dehydrogenase (LDH) secreted into conditioned cell media was measured immediately after harvest. Cells treated with CSE (2.5%) at 6 hours were more than 90% viable as compared with cells treated with media alone (data not shown). To confirm that HDAC2 degradation was a direct consequence of CSE exposure, an optimal time point (6 h) was selected at which significant HDAC2 degradation was observed and MonoMac6 cells were exposed to increasing concentrations of CSE. Increasing doses of CSE exposure induced a dose-dependent decrease in HDAC2 levels (data not shown).
Figure 1.
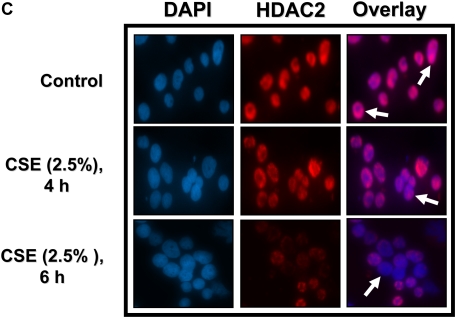
Cigarette smoke extract (CSE) time-dependently caused degradation of class I histone deacetylases (HDACs) in MonoMac6 cells. MonoMac6 cells were treated with CSE (2.5%) for indicated time points. Ten micrograms of protein from whole cell extracts was electrophoresed on SDS-7.5% polyacrylamide gel, transferred onto PVDF membranes, and immunochemically stained with HDAC1, HDAC2, and HDAC3 antibodies. (A) CSE significantly decreased class I HDAC levels as early as 1 hour after CSE exposure. (B) Relative density of HDAC levels in MonoMac6 cells at various time points of exposure to CSE. Open bars, HDAC1; solid bars, HDAC2; hatched bars, HDAC3. (C) MonoMac6 cells were treated with CSE (2.5%) for 0, 4, and 6 hours, cytospin slides were prepared, and cells were fixed in 4% paraformaldehyde and immunostained with anti-HDAC2 antibodies. DAPI stain was used to visualize the nucleus (shown by arrows). CSE showed significant loss of HDAC2 expression in the nucleus without any nucleo-cytoplasmic shuttling in MonoMac6 cells. Data are shown as mean ± SEM (n = 3). ***P < 0.001 or ###P < 0.001, significant compared with respective control treatments.
Class I HDACs are predominantly nuclear proteins (7), but thought to be present in the cytoplasm. To determine if CSE induced significant HDAC2 nucleocytoplasmic shuttling, MonoMac6 cells were treated with CSE (2.5%) for 0.5, 1, 4, and 6 hours, cells were fixed in 4% paraformaldehyde and immunostained with anti-HDAC2 antibodies to determine HDAC2 localization. Contrary to our expectations, HDAC2 did not show any significant translocation to the cytoplasm in response to CSE at any time points studied (Figure 1C).
CSE-Induced HDAC2 Degradation Is Associated with Decreased Specific HDAC2 Activity in MonoMac6 Cells
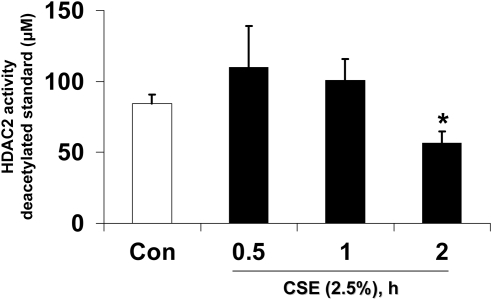
To determine if CSE-induced loss of HDAC2 is associated with a change in specific HDAC2 activity, MonoMac6 cells were treated with CSE (2.5%) for 0.5, 1, and 2 hours and endogenous HDAC2 was immunoprecipitated. HDAC2 activity was significantly decreased as early as 2 hours after CSE treatment (P < 0.05) (Figure 2), indicating a possible role for CSE-induced post-translational modifications.
Figure 2.
CSE-induced HDAC2 degradation is associated with decreased specific HDAC2 activity in MonoMac6 cells. MonoMac6 cells were treated with CSE (2.5%) for 0.5, 1, and 2 hours, and immunoprecipitated HDAC2 (500 μg protein) from whole cell extracts was assayed for HDAC2 activity with a colorimetric assay kit (Biomol) at 405 nm with Color de Lys substrate (500 μM) and expressed as deacetylated standard using 1 to 500 μM standard curve. HeLa nuclear extract was used as a positive control. Data are shown as mean ± SEM (n = 4). *P < 0.05, significant compared with media alone control treatments.
