Abstract
The PIN family of auxin efflux transporters exhibit polar plasma membrane (PM) localization and play a key role in auxin gradient-mediated developmental processes. Auxin inhibits PIN2 endocytosis and promotes its PM localization. However, the underlying mechanisms remain elusive. Here, we show that the inhibitory effect of auxin on PIN2 endocytosis was impaired in SCFTIR1/AFB auxin signaling mutants. Similarly, reducing membrane sterols impaired auxin inhibition of PIN2 endocytosis. Gas chromatography–mass spectrometry analyses indicate that membrane sterols were significantly reduced in SCFTIR1/AFB mutants, supporting a link between membrane sterols and auxin signaling in regulating PIN2 endocytosis. We show that auxin promoted PIN2 recycling from endosomes to the PM and increased PIN2 steady state levels in the PM fraction. Furthermore, we show that the positive effect of auxin on PIN2 levels in the PM was impaired by inhibiting membrane sterols or auxin signaling. Consistent with this, the sterol biosynthetic mutant fk-J79 exhibited pronounced defects in primary root elongation and gravitropic response. Our data collectively indicate that, although there are distinct processes involved in endocytic regulation of specific PM-resident proteins, the SCFTIR1/AFB-dependent processes are required for auxin regulation of endocytosis, recycling, and PM accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana.
INTRODUCTION
Auxins are prominent growth regulators playing essential roles in plant developmental processes, including axis formation during embryogenesis, differentiation of vascular tissues, development of lateral organs, and tropic responses. Unique among plant growth regulators, auxin is transported from cell to cell in a polar fashion, regulating cell division, differentiation, and elongation (Woodward and Bartel, 2005; Leyser, 2006; Teale et al., 2006). Recent studies support important roles for the PIN family of auxin efflux proteins, the AUX1 family of auxin influx proteins, and the ABC-type multidrug resistance p-glycoproteins (MDR/PGP) in auxin transport (Blakeslee et al., 2007; Mravec et al., 2008; Titapiwatanakun et al., 2008). The PIN efflux transporters exhibit polar plasma membrane (PM) localization and determine the direction and rate of intercellular auxin flow (Petrasek et al., 2006; Wisniewska et al., 2006). In cells where PIN and MDR/PGP proteins are colocalized, the MDR/PGP proteins promote the amplitude and specificity of PIN-mediated auxin transport (Blakeslee et al., 2007; Mravec et al., 2008; Titapiwatanakun et al., 2008). Thus, the polarity of auxin transport as postulated in the classical chemiosmotic model of auxin transport is supported by polar PM localization of the auxin transporters.
The PM localization of PIN1 and PIN2 proteins is maintained by endocytosis and recycling through vesicle trafficking in a process termed constitutive cycling. Auxin itself has been shown to inhibit PIN2 endocytosis and to promote PIN2's PM localization (Paciorek et al., 2005). However, mechanisms that underpin auxin inhibition of PIN2 endocytosis remain elusive. Recently, a group of auxin transport inhibitors, including 2,3,5-triiodobenoic acid (TIBA) and 2-(1-pyrenoyl) benzoic acid, but not 1-naphthylphthalamic acid, have been shown to impair vesicle movement and actin cytoskeleton dynamics in several eukaryotic organisms, suggesting a role for actin-dependent intracellular vesicle trafficking in auxin transport regulation (Geldner et al., 2001; Dhonukshe et al., 2008).
In Arabidopsis thaliana roots, PIN1 is localized to the bottom side of protophloem cells (Gälweiler et al., 1998), whereas PIN2 is localized to the top side of root epidermal and mature cortical cells and to the bottom side of young cortical cells in the meristematic and elongation zones (Müller et al., 1998; Chen and Masson, 2006; Michniewicz et al., 2007). Protein phosphatase PP2A and protein kinase PINOID have been shown to play key roles in auxin transport and polar localization of PIN1 and PIN2 in sequence- and cell-specific manners (Garbers et al., 1996; Shin et al., 2005; Michniewicz et al., 2007). Polar targeting of PIN1 and PIN2 proteins to a particular side of a cell is dependent on distinct vesicle trafficking machineries, requiring either brefeldin A (BFA)-sensitive or -insensitive ADP-ribosylation factor guanine nucleotide exchange factor (ARF GEF) vesicle-trafficking regulators (Kleine-Vehn et al., 2008). Furthermore, it has been shown that PIN2 is targeted to the cell plate during cell division (Men et al., 2008). At the end of cytokinesis, PIN2 disappears from one daughter membrane in processes dependent on membrane sterols, indicating that membrane sterols are required for the establishment of PIN2 polarity after cytokinesis (Men et al., 2008). Membrane sterols also play a role in polar targeting of PIN1 protein, since PIN1 subcellular localization is altered in the sterol biosynthetic mutant smt1/orc (Willemsen et al., 2003).
Downstream from auxin transport are auxin signaling processes. By binding to auxin receptors belonging to the TIR1/AFB family of F-box proteins, a high concentration of auxin promotes interactions of the F-box proteins with members of the auxin/indole-3-acetic acid (Aux/IAA) family of transcription repressors and promotes ubiquitination and degradation of the Aux/IAA proteins by E3 ubiquitin-ligase SCFTIR1/AFB-26S proteasomes (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005; Tan et al., 2007). This releases the inhibitory effect of Aux/IAA transcription repressors on the auxin response factor (ARF) family of transcription factors and activates the expression of auxin-responsive genes. The Aux/IAA-ARF signaling pathway has been implicated in auxin regulation of lateral distribution of PIN1 and PIN2 within the PM (Sauer et al., 2006). However, the underlying mechanisms remain unclear.