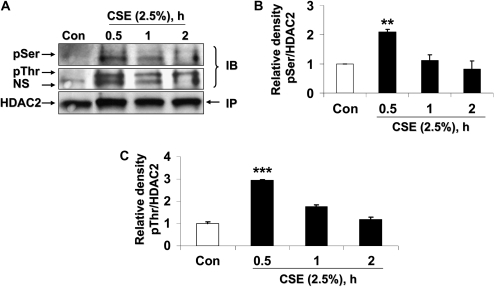
CSE Induces HDAC2 Phosphorylation in MonoMac6 Cells
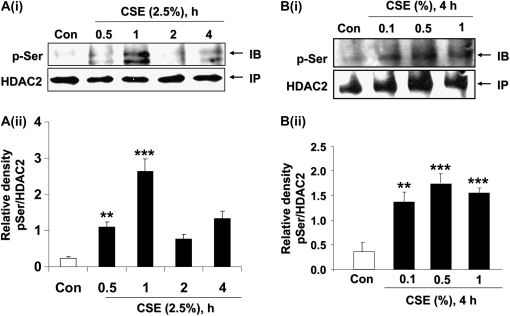
To determine whether CSE phosphorylated HDAC2, MonoMac6 cells were treated with CSE (2.5%) for 0.5, 1, and 2 hours. CSE induced the phosphorylation of HDAC2 on both serine and threonine residues maximally at 0.5 hours, which thereafter decreased time-dependently with CSE exposure (Figures 3A–3C). CSE-induced HDAC2 phosphorylation was transient and was diminished after longer than 2 hours of exposure to CSE.
Figure 3.
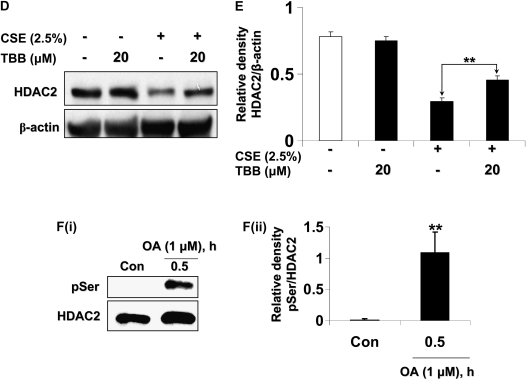
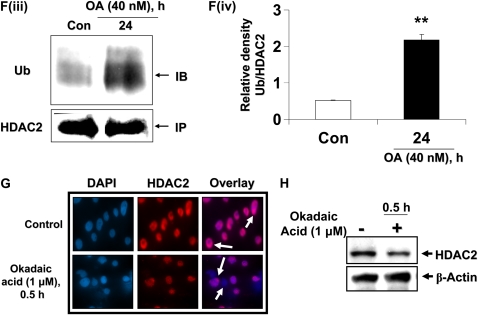
CSE induces HDAC2 phosphorylation via CK2 in MonoMac6 cells. (A) MonoMac6 cells were treated with CSE (2.5%) for 0.5, 1, and 2 hours. Immunoprecipitated HDAC2 was analyzed for phosphorylation of serine and threonine residues using specific phospho-serine and phospho-threonine antibodies by SDS-polyacrylamide gel. CSE induced significant phosphorylation of HDAC2 on serine and threonine residues. NS, nonspecific bands. Relative density of (B) phospho-serine and (C) phospho-threonine levels normalized to immunoprecipitated HDAC2 and expressed as fold change relative to control. (D) MonoMac6 cells were pretreated with or without CK2 inhibitor (20 μM 4,5,6,7-tetrabromobenzotriazole [TBB]) for 2 hours. Cells were then exposed to CSE (2.5%) for 6 hours. Ten micrograms of protein from whole cell lysates was electrophoresed on SDS-polyacrylamide gel and assayed for HDAC2 levels. TBB significantly inhibited HDAC2 degradation in response to CSE. (E) Relative density of HDAC2 levels normalized to β-actin. [F(i)] MonoMac6 cells were treated with phosphatase inhibitor, okadaic acid (1 μM), for 0.5 hours. Immunoprecipitated HDAC2 was analyzed for phosphorylation of serine residues using specific phospho-serine antibody by SDS-polyacrylamide gel. Okadaic acid significantly induced phosphorylation of HDAC2 on serine residues. [F(ii)] Relative density of phospho-serine expression normalized to immunoprecipitated HDAC2. [F(iii)] MonoMac6 cells were treated with or without phosphatase inhibitor, okadaic acid (40 nM), for 24 hours. Immunoprecipitated HDAC2 was assessed for bound ubiquitin using specific ubiquitin anti-sera. Okadaic acid induced significant ubiquitination of HDAC2. [F(iv)] Relative density of ubiquitin levels normalized to immunoprecipitated HDAC2. (G) MonoMac6 cells were treated with okadaic acid (1 μM) for 0.5 hours, cytospin slides were prepared, and cells were fixed in 4% paraformaldehyde and immunostained with anti-HDAC2 antibodies. DAPI stain was used to visualize the nucleus (arrows). Okadaic acid showed an early and significant loss of HDAC2 abundance in the nucleus without any nucleo-cytoplasmic shuttling in MonoMac6 cells. (H) MonoMac6 cells were treated with okadaic acid (1 μM) for 0.5 hours. Western blots for nuclear extracts showed significant decrease in HDAC2 expression. Data expressed as mean ± SEM (n = 3). **P < 0.01, ***P < 0.001 significant compared with media alone treatments or respective controls.