Here, we address the requirement for the SCFTIR1/AFB-dependent auxin signaling in auxin inhibition of PIN2 endocytosis. We also examine the involvement of auxin signaling in auxin promotion of PIN2 recycling and PM accumulation. To gain further insights, we studied the role of membrane sterols in auxin regulation of PIN2 endocytosis. We show that reduced membrane sterols or impaired auxin signaling similarly impaired auxin regulation of PIN2 endocytosis and that membrane sterols are significantly reduced in auxin signaling mutants. Analysis of the tir1 cvp1 double mutant reveals synergistic interactions between auxin signaling- and membrane sterol-dependent processes. Taken together, our results collectively support a critical role for auxin signaling and membrane sterols in auxin regulation of endocytosis, recycling, and PM accumulation of the auxin efflux transporter PIN2.
RESULTS
SCFTIR1/AFB-Dependent Auxin Signaling Is Required for Auxin Inhibition of PIN2 Endocytosis
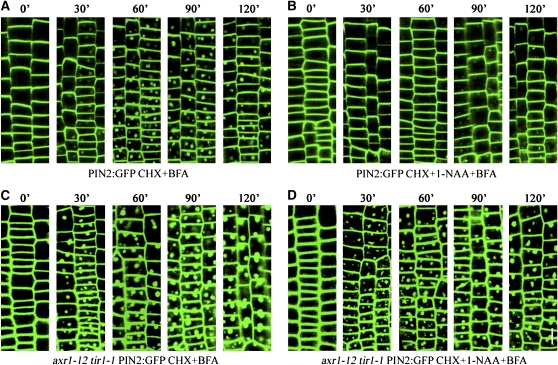
The PM-resident PIN1 and PIN2 proteins undergo constitutive cycling between the PM and endosomes (Geldner et al., 2001; Muday et al., 2003; Murphy et al., 2005; Shin et al., 2005; Chen and Masson, 2006; Dhonukshe et al., 2007). In the presence of the recycling inhibitor BFA, internalized PIN1 and PIN2 accumulate in intracellular compartments termed BFA bodies. Previously, auxin has been shown to inhibit endocytosis of PIN2 and a functional PIN2:green fluorescent protein (GFP) marker (Blilou et al., 2005; Paciorek et al., 2005; Xu and Scheres, 2005; Dhonukshe et al., 2007). To elucidate molecular mechanisms that underpin the inhibitory effect of auxin on endocytosis, we investigated the kinetics of endocytosis of fluorescently tagged PIN2 and examined the requirement of auxin signaling in this process using confocal live-cell imaging. Consistent with previous studies (Paciorek et al., 2005; Dhonukshe et al., 2007; Kleine-Vehn et al., 2008), the accumulation of PIN2:GFP in BFA bodies occurred within 30 min after BFA treatments (Figure 1A). Accumulation of PIN2:GFP in BFA bodies increased over a period of 2 h and was not affected by the protein synthesis inhibitor cycloheximide (CHX; 50 μM; Figures 1A and 2A). We show that BFA treatments slightly but significantly reduced the steady state level of PIN2 transcripts in roots (see Supplemental Figure 1 online). These results indicate that the accumulation of PIN2:GFP in BFA bodies was not resulting from de novo protein synthesis but instead derived from the PM via endocytosis. In agreement with earlier studies (Paciorek et al., 2005; Dhonukshe et al., 2007; Kleine-Vehn et al., 2008), auxin clearly inhibited the accumulation of PIN2:GFP in BFA bodies during the 2-h time course (Figure 1B), albeit the inhibitory effect of auxin on PIN2:GFP endocytosis was often incomplete (Figure 1B).
Figure 1.
Auxin Inhibits Endocytosis of PIN2:GFP in Roots of Wild-Type Plants but Not of Auxin Signaling Mutants.
Four-day-old seedlings were treated with the protein synthesis inhibitor CHX and followed by washout with CHX and solvent ([A] and [C]) or CHX and 1-NAA ([B] and [D]) for 30 min and finally by washout with CHX and BFA ([A] and [C]) or CHX, 1-NAA, and BFA ([B] and [D]) for various lengths of time (in minutes) as indicated at the top of each panel before laser confocal microscopy. Shown is PIN2:GFP in root epidermis cells in the meristematic and elongation zones of wild-type ([A] and [B]) and axr1-12 tir1-1 mutant ([C] and [D]) backgrounds. Bars = 25 μm.
We tested the effect of auxin on endocytosis of FM4-64, a lipophilic styryl dye that labels the PM. In contrast with previous reports (Paciorek et al., 2005), our data indicate that auxin did not noticeably inhibit endocytosis of FM4-64 in either wild-type or axr1-12 auxin signaling mutants (see Supplemental Figure 2 online) and suggest that auxin specifically inhibits endocytosis of PM-resident proteins but not of FM4-64–labeled lipid components of the PM.
In axr1-12 tir1-1 auxin signaling mutants, accumulation of PIN2:GFP in BFA bodies occurred within 30 min after BFA treatments in the presence of CHX and was further increased during a 2-h time course (Figure 1C), similar to that in wild-type plants, although PIN2:GFP signal was much higher in the auxin signaling mutant than in the wild-type plants (Figures 1A and 1C). However, contrary to the inhibitory effect of auxin on PIN2:GFP endocytosis in wild-type plants, accumulation of PIN2:GFP in BFA bodies was not noticeably inhibited by auxin in the auxin signaling mutants in presence of CHX (Figures 1B and 1D), indicating that auxin signaling is required for auxin inhibition of PIN2:GFP endocytosis.
We next examined the effect of auxin on endocytosis of PIN2:GFP in single, double, triple, and quadruple mutants of members of the TIR1/AFB family of auxin receptor proteins (Dharmasiri et al., 2005b). We show that the inhibitory effect of auxin on PIN2:GFP endocytosis was strongly reduced in tir1-1 afb2-1 double and tir1-1 afb1-1 afb2-1 triple mutants compared with that in the tir1-1 single mutant (Figures 2A to 2D), consistent with functional redundancies among the TIR1 family members (Dharmasiri et al., 2005b). In tir1-1 afb1-1 afb2-1 afb3-1 quadruple mutants, no differences in PIN2:GFP signals and sizes of the BFA bodies were observed between mock- and auxin-treated seedlings (Figure 2E, middle and right panels), indicating that auxin was ineffective in inhibiting PIN2:GFP endocytosis when auxin signaling was impaired.