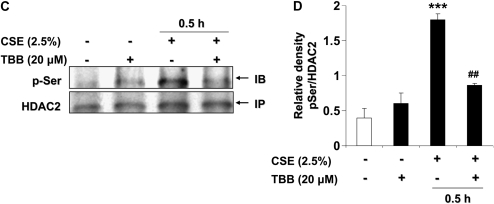
Since CK2 has been implicated in hypoxia-induced HDAC2 phosphorylation (21, 27), we reasoned that CK2 inhibition with a specific CK2 inhibitor, TBB (20 μM) (28), would attenuate CSE-induced HDAC2 phosphorylation and degradation. TBB significantly attenuated HDAC2 degradation in response to CSE treatment (Figures 3D and 3E). At concentrations and time points of TBB treatment, cells remained greater than 90% viable as determined by trypan blue cell viability assay and cellular morphology (data not shown), and no change in HDAC2 relative levels compared with control was observed. To further validate the CSE-induced phosphorylation of HDAC2, MonoMac6 cells were treated with PP1/2A phosphatase inhibitor, okadaic acid, for 0.5 and 1 hour. Immunoprecipitated HDAC2 was then assessed for phosphorylation of serine residues. Okadaic acid significantly increased the phosphorylation of HDAC2 on serine residues, as earlier observed with CSE (Figure 3F, panels i and ii). HDAC2 phosphorylation in response to okadaic acid was also diminished beyond 1 hour.
To further confirm our hypothesis, we determined if exposure to okadaic acid would induce ubiquitination and degradation of HDAC2. MonoMac6 cells were treated with okadaic acid (40 nM), at a dose that was not toxic to the cells for 24 hours (data not shown). Immunoprecipitated HDAC2 was then assayed for the presence of ubiquitinated residues using specific ubiquitin antisera by Western blot. Okadaic acid significantly induced the ubiquitination of HDAC2 (Figure 3F, panels iii and iv) and also induced the loss of nuclear HDAC2 (Figures 3G and 3H).
HDAC2 Degradation Is Ubiquitin-Proteasome Dependent
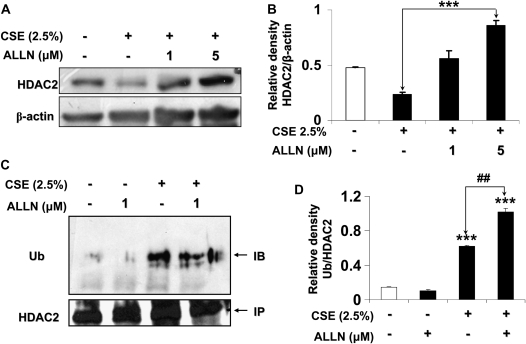
Since HDAC2 undergoes basal turnover via the proteasome (21), we hypothesized that CSE-induced HDAC2 degradation is proteasome dependent. To test this, MonoMac6 cells were pretreated with the specific proteasome inhibitor, N-Acetyl-Leu-Leu-Nle-CHO (ALLN) (1 μM and 5 μM) for 2 hours before treatment with CSE (2.5%) for 6 hours. ALLN dose-dependently inhibited HDAC2 degradation in response to CSE (Figures 4A and 4B). ALLN (20 μM) did not induce cell death, nor was HDAC2 degraded in cells treated with the compound alone (data not shown). Since basal HDAC2 turnover was proteasome dependent, we also expected that ALLN treatment would inhibit both CSE-induced HDAC2 degradation and basal turnover. To determine if CSE-induced HDAC2 degradation was dependent on a ubiquitin-mediated pathway, endogenous HDAC2 from MonoMac6 cells treated with CSE with or without ALLN (1 μM) for 6 hours was immunoprecipitated and assayed for the presence of ubiquitinated residues. CSE treatment significantly induced the ubiquitination of HDAC2, which was further enhanced by pretreatment with ALLN (Figures 4C and 4D), an important mechanism required to target specific proteins for proteasomal degradation.
Figure 4.
CSE-mediated degradation of HDAC2 is ubiquitin-proteasome dependent in MonoMac6 cells. (A) MonoMac6 cells were pre-treated with proteasome inhibitor N-Acetyl-Leu- Leu-Nle-CHO (ALLN, 1 μM and 5 μM) for 2 hours, followed by a 6-hour exposure to CSE (2.5%). Ten micrograms of protein from whole cell extracts was electrophoresed on SDS-7.5% polyacrylamide gel, transferred onto PVDF membranes, and immunochemically stained with HDAC2 antibodies. Dose-dependent inhibition of the proteasome significantly attenuated HDAC2 degradation. (B) Relative density of HDAC2 levels in MonoMac6 cells after proteasome inhibition of CSE-induced HDAC2 degradation normalized to β-actin. (C) MonoMac6 cells were pre-treated with or without ALLN (1 μM) and then exposed to CSE (2.5%) for 6 hours. Immunoprecipitated HDAC2 was assayed for bound ubiquitin using specific ubiquitin anti-sera. CSE induced significant increase in HDAC2 ubiquitination, which was enhanced with ALLN pretreatment. (D) Relative density of ubiquitin expression in MonoMac6 cells after proteasome inhibition of CSE-induced HDAC2 degradation normalized to immunoprecipitated HDAC2. Data shown as mean ± SEM (n = 3). ***P < 0.001, significant compared with media alone control treatments; ##P < 0.001, significant compared with CSE+ALLN versus CSE alone.
CSE Time-Dependently Causes Degradation of HDAC2 in Bronchial Epithelial Cells and Primary Small Airway Epithelial Cells
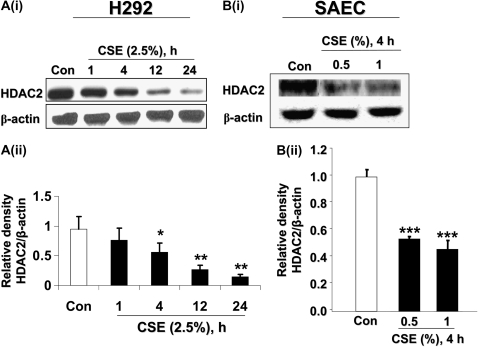
Bronchial epithelial cells (H292) were exposed to CSE (2.5%) for 1, 4, 12, and 24 hours and the levels of HDAC2 determined by Western blot. While epithelial cells showed less sensitivity to CSE as compared with MonoMac6 cells, CSE-induced HDAC2 degradation was observed as early as 4 hours after treatment (Figure 5A, panels i and ii). To further confirm results observed in transformed epithelial cells, we used human primary small airway epithelial cells (SAEC) exposed to CSE (0.5% and 1%) for 4 hours. Lower doses of CSE were used this time, since the primary cells had been shown to be more sensitive compared with immortalized epithelial cell lines (23). We found that CSE decreased HDAC2 levels in a dose-dependent fashion in primary SAEC cells, similar to our observations in bronchial epithelial cell lines (Figure 5B, panels i and ii).
Figure 5.
CSE time-dependently caused degradation of HDAC2 in human bronchial epithelial and primary small airway epithelial cells. [A(i)] Bronchial epithelial cells (H292) were treated with CSE (2.5%) for 0, 1, 4, 12, and 24 hours. Ten micrograms of protein from whole cell extracts was electrophoresed on SDS-7.5% polyacrylamide gel, transferred onto PVDF membranes and immunochemically stained with HDAC2 antibodies. CSE time-dependently decreased HDAC2 levels in H292 cells. [A(ii)] Relative density of HDAC2 expression in H292 cells exposed to CSE normalized to β-actin. [B(i)] Small airway epithelial cells (SAEC) were exposed to CSE (0.5% and 1%) for 4 hours. Whole cell lysates were electrophoresed on SDS-polyacrylamide gels and membranes immunochemically stained for HDAC2 expression. CSE time-dependently induced HDAC2 degradation. [B(ii)] Relative density of HDAC2 levels in SAECs exposed to CSE normalized to β-actin. Data shown as mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 significant compared with control treatments.
CSE Induces HDAC2 Phosphorylation in Human Bronchial Epithelial and Small Airway Epithelial Cells
Bronchial epithelial cells (H292) were exposed to CSE (2.5%) for 0.5, 1, 2, and 4 hours. CSE transiently induced HDAC2 serine phosphorylation with the most intense serine phosphorylation observed in H292 cells as early as 0.5 to 1 hour (Figure 6A, panels i and ii). As was observed in MonoMac6 cells, HDAC2 phosphorylation was cyclical. CSE (0.1%, 0.5%, and 1%) for 4 hours dose-dependently induced HDAC2 serine phosphorylation in primary SAEC in a manner similar to that in H292 cells (Figure 6B, panels i and ii). Higher doses of CSE induced phosphorylation at earlier time-points (data not shown) compared with lower doses used, an indication that an unknown mechanism whereby HDAC2 is phosphorylated was being induced by CSE in a dose-dependent manner. Treatment of bronchial epithelial cells with CK2 inhibitor TBB (20 μM) for 1 hour completely reversed CSE-induced HDAC2 phosphorylation (Figures 6C and 6D).
Figure 6.