Figure 2.
SCFTIR1/AFBs-Dependent Auxin Signaling Plays a Key Role in Auxin Inhibition of Endocytosis of PIN2:GFP.
The effect of auxin on endocytosis of PIN2:GFP was examined in wild-type (A), tir1-1 (B), tir1-1 afb2-1 (C), tir1-1 afb1 afb2 (D), tir1-1 afb1 afb2 afb3 (E), axr6-3 (F), axr1-12 (G), or axr1-12 tir1-1 (H) backgrounds. Five-day-old seedlings were treated with solvent (left and middle panels) or 1-NAA (right panel) and subsequently washed out with solvent (left panel), BFA (middle panel), or BFA and 1-NAA together (right panel) for 60 min before confocal laser microscopy. PIN2:GFP endocytosis was inhibited by auxin in wild-type plants but not in auxin signaling mutants. Shown is PIN2:GFP in root epidermis cells in the meristematic and elongation zones. Bars = 25 μm.
[See online article for color version of this figure.]
The E3 ubiquitin ligase SCFTIR1/AFB complex is composed of Arabidopsis SKP1-like1, Cullin1 (CUL1), F-box protein, and the RING protein RBX1/ROC1/HRT1 (Gray et al., 2001). The E3 ubiquitin ligase function of the SCFTIR1/AFB complex depends on modifications of CUL1 by the ubiquitin-related protein RUB in a process that requires heterodimeric E1 enzymes consisting of AXR1 and ECR1, and a RUB1-conjugating E2 enzyme RCE1 (Gray et al., 2001). To further demonstrate that the SCFTIR1/AFB-dependent auxin signaling is required for auxin inhibition of PIN2:GFP endocytosis, we tested endocytosis of PIN2:GFP in axr6-3 (an allele of the cul1 mutant) (Quint et al., 2005), axr1-12 (an allele of the axr1 mutant) (Timpte et al., 1995), and axr1-12 tir1-1 double mutants. Similar to other auxin signaling mutants examined earlier, auxin was ineffective in inhibiting PIN2:GFP endocytosis in these additional auxin signaling mutants (Figures 2F to 2H). Collectively, these results indicate that the SCFTIR1/AFB E3 ubiquitin ligase-dependent auxin signaling is required for auxin inhibition of PIN2:GFP endocytosis.
We then tested the specificity of the auxin signaling–dependent regulation of endocytosis by examining several other previously characterized PM-resident markers, including PIN1:GFP (Blilou et al., 2005; Heisler et al., 2005), PIP2A:GFP (Cutler et al., 2000), RCI2A:GFP (Cutler et al., 2000), and AUX1:yellow fluorescent protein (YFP) (Swarup et al., 2004). We show that, similar to the PIN2:GFP marker, auxin inhibited endocytosis of PIP2A:GFP (Figure 3A), RCI2A:GFP (Figure 3C), and PIN1:GFP (Figure 3E). However, auxin did not noticeably inhibit endocytosis of the auxin influx carrier AUX1:YFP marker (see Supplemental Figure 3 online). The latter result is in agreement with previous studies suggesting that AUX1 and PIN1 are internalized through distinct mechanisms (Kleine-Vehn et al., 2006). On the other hand, we show that endocytosis of the PIP2A:GFP marker was effectively inhibited by auxin in both wild-type and the axr1-12 auxin signaling mutant (Figures 3A and 3B, right panels), suggesting that auxin signaling is not required for the inhibitory effect of auxin on PIP2A:GFP endocytosis. This was in contrast with the requirement for auxin signaling in auxin inhibition of endocytosis of PIN1:GFP (Figure 3F), PIN2:GFP (Figures 1D and 2B to 2H), and RCI2A:GFP (Figure 3D). Collectively, these results suggest that the SCFTIR1/AFB-dependent auxin signaling is required for auxin inhibition of endocytosis of only a subset of PM-resident proteins.
Figure 3.
The Role of Auxin Signaling in Auxin Regulation of Endocytosis of Other PM Markers.
Endocytosis of PIP2A:GFP ([A] and [B]), RCI2A:GFP ([C] and [D]), and PIN1:GFP ([E] and [F]) was examined in wild-type ([A], [C], and [E]), axr1-12 ([B] and [D]), and tir1-1 afb1 afb2 afb3 (F) backgrounds. Five-day-old seedlings were treated with solvent (left and middle panels) or 1-NAA (right panel) and subsequently washed out with solvent (left panel), BFA (middle panel), or BFA and 1-NAA together (right panel) for 60 min before confocal laser microscopy. Auxin inhibited endocytosis of PIP2A:GFP in both the wild type (A) and the auxin signaling mutant (B) and endocytosis of both RCI2A:GFP and PIN1:GFP in the wild type ([C] and [E]) but not in the auxin signaling mutants ([D] and [F]). Shown are root epidermis cells ([A] to [D]) and central provascular cells ([E] and [F]) in the meristematic and elongation zones of young seedlings. Bars = 25 μm.
[See online article for color version of this figure.]
The differential effects of auxin on endocytosis between wild-type and auxin signaling mutants may be due to differences in auxin uptake by these plants. To rule out this possibility, we examined the effect of endogenously induced auxin (IAA) on PIN2:GFP endocytosis. We show that the endogenously induced auxin effectively inhibited endocytosis of PIN2:GFP in wild-type plants (see Supplemental Figures 4A to 4C online) but failed to inhibit PIN2:GFP endocytosis in auxin signaling mutants (see Supplemental Figures 4E to 4G online). The IAAH substrate indole-3-acetamide did not affect PIN2:GFP endocytosis in wild-type or auxin signaling mutants that did not contain the IAAH transgene (see Supplemental Figures 4D and 4H online). Thus, these results rule out the possibility that the differential effects of exogenously applied auxin on PIN2:GFP endocytosis between wild-type and auxin signaling mutants was due to differential uptake of auxin by these different plants.