CSE induced CK2-dependent HDAC2 phosphorylation in human bronchial epithelial and small airway epithelial cells. [A(i)] H292 cells were treated with CSE (2.5%) for 0.5, 1, 2, and 4 hours. Immunoprecipitated HDAC2 was analyzed for phosphorylation of serine residues using specific phospho-serine antibodies by SDS-polyacrylamide gel. CSE induced significant cyclical phosphorylation of HDAC2 on serine residues at 0.5, 1, and 4 hours. [A(ii)] Relative density of phospho-serine level normalized to immunoprecipitated HDAC2. [B(i)] SAECs were exposed to CSE (0.1%, 0.5%, and 1%) for 4 hours. Immunoprecipitated HDAC2 from whole cell lysates was probed for phosphorylation of serine residues. CSE induced a dose-dependent increase in HDAC2 serine phosphorylation. [B(ii)] Relative density of phospho-serine level normalized to immunoprecipitated HDAC2. (C) H292 cells were treated with or without specific CK2 inhibitor, TBB, for 1 hour, and cells were washed with PBS and then treated with freshly prepared CSE (2.5%) for 0.5 hours. Immunoprecipitated HDAC2 was assayed for phosphorylation on serine residues. TBB significantly inhibited CSE-induced HDAC2 phosphorylation. (D) Relative density of phospho-serine level normalized to immunoprecipitated HDAC2. Data expressed as mean ± SEM (n = 3). **P < 0.01, ***P < 0.001, significant compared with control treatments, or respective controls. ##P < 0.01 significant compared with CSE + TBB versus CSE alone.
CS Exposure Induces HDAC2 Degradation and Decreased Activity Associated with its Phosphorylation in Mouse Lungs
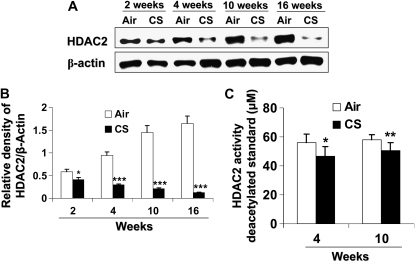
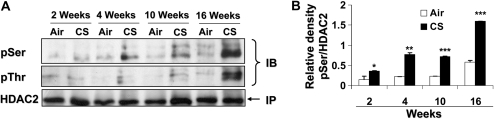
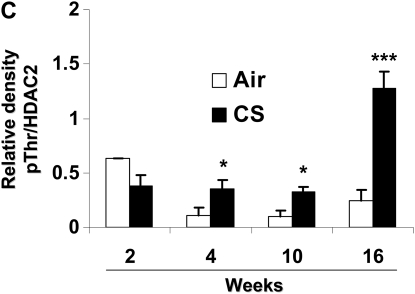
CS exposure (2, 4, 10, and 16 wk) time-dependently decreased HDAC2 levels in mouse lungs (Figures 7A and 7B). Furthermore, CS-exposed mice at 4 and 10 weeks significantly showed decreased HDAC2 activity compared with air-exposed mice (Figure 7C). HDAC2 was phosphorylated at serine/threonine residues in the lungs of mice exposed to CS from 2 to 16 weeks as compared with mice exposed to filtered air alone (Figure 8). Phosphorylation was more pronounced on serine residues compared with threonine residues. Interestingly, HDAC2 level was restored in lungs of mice allowed to recover in fresh filtered air for 4 months after a 5-month chronic CS exposure (airspace enlargement is seen), suggesting that CS-induced loss of HDAC2 is reversible (data not shown).
Figure 7.
CS exposure caused HDAC2 degradation and decreased activity in mouse lungs. Mice were exposed to CS as described in Materials and Methods. Lung tissue (100–150 mg) was homogenized and nuclear extracts prepared and frozen until analyzed. Five micrograms of protein of nuclear extracts were analyzed for HDAC2 abundance on SDS-polyacrylamide gels. (A) CS time-dependently decreased HDAC2 levels in mouse lungs. (B) Relative density of HDAC2 levels normalized to β-actin. (C) HDAC2 was immunoprecipitated and HDAC2 activity measured by colorimetric assay kit (Biomol) using Color de Lys substrate. Mice exposed to CS showed decreased specific HDAC2 activity as compared with air exposed mice. Data expressed as mean ± SEM (n = 4–5 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with air-exposed mice.
Figure 8.
CS induces HDAC2 phosphorylation in mouse lungs. Mice were exposed to CS as described in Materials and Methods. Mouse lung tissue (100–150 mg) was homogenized in RIPA buffer (100 μl) as whole tissue extracts, and HDAC2 (500 μg) was immunoprecipitated. Phosphorylation of serine and threonine residues was analyzed using specific phospho-serine and phospho-threonine antibodies on SDS-polyacrylamide gel. (A) Mice exposed to CS for 2, 4, 10, and 16 weeks showed significant phosphorylation of HDAC2 on both serine and threonine residues; however, mice exposed at 2 weeks did not show significant phosphorylation on threonine residues. Relative density of (B) phospho-serine and (C) phospho-threonine HDAC2 levels normalized to immunoprecipitated HDAC2. Data expressed as mean ± SEM (n = 4–5 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with air-exposed littermates.
DISCUSSION
Smokers and patients with severe COPD, known to be unresponsive to corticosteroid therapy, have decreased HDAC2 levels/expression and activity in alveolar macrophages and lungs (13). CSE-induced HDAC2 degradation also causes increased global histone H4 acetylation and increased histone acetyltransferase (HAT) activity in alveolar epithelial A549 cells and monocyte-macrophage MonoMac6 cells (10, 25, 29). Phosphorylation is associated with decreased HDAC2 activity (20). We sought to test the hypothesis that CS-induced down-regulation of HDAC2 involves phosphorylation of HDAC2 particularly on serine residues. Our data show that CSE caused degradation of HDAC1, HDAC2, and HDAC3 in MonoMac6 cells and also induced a decrease in specific HDAC2 activity. Interestingly, HDAC3 was less sensitive to CSE exposure compared with other class I HDACs studied, suggesting that HDAC3 may not play so prominent a role in regulating the inflammatory response in cells exposed to CSE. HDAC3 is also regarded as distinct from HDAC1 and HDAC2, as it is not found in either Sin3 or NuRD co-repressor complexes (30). While CSE treatment induced degradation of HDAC1, HDAC2, and to a lesser extent HDAC3, previously described mechanisms of degradation for all three histone deacetylases appear to differ markedly. While HDAC1 has been reported to be degraded by diesel exhaust particulate matter (DEP) (31) and TNF-α through an IκB-kinase (IKK)2-dependent mechanism (32), HDAC2 and HDAC3 were not affected. Valproic acid also selectively degrades HDAC2 via the proteasome by increasing expression of E2 ubiquitin-conjugating enzyme Ubc8 (21); surprisingly, both HDAC1 and HDAC3 were not affected by valproic acid treatment. This indicates the possibility of clear differences in CSE-induced regulation of all three HDACs by multiple complex pathways with the possibility of a greater similarity between HDAC1 and HDAC2. However, we have primarily focused on HDAC2, since its loss and decreased deacetylase activity in the lung correlates strongly with disease severity in patients with COPD (13).
Phosphorylation/dephosphorylation of proteins in eukaryotic cells on serine, threonine, or tyrosine residues is an important mechanism by which several cellular processes are regulated. Phosphorylation may act as a master switch to turn on or off certain kinases, activate enzymes, produce conformational changes in proteins, or act as a trigger to induce proteasomal degradation of certain proteins such as IκB-α.
HDAC2 was first discovered to be a phospho-protein using in vitro kinase assays that suggested that protein kinase CK2 was the major source of HDAC2 kinase (19). Tsai and Seto showed that HDAC2 was specifically phosphorylated on serine but not on threonine or tyrosine residues (19). Pluemsampant and colleagues also implicated protein kinase CK2 in hypoxia-induced phosphorylation of serine residues on HDAC2 in tumor cells by knocking down CK2α and CK2α′ expression, which abolished HDAC2 phosphorylation (27). Tsai and Seto, using in vitro techniques, determined three potential phosphorylation sites (Ser394, Ser422, and Ser424) on HDAC2 that present within the recognition sequences for protein kinase CK2, which has been largely shown to be responsible for HDAC2 phosphorylation (19). However, the physiological relevance of HDAC2 phosphorylation remains largely controversial (19, 20, 27, 33). We observed significant serine phosphorylation of HDAC2 in MonoMac6, bronchial (H292), and small airway epithelial cells (SAEC), and in mouse lungs both on serine and threonine residues. Phosphorylation of threonine residues was, however, negligible, indicating that while serine phosphorylation is global, phosphorylation of threonine residues may be cell- and tissue-specific. This is reinforced in part by a study that showed that serine residues, but not threonine or tyrosine residues, on HDAC2 were phosphorylated by CK2 (19).
CSE induced the degradation of HDAC2 expression in MonoMac6 cells as quickly as 1 hour after exposure, while HDAC2 degradation was observed 2 weeks after CS exposure in mouse lung tissue. Immunoprecipitated HDAC2 from lungs of CS-exposed mice showed increased levels of serine phosphorylation as early as 2 weeks after CS exposure, and serine phosphorylation increased with increasing degradation of HDAC2 in the lungs, suggesting a strong association between phosphorylation and CS-induced down-regulation of HDAC2. The highest level of phosphorylation was observed in 16-week CS-exposed groups, which also showed the highest level of HDAC2 degradation. Consistent with the other study in which basal phosphorylation of HDAC2 is seen (20), we also observed slight basal phosphorylation of HDAC2 in lungs of air-exposed mice associated with increased HDAC2 levels.