Auxin Signaling Is Required for Auxin Promotion of Recycling and Steady State Accumulation of PIN2 in the PM
Although auxin inhibits endocytosis and promotes PM localization of PIN2 (Paciorek et al., 2005), it remains to be elucidated whether auxin and auxin signaling play a role in the recycling of PIN2 from endosomes to the PM. We show that PIN2:GFP disappeared from BFA bodies within 80 min after BFA washout in wild-type plants (Figure 4A). By contrast, PIN2:GFP disappeared from BFA bodies in wild-type plants within 40 min after BFA washout with an auxin solution, suggesting that auxin promotes recycling of PIN2:GFP from endosomes to the PM (Figure 4C). In auxin signaling mutants, however, auxin did not promote recylcing of PIN2:GFP from the BFA bodies (Figures 4B and 4D). Because PIN2:GFP remained in the BFA bodies of both wild-type and auxin signaling mutants after BFA washout with BFA and auxin together (Figures 4E and 4F), these results collectively indicate that auxin signaling is required for auxin promotion of PIN2:GFP recycling from endosomes to the PM.
Figure 4.
SCFTIR/AFBs-Dependent Auxin Signaling Is Required for Auxin Promotion of PIN2:GFP Recycling.
Four-day-old seedlings were treated with BFA and subsequently washed out with solvent ([A] and [B]), 1-NAA ([C] and [D]), or 1-NAA and BFA together ([E] and [F]) for various lengths of time (in minutes) as indicated at the top of each panel before laser confocal microscopy. Shown is PIN2:GFP in root epidermis cells at the meristematic and elongation zones of wild-type ([A], [C], and [E]) and axr1-12 mutant ([B], [D], and [F]) backgrounds. Bar = 25 μm.
[See online article for color version of this figure.]
The combined effects of auxin on endocytosis and recycling should result in the accumulation of PIN2 in the PM of wild-type plants but not of the auxin signaling mutants. To show this, we quantified the endogenous PIN2 using protein gel blotting analysis (see Supplemental Figure 5A online). Since we have previously shown that PIN2 PM localization is sensitive to the light environment in which plants are grown (Laxmi et al., 2008), we used 5-d-old seedlings grown in liquid culture under a constant light condition. We separated the PM fraction from the total membrane fraction using two-phase partitioning (Larsson et al., 1994) and confirmed the enrichment of the PM fraction by monitoring a PM marker PM H+-ATPase and an endoplasmic reticulum marker SEC12 (see Supplemental Figure 5B online). Protein gel blotting analyses reveal that the steady state level of the endogenous PIN2 in the PM fraction was steadily increased in wild-type plants treated with auxin (1-Naphthalene acetic acid; 1-NAA, 10 μM) over a 2-h time course (Figure 5A, lanes 1 to 3). However, our protein quantification results are contrary to an earlier report, which suggests that the steady state level of PIN2 was reduced by auxin (Abas et al., 2006). The reason for this discrepancy is currently unknown, but differences in experimental conditions used in these independent studies may contribute to the differences.
Figure 5.
Auxin Signaling and Membrane Sterols Are Required for Auxin Promotion of the Steady State Level of PIN2 in the PM.
(A) to (C) Protein gel blotting analysis of the endogenous PIN2 in the PM fraction of 5-d-old wild-type (A), eir1-4 35S:PIN2 (B), or axr6-3 (C) plants treated with solvent (lane 1), auxin (1-NAA; lanes 2 and 3), or CHX and 1-NAA together (lane 4) for various lengths of time (in minutes) as indicated.
(D) Protein gel blotting analysis of PIN2 in the PM fraction of wild-type plants treated with solvent (lane 1), FEN (lanes 2 and 3), or FEN and 1-NAA together (lane 4) for 2 h. The top panels show PIN2 signals in the PM fraction, and the bottom panels show equal loading as indicated by silver staining.
We show that treatments with CHX did not inhibit auxin-mediated accumulation of the endogenous PIN2 in the PM fraction of wild-type plants (Figure 5A, lanes 3 and 4). Auxin treatments also significantly increased the steady state level of PIN2 in the PM fraction of eir1-4 35S:PIN2 transgenic plants (Figure 5B, lanes 1 to 3; eir1-4 is a pin2 allele). Since the cauliflower mosaic virus 35S promoter is not responsive to auxin, these data collectively support that auxin promotion of PIN2 PM accumulation was not due to de novo protein synthesis but rather due to its combined effects on endocytosis and recycling. Furthermore, we show that auxin treatments of the axr6-3 auxin signaling mutants did not promote the accumulation of steady state PIN2 level in the PM fraction, in contrast with that in wild-type plants (Figure 5C), consistent with the role of auxin signaling in auxin regulation of PIN2 PM accumulation.
Auxin transport assays indicate that treatment with 1-NAA (2 μM) for 1 h significantly increased the rate of root basipetal transport of 3H-IAA in wild-type plants but not in the axr1-12 tir1-1 auxin signaling mutants (Figure 6), unlike the inhibitory effect of the auxin transport inhibitor TIBA on auxin transport (Figure 6). Without auxin treatments, root basipetal transport of 3H-IAA was significantly higher in the auxin signaling mutant than in the wild-type plants (Figure 6). Thus, auxin transport assays support the role of auxin and auxin signaling in endocytosis, recycling, and PM accumulation of the auxin efflux transporter PIN2.
Figure 6.
Auxin Signaling Is Required for Auxin Positive Regulation of Basipetal Auxin Transport in Arabidopsis Roots.
Root basipetal transport of 3H-IAA was significantly higher in the auxin signaling mutant axr1-12 tir1-1 than in the wild-type plants (Student's t test, n = 3, P < 0.05). Short-term treatments with auxin (1-NAA, 2 μM; 1 h) significantly increased root basipetal transport of 3H-IAA in wild-type plants (Student's t test, n = 3, P < 0.05) but not in the auxin signaling mutant (Student's t test, n = 3, P > 0.5). By contrast, treatments with the auxin transport inhibitor TIBA (25 μM; 1 h) significantly decreased basipetal auxin transport in Arabidopsis roots. Auxin transport was set to 100% for the wild-type plants. Shown are means ± se.
Membrane Sterols Are Required for Auxin Inhibition of Endocytosis of PIN2:GFP
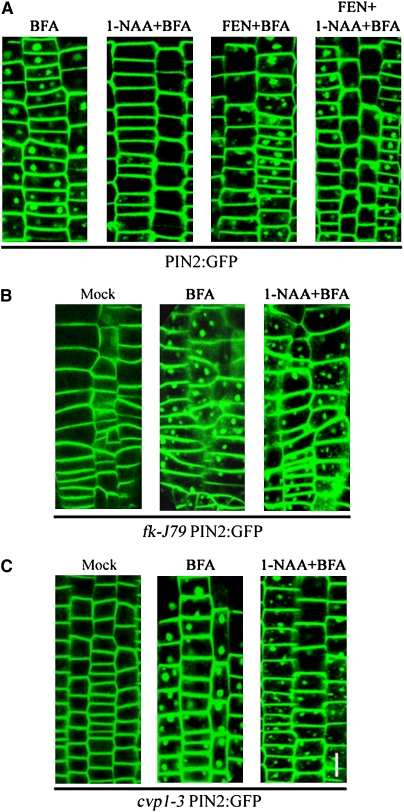
In animal and yeast cells, membrane sterols play an important role in various endocytic processes (Pichler and Riezman, 2004; Souza and Pichler, 2007). In Arabidopsis, membrane sterols are required for targeting and polarity establishment of PIN1 and PIN2 in the PM of specific cells in roots (Willemsen et al., 2003; Men et al., 2008). To provide insights on auxin regulation of PIN2 endocytosis, we examined the role of membrane sterols in this process. We tested fenpropimorph (FEN), a well-characterized inhibitor of the sterol biosynthetic enzyme C14 sterol reductase FACKEL (He et al., 2003). We show that short-term treatments with FEN significantly reduced membrane sterols in roots (see Supplemental Table 1 online). Although steady state levels of PIN2 transcripts and PIN2:GFP endocytosis were not altered by FEN treatments in wild-type plants (see Supplemental Figure 6 online; Figure 7A, left and middle right panels), the inhibitory effect of auxin on PIN2:GFP endocytosis was effectively reversed in wild-type plants pretreated with FEN (Figure 7A, middle left and right panels), suggesting that membrane sterols are required for auxin inhibition of PIN2:GFP endocytosis.
Figure 7.
Membrane Sterols Play a Key Role in Auxin Inhibition of Endocytosis of PIN2:GFP.
Endocytosis of PIN2:GFP was examined in wild-type (A), fk-J79 (B), or cvp1-3 (C) mutant backgrounds.
(A) Five-day-old seedlings were treated with solvent (left and middle panels) or FEN (right panel), subsequently washed out with solvent (left panel), 1-NAA (middle left panel), FEN (middle right panel), or FEN and 1-NAA together (right panel), and finally washed out with BFA (left panel), 1-NAA, and BFA together (middle left panel), FEN and BFA together (middle right panel), or FEN, 1-NAA, and BFA together (right panel) for 60 min before confocal laser microscopy.
(B) and (C) Similarly, 5-d-old seedlings were treated with solvent (left panel), BFA (middle panel), or 1-NAA and BFA together (right panel) for 60 min before confocal laser microscopy. Bars = 25 μm.
[See online article for color version of this figure.]
To further support the role of membrane sterols in PIN2:GFP endocytosis, we also tested two sterol biosynthetic mutants, cvp1-3 and fk-J79. FK encodes the FEN-sensitive C-14 sterol reductase (Jang et al., 2000; Schrick et al., 2000; He et al., 2003), and CVP1 encodes sterol methyltransferase2 (SMT2) (Carland et al., 2002). We show that the auxin inhibitory effect on PIN2:GFP endocytosis was effectively reversed in both cvp1-3 and fk-J79 mutants, consistent with the effect of sterol biosynthesis inhibitors (Figures 7B and 7C; compare with Figure 7A). Protein gel blotting analysis reveals that the steady state level of PM-accumulated PIN2 was not affected by auxin in wild-type plants treated with FEN, although FEN treatments slightly elevated the steady state level of PIN2 in the PM fraction (Figure 5D).
Since the FEN-targeted enzymatic reaction is upstream of a branching point that leads to the biosynthesis of both sterols and brassinosteroids (BRs), a group of signaling molecules that regulate many developmental processes, treatments with FEN also affect biosynthesis of BRs. We tested whether BRs are involved in auxin regulation of PIN2 endocytosis. We show that treatments of wild-type seedlings with brassinozole, a BR biosynthesis inhibitor (Nagata et al., 2001), did not release the inhibitory effect of auxin on PIN2:GFP endocytosis (see Supplemental Figures 7A to 7D online). Additionally, PIN2:GFP endocytosis was still effectively inhibited by auxin in the BR-deficient det2-1 mutant (Noguchi et al., 1999) (see Supplemental Figures 7E to 7G online). Taken together, these results rule out the involvement of BRs and further support the involvement of membrane sterols in auxin regulation of PIN2:GFP endocytosis and PM accumulation.
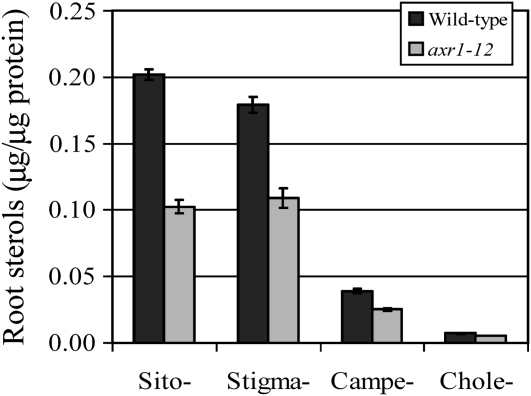
Using gas chromatography–mass spectrometry (GC-MS), we quantified the four most abundant plant sterols, sitosterol, stigmasterol, campesterol, and cholesterol, in the membrane fraction of wild-type and axr1-12 mutant roots. We show that the levels of the four plant sterols were all significantly reduced in the axr1-12 mutant compared with the wild-type plants (Figure 8; Student's t test, n = 3; P < 0.001). In agreement with the insensitivity of PIN2:GFP endocytosis to auxin in plants with compromised auxin signaling or membrane sterols, these data suggest involvement of membrane sterols in auxin signaling-dependent regulation of PIN2:GFP endocytosis and PM accumulation of PIN2.