While the mechanism of CS-induced HDAC2 phosphorylation remains unknown, it is possible that CS directly induces several candidate kinases that phosphorylate HDAC2 or indirectly acts as a protein phosphatase inhibitor. We tested both the possibilities, first by treating MonoMac6 cells with the PP1/2A inhibitor, okadaic acid. Okadaic acid induced HDAC2 phosphorylation on serine residues and caused loss of HDAC2 in the nucleus as early as 0.5 hours after inhibitor exposure. These data were supported by the finding of Galasinski and coworkers, who showed that okadaic acid exposure induced phosphorylation of HDAC2 as observed by gel mobility retardation and disruption of the HDAC co-repressor complex (20). Another possibility of HDAC2 phosphorylation would be by protein kinase CK2, which is known to be only kinase that directly phosphorylates HDAC2 on three known recognition sites (19). We tested the hypothesis that CK2 inhibitors would attenuate CSE-induced HDAC2 phosphorylation in MonoMac6 cells as well as in epithelial cells. A specific CK2 inhibitor, TBB, significantly reversed CSE-induced HDAC2 phosphorylation and degradation, implicating phosphorylation as an important step in CSE-induced HDAC2 degradation. Since phosphorylation is a reversible process, inactivation of protein phosphatases by CSE will lead to irreversible phosphorylation of HDAC2, and subsequent oxidative post-translational modifications will render HDAC2 subject to proteasomal degradation (10, 16, 34). Nevertheless, our data suggest that protein kinase CK2 is involved in CSE-mediated phosphorylation of HDAC2, but we cannot rule out the involvement of other potential kinases (PI-3K and MAPK) in HDAC2 phosphorylation in these cells. While Ser394, Ser422, and Ser424 are CK2 recognition motifs, Ser411 is not, which may be potentially phosphorylated by PKA and PKC (19). Oxidative stress (H2O2) has been shown to induce the activation of PI-3K, which was associated with reduced HDAC activity in U937 monocytic cell line (35). The PI-3K/Akt inhibitor, LY-294002, restored oxidant-mediated reduced HDAC2 activity, but the effect of PI-3K/Akt inhibition on CSE-mediated phosphorylation of HDAC2 was not studied (35). Since PI-3K and Akt are cell membrane-associated kinases while HDAC2 is predominantly localized to the nucleus, we speculate that any possible role for PI-3K in HDAC2 phosphorylation is most likely as an upstream activator of other effector kinases. Furthermore, recent evidence indicates that CSE induces binding of CK2α to HDAC2 (D. Adenuga and colleagues, unpublished observation), strongly implicating CK2α as the effector kinase responsible for HDAC2 phosphorylation. While LY-294002, a nonspecific PI-3K inhibitor, restores oxidant-mediated reduced HDAC2 activity (35), it must be noted that it also inhibits CK2α with potency similar to that of PI-3K (36), suggesting that its effects may not be solely due to inhibition of PI-3K alone. It remains to be seen whether PI-3K/Akt inhibitors can directly inhibit HDAC2 serine phosphorylation and restore steroid efficacy in vivo in response to CS in mouse lungs. Indeed, as we found while proofreading this article, Marwick and colleagues showed that inhibition of PI-3K delta restored glucocorticoid function in smoking-induced airway inflammation in mice (39). Nevertheless, understanding the involvement of specific kinase inhibitors will lead to the development of small molecule kinase inhibitors, which may restore steroid efficacy in patients with COPD by restoring both HDAC2 activity and protein levels in the lungs.
Basal HDAC2 turnover and valproic acid (VPA)-induced HDAC2 degradation is mediated by the proteasome and requires the E2 ubiquitin conjugase, Ubc8 (21). We determined that this mechanism is involved in CSE-mediated HDAC2 degradation in MonoMac6 cells. Our data showed that ALLN, a potent proteasomal inhibitor, attenuated CSE-induced HDAC2 degradation. Interestingly, treatment of MonoMac6 cells with the phosphatase inhibitor (okadaic acid) also induced increased phosphorylation, ubiquitination, and degradation of HDAC2. Ubc8 expression was low in MonoMac6 cells, and no significant induction of Ubc8 was observed in response to CSE, indicating that this pathway was not required for both okadaic acid and CSE-induced HDAC2 degradation. Immunocytochemistry did not show any nucleo-cytoplasmic shuttling of HDAC2, unlike class II HDACs. It is known that 14-3-3ɛ protein binds to phosphoserine residues of HDAC4 and HDAC5, leading to nucleo-cytoplasmic shuttling but not to HDAC1 or HDAC2 (37); this may possibly explain the absence of nucleo-cytoplasmic shuttling in phosphorylated HDAC2 unlike HDAC4 and HDAC5.
Our data suggest that both phosphorylation and dephosphorylation events are involved in CSE-mediated HDAC2 degradation. Hence, apart from kinase inhibitors, activation or restoration of inactive protein phosphatases, such as the MAPK phosphatase-1 (MKP-1), could prove a potential mechanism of restoring HDAC2 levels/activity. The PP1/2A inhibitor, okadaic acid, induced a very similar response to CSE exposure in cells, and CS exposure is known to decrease expression of lung tissue protein phosphatases such as MKP-1 in vivo (38). Hence the use of specific proteasome inhibitors and/or MKP-1 activators to attenuate CS-induced HDAC2 degradation with the long-term goal of restoring steroid function in patients with COPD and in individuals with asthma who smoke are areas for further research.