Figure 8.
GC-MS Analysis of Membrane Composition of Four Major Plant Sterols.
The membrane levels of sitosterol, stigmasterol, campesterol, and cholesterol were all significantly reduced in roots of the 5-d-old axr1-12 mutant compared with those of wild-type plants (Columbia-0). Shown are means ± se; n = 3; Student's t test, P < 0.001. Sito-, sitosterol; stigma-, stigmasterol; campe-, campesterol; chole-, cholesterol.
Auxin Signaling and Membrane Sterol-Dependent Processes Interact Synergistically in Mediating Auxin and Gravitropic Responses
To provide genetic evidence that membrane sterols are involved in auxin response, we constructed tir1-1 cvp1-3 double mutants and measured auxin sensitivity of mutant roots. Our data show that the synthetic auxin 2,4-D at 0.1 μM significantly inhibited primary root elongation of wild-type plants (11% ± 1.3% of the mock control; Figure 9), whereas 2,4-D at the same concentration was slightly less inhibitory of primary root elongation of the cvp1-3 mutants (15.5% ± 1.3% of the control; Figure 9). Compared with primary roots of the weak auxin signaling mutant tir1-1, which exhibited resistance to 0.1 μM 2,4-D (38% ± 2.7% of the control), primary roots of the cvp1-3 tir1-1 double mutant were highly resistant to 2,4-D (75.4% ± 3.5% of the control; Figure 9). These results reveal the involvement of membrane sterol-dependent processes in auxin signaling and synergistic interactions between TIR1-dependent auxin signaling and membrane sterol-dependent processes in auxin response.
Figure 9.
Auxin Signaling and Membrane Sterols Function Synergistically in Auxin Response.
Four-day-old wild-type, tir1-1, cvp1-3, and tir1-1 cvp1-3 double mutants were transferred to regular growth medium with or without 0.1 μM 2, 4-D and grown for various lengths of time. The rate of primary root elongation was determined using Image J software. Shown are means ± se; n = 3. Eight to ten seedlings were tested in each experiment.
To explore the physiological relevance of the involvement of membrane sterols in regulation of endocytosis and PM accumulation of the auxin efflux transporter PIN2, we examined the effects of the sterol biosynthesis inhibitor FEN on root gravitropic responses. At a low concentration (1 μM), FEN slightly altered root gravitropic responses compared with the mock control (Figures 10A, 10B, and 10H), while, at a high concentration (10 μM), it significantly delayed root gravitropic responses of wild-type plants without much effect on primary root elongation (Figures 10C and 10H). At higher concentrations (25 to 150 μM), FEN strongly inhibited both primary root elongation and root gravitropic responses of wild-type plants (Figures 10D to 10H). This mimicked in large part the phenotypic defects of the sterol biosynthesis mutant fk-J79, with an exception that excessive root hairs were formed only in the fk-J79 mutant but not in FEN-treated plants (Figure 10P).
Figure 10.
Inhibition of Membrane Sterols Impairs Auxin Gradient and Root Gravitropic Responses.
(A) to (G) Five-day-old wild-type seedlings grown on 45° tilted agar medium plates supplemented with the indicated concentrations of FEN exhibited increasingly severe defects in primary root elongation and gravitropic response.
(H) Quantification of root gravitropic response of 4-d-old wild-type plants transferred to regular growth medium supplemented with increasing concentrations of FEN and gravistimulated for 24 h before root tip curvatures were measured. Shown is the number of plants with root tip angles that deviated from the vertical in 10° increments.
(I) and (J) An auxin gradient as indicated by the auxin response marker DR5rev:GFP was established at the bottom flank of wild-type roots after 2-h gravistimulation.
(K) and (L) By contrast, auxin gradients were formed at both upper and bottom flanks of roots of the sterol biosynthesis mutant fk-J79 before and after 2-h gravistimulation.
(M) to (O) Endocytosis of PIN2:GFP was inhibited in root epidermal and cortical cells at the bottom flank of 2-h gravistimulated wild-type roots (M) but not in gravistimulated roots of the axr1-12 tir1-1 double mutant (N) or the sterol biosynthesis mutant fk-J79 (O).
(P) Five-day-old fk-J79 seedlings exhibited pronounced primary root elongation and gravitropic response defects. Arrows indicate the gravity vector.
Bars = 5 mm in (A) to (G), 50 μm in (I) to (O), and 1 mm in (P).
Upon gravistimulation, an auxin gradient is established at the bottom flank of gravistimulated wild-type roots (Figures 10I and 10J). Consistent with this, endocytosis of PIN2:GFP was inhibited in root epidermal and cortical cells at the bottom flank but not at the upper flank of gravistimulated roots (Figure 10M). By contrast, PIN2:GFP endocytosis was not inhibited at the bottom flank of gravistimulated roots of the auxin signaling mutant tir1-1 axr1-12 or sterol biosynthesis mutant fk-J-79 (Figures 10N and 10O), in agreement with the insensitivity of PIN2:GFP endocytosis to auxin in these mutants.
All auxin signaling mutants exhibit defective root gravitropic responses. However, it is difficult to evaluate auxin distribution in auxin signaling mutants, since the auxin response reporter DR5rev:GFP frequently used to monitor auxin gradients is not expressed in these mutants. Therefore, we addressed this question in sterol biosynthesis mutants. The sterol biosynthesis mutant fk-J79, similar to the cpi1-1 mutant (Men et al., 2008), exhibited strong primary root elongation and gravitropic defects (Figure 10P). We show that, in contrast with the asymmetric auxin gradient formed at the bottom flank of wild-type roots after 2-h gravistimulation (Figures 10I and 10J), auxin gradients were formed at both upper and bottom flanks of fk-J79 mutant roots, regardless of whether the roots were gravistimulated or not (Figures 10K and 10L). The impaired establishment of the asymmetric auxin gradient in fk-J79 mutant roots upon gravistimulation is consistent with failure of auxin to inhibit PIN2 endocytosis in the sterol biosynthesis mutants, explaining the strong root gravitropic defects when biosynthesis of membrane sterols are altered.
DISCUSSION
Polar transport of the plant hormone auxins has been linked to diverse auxin-regulated developmental processes. IAA, a major endogenous auxin, enters into cells from the apoplastic space by diffusion or active uptake mediated by auxin influx carriers, such as AUX and PGP4. Once inside of the cell, IAA dissociates and requires auxin efflux carriers, such as PIN, PGP1, or PGP19, for export (Tanaka et al., 2006; Blakeslee et al., 2007; Vieten et al., 2007; Mravec et al., 2008; Titapiwatanakun et al., 2008). Auxin transport across the PM contributes to regulation of auxin homeostasis within the cell. In turn, auxin feedback regulates transcription of some PIN genes and stability, internalization, and polar targeting of several PIN proteins (Chen and Masson, 2006; Tanaka et al., 2006; Vieten et al., 2007). Recent evidence suggests involvement of the Aux/IAA and ARF signaling pathway in lateral distribution of PIN proteins within the PM (Sauer et al., 2006). However, how auxin signaling regulates polar targeting of PIN proteins to the PM remains a topic for future investigation.
Here, we show that the E3 ubiquitin ligase SCFTIR1/AFB-dependent auxin signaling plays a key role in auxin regulation of endocytosis, recycling, and PM accumulation of PIN1, PIN2, and other unrelated proteins, such as RCI2A, but not of the auxin influx carrier AUX1. In addition, we show that auxin regulation of PIN2 endocytosis is impaired in sterol biosynthetic mutants or in wild-type plants treated with a sterol biosynthetic inhibitor. Using GC-MS, we show that membrane sterols are significantly reduced in auxin signaling mutants. These data collectively suggest that auxin signaling and membrane sterols play a similar role in auxin regulation of endocytosis of specific PM proteins, including some PIN proteins.
Sterols are lipid components of cellular membranes. In mammalian and yeast cells, they are associated with lipid microdomains or lipid rafts and play important roles in lateral movements within the PM, endocytosis, and intracellular vesicle trafficking of membrane proteins (Pichler and Riezman, 2004; Souza and Pichler, 2007). The role of membrane sterols in plant cells is not well defined. Our findings support a key role for membrane sterols in endocytic regulation of PIN1, PIN2, and some other PM proteins. However, the mode of action of membrane sterols remains to be further investigated. Recent studies suggest that PIN and PGP proteins play distinct and in some cases synergistic roles in auxin transport (Blakeslee et al., 2007; Mravec et al., 2008; Titapiwatanakun et al., 2008). In cells where both types of proteins are colocalized in the PM, they physically interact with each other and act synergistically in auxin transport. Thus, it is plausible that membrane sterols may facilitate specific protein–protein interactions within the membrane microdomains, which in turn regulate endocytosis of PM-resident proteins.
Our results suggest that distinct processes are involved in regulation of endocytosis of specific PM-resident proteins in Arabidopsis. In the case of the auxin efflux transporter PIN2 protein, both auxin signaling and membrane sterols are required for the inhibitory effect of auxin on its endocytosis. The auxin-sensitive endocytic processes provide a means for auxin to feedback regulate its own transport at the level of PM accumulation of its own transporters, a process required for gravitropism and wounding responses. The existence of multiple endocytic processes in Arabidopsis explains why endocytosis of some PM markers, such as AUX1:YFP, is not inhibited by auxin, while that of other markers, such as PIP2A:GFP, is effectively inhibited by auxin in both wild-type and auxin signaling mutants.
Because the expressions of FK, SMT1, SMT2 (CVP1), and SMT3 genes that encode key sterol biosynthesis enzymes are regulated both developmentally and by signaling molecules, including auxin, cytokinin, ethylene, and gibberelic acid (Jang et al., 2000; Carland et al., 2002), the establishment of local PIN-mediated auxin gradients required for axis formation during embryogenesis, tissue regeneration, and wounding and root curvature responses is feedback regulated by developmental and environmental cues through modulation of membrane sterols. Consistent with the role of membrane sterols in endocytic regulation of PIN proteins, sterol biosynthesis mutants fk and hydra2 exhibit apical-basal patterning defects during embryogenesis, mimicking phenotypes of auxin transport mutants (Jang et al., 2000; Schrick et al., 2000; Souter et al., 2002; Friml et al., 2003; Blilou et al., 2005). In summary, our data support the involvement of SCFTIR1/AFBs- and membrane sterol-dependent processes in auxin regulation of endocytosis, recycling, and PM accumulation of specific PM-resident proteins, including the auxin efflux PIN proteins.
METHODS
Plant Materials and Growth Conditions
The following mutants and transgenic lines were used in the study: PIN1:PIN1-GFP (Heisler et al., 2005) and eir1-1 PIN2:PIN2-GFP (Blilou et al., 2005; Xu and Scheres, 2005), eir1-4 35S:PIN2 (Abas et al., 2006), WOX5:IAAH (Xu et al., 2006), DR5rev:GFP, AUX1:AUX1-YFP (Swarup et al., 2004), RCI2A:GFP (Cutler et al., 2000), PIP2A:GFP (Cutler et al., 2000), agr1-5, tir1-1, tir1-1 afb1-1 afb2-1 afb3-1 (Dharmasiri et al., 2005b), axr1-12, axr6-3 (Quint et al., 2005), det2-1 (Noguchi et al., 1999), fk-J79 (Jang et al., 2000), and cvp1-3 (Carland et al., 2002). Reporter lines were introgressed into various mutant backgrounds to allow comparison of reporter gene expression. Consistent with a previous report (Dharmasiri et al., 2005b), two groups of seedlings from the tir1 afb1, 2, 3 quadruple mutants exhibited either no roots or early collapsed roots. Only seedlings with healthy roots were analyzed.