In conclusion, our data clearly show that HDAC2 is negatively regulated by cigarette smoke via the phosphorylation-ubiquitin-proteasomal pathway. We further show that phosphorylation/dephosphorylation of HDAC2 plays a predominant role in regulating cellular HDAC2 abundance. These findings suggest that small molecule kinase inhibitors and/or protein phosphatase inducers (methylxanthines-theophylline, aminophylline and caffeine, polyphenolics-curcumin and catechins), targeted solely at improving HDAC2 activity and level in smokers and patients with COPD, may reverse steroid resistance.
Acknowledgments
The authors thank Dr. Se-Ran Yang for technical assistance.
This work was supported by the National Institutes of Health-NHLBI Grant R01-HL-085613 (to I.R.), NIEHS Toxicology training grant ES-07026, and NIEHS Environmental Health Sciences Center Grant ES-01247.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0255OC on October 16, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pryor WA, Stone K. Oxidants in cigarette smoke: radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993;686:12–27. [DOI] [PubMed] [Google Scholar]
- 2.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 3.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 2006;533:222–239. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:342–344. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 2000;20:6891–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol 2008; (In press). (PMID 18817512.) [DOI] [PubMed]
- 7.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003;370:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J 2001;15:1110–1112. [PubMed] [Google Scholar]
- 9.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, MacNee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 2004;31:633–642. [DOI] [PubMed] [Google Scholar]
- 10.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 2006;291:L46–L57. [DOI] [PubMed] [Google Scholar]
- 11.Cosio BG, Mann B, Ito K, Jazrawi E, Barnes PJ, Chung KF, Adcock IM. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am J Respir Crit Care Med 2004;170:141–147. [DOI] [PubMed] [Google Scholar]
- 12.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1174–L1186. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 2002;166:392–396. [DOI] [PubMed] [Google Scholar]
- 15.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-κB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 2008;10:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meja KK, Rajendrasozhan S, Adenuga D, Biswas SK, Sundar IK, Spooner G, Marwick JA, Chakravarty P, Fletcher D, Whittaker P, et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am J Respir Cell Mol Biol 2008;39:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun 2004;315:240–245. [DOI] [PubMed] [Google Scholar]
- 18.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 2008;455:411–415. [DOI] [PubMed] [Google Scholar]
- 19.Tsai SC, Seto E. Regulation of histone deacetylase 2 by protein kinase CK2. J Biol Chem 2002;277:31826–31833. [DOI] [PubMed] [Google Scholar]
- 20.Galasinski SC, Resing KA, Goodrich JA, Ahn NG. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J Biol Chem 2002;277:19618–19626. [DOI] [PubMed] [Google Scholar]
- 21.Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J 2003;22:3411–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock HW, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (MonoMac6) with characteristics of mature monocytes. Int J Cancer 1988;41:456–461. [DOI] [PubMed] [Google Scholar]
- 23.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 2006;7:132–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci 2006;92:545–559. [DOI] [PubMed] [Google Scholar]
- 25.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, et al. IKKα causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 2008;38:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 2008;172:1222–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluemsampant S, Safronova OS, Nakahama K, Morita I. Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int J Cancer 2008;122:333–341. [DOI] [PubMed] [Google Scholar]
- 28.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘Casein Kinase-2’). FEBS Lett 2001;496:44–48. [DOI] [PubMed] [Google Scholar]
- 29.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897–1899. [DOI] [PubMed] [Google Scholar]
- 30.Knoepfler PS, Eisenman RN. Sin meets NuRD and other tails of repression. Cell 1999;99:447–450. [DOI] [PubMed] [Google Scholar]
- 31.Cao D, Bromberg PA, Samet JM. Cox-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol 2007;37:232–239. [DOI] [PubMed] [Google Scholar]
- 32.Vashisht GYN, Arora TS, Van Dyke MW. Tumour necrosis factor-alpha depletes histone deacetylase 1 protein through IKK2. EMBO Rep 2006;7:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JM, Chen HY, Davie JR. Differential distribution of unmodified and phosphorylated histone deacetylase 2 in chromatin. J Biol Chem 2007;282:33227–33236. [DOI] [PubMed] [Google Scholar]
- 34.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem 2008;389:203–209. [DOI] [PubMed] [Google Scholar]
- 35.Failla M, To Y, Ito M, Adcock IM, Barnes PJ, Ito K. Oxidative stress-induced PI3-kinase activation reduces HDAC activity and is inhibited by theophylline. Am J Respir Crit Care Med 2007;176:A45. [Google Scholar]
- 36.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000;351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14–3-3-dependent cellular localization. Proc Natl Acad Sci USA 2000;97:7835–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Russell RM, Wang XD. Low dose beta-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38 MAPK, and p53 proteins. J Nutr 2004;134:2705–2710. [DOI] [PubMed] [Google Scholar]
- 39.Marwick JA, Caramori G, Stevenson CC, Casolari P, Jazrawi E, Barnes PJ, Ito K, Adcock IM, Kirkham PA, Papi A. Inhibition of PI3K{delta} restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med [Epub ahead of print on 2009 Jan 22]. [DOI] [PubMed]