Seeds were surface sterilized and imbibed for 3 d at 4°C in dark and then sowed vertically in Petri dishes containing half-strength Murashige and Skoog basal salts with minimal organics (Sigma-Aldrich) supplemented with 1% sucrose and 1.5% agar (Sigma-Aldrich). Seedlings were grown in a climate-controlled growth room (24/20°C day/night temperature; 16/8 h photoperiod; and 80 μE/s/m−2 light intensity).
Chemical Solutions
All chemicals were purchased from Sigma-Aldrich, except specified otherwise, and prepared as stock solutions. DMSO was used to dissolve indole-3-acetamide (10 mM), CHX (50 mM), FEN (100 mM), and brassinozole (20 mM). FM4-64 (1 mM; Molecular Probes), TIBA (25 mM), and 2,4-D (10 mM) were dissolved in water. 1-NAA (10 mM) was first dissolved in a few drops of 1 n NaOH and then diluted with water. BFA (50 mM; Molecular Probes) was dissolved in methanol. Final concentrations were 50 μM for CHX, 10 μM for 1-NAA, 25 and 50 μM for BFA, 30 and 100 μM for FEN, and others as specified in the text. Pretreatments with various chemicals were for 30 min, and treatments with BFA were for 60 min.
PM Purification and Protein Gel Blotting Analyses
Approximately 10 g of 5-d-old seedlings grown in liquid Murashige and Skoog medium were collected for total membrane isolation, following a previously described protocol (Abas et al., 2006). Purification of the PM fraction was performed essentially as previously described (Larsson et al., 1994). The total membrane pellet was resuspended in a microsomal resuspension buffer (5 mM phoshate, pH 7.8, 330 mM sucrose, 2 mM DTE, 10 mM NaF, and protease inhibitors) and loaded to an aqueous two-phase solution with a final composition of 6.1% (w/w) Dextran 500, 6.1% (w/w) PEG-3350, 5 mM phoshate, pH 7.8, 5 mM KCl, 300 mM sucrose, and spin at 100,000g for 24 h. The PM fraction was collected and diluted with 6 mL PM buffer (10 mM TRIZMA base, 10 mM boric acid, 300 mM sucrose, 9 mM KCl, 5 mM EDTA-Na2, 5 mM EGTA, 50 mM NaF, and protease inhibitors) and pelleted again at 100,000g for 90 min. The pellet was resuspended in the PM buffer. An equal amount of proteins was loaded onto a 10% denaturing SDS-PAGE gel and separated by electrophoresis. SDS-PAGE gels were stained with Coomassie Brilliant Blue or silver nitrate following standard procedures (Sambrook and Russell, 2001). Protein gel blotting analyses were performed using affinity-purified anti-PIN2 (Boonsirichai et al., 2003; Shin et al., 2005), anti-PM-ATPase (DeWitt et al., 1996), and anti-SEC12 (Bar-Peled and Raikhel, 1997; Rancour et al., 2002) antibodies with 1:1000 dilution and the Chemiluminescent Western Blot Immunodetection Kit following the manufacturer's instructions (Invitrogen).
RT-PCR and Real-Time Quantitative RT-PCR
Six-day-old wild-type (Columbia-0) seedlings were treated for 0, 30, 60, and 120 min with DMSO (0.1%), BFA (25 μM), or FEN (100 μM). Roots were excised and immediately frozen in liquid nitrogen. Total RNA, RT-PCR, and real-time quantitative RT-PCR were performed essentially as described before (Laxmi et al., 2008). The experiments were repeated three times.
Root Gravistimulation and Measurement
Root gravistimulation and BFA treatments were performed as previously described (Paciorek et al., 2005). For gravitropic stimulation, 5-d-old vertically grown seedlings were gravistimulated with 90° rotation. Digital images were collected for at least 20 seedlings for each time point (0, 2, 4, and 8 h after gravistimulation) and analyzed using the Image J program (http://rsb.info.nih.gov/ij/).
Auxin Transport Assay
Basipetal auxin transport assays were performed essentially as described previously (Shin et al., 2005). Briefly, 5-d-old light-grown seedlings were transferred to a growth medium plate supplemented with solvent, 1-NAA, or TIBA and incubated for the length of time specified. Agar blocks of 1-mm in diameter of the same growth medium supplemented with 7.7 × 10−8 m 3H-IAA were placed next to the root tips. After incubation for 1.5 h, agar blocks were removed. Root tips of ∼0.3 mm in length were excised and discarded. Two consecutive 2-mm root segments were then excised from the remaining root tips and placed separately into two scintillation vials each containing 5 mL of scintillation fluid. The amount of radioactivity in the two root segments were pooled from eight roots and measured using a Beckman Coulter LS6500 scintillation counter. The experiments were repeated three times. A student's t test (paired with two-tailed distribution) was used to evaluate statistical significance of the data.
Sterol Composition Analysis
GC-MS analysis of membrane sterol composition was performed as previously described (Fujioka et al., 2002). Briefly, ∼0.5 to 2 g of root segments were collected from 5-d-old seedlings without treatments or treated with either DMSO (0.1%) or FEN (100 μM) for 1.5 h. Membrane fractions were extracted with methanol-CHCl3. Deuterium-labeled internal standards (d8-sitosterol, d6-campesterol, and d3-cholesterol) were added to the extracts. Samples were derivatized to trimethyl silyl ether and analyzed by full-scan GC-MS. Levels of sitosterol, campesterol, and cholesterol were calculated from the peak area ratios of molecular ions of the internal standards and the endogenous sterols as follows: sitosterol, m/z 494 and 486; campesterol, m/z 478 and 472; and cholesterol, m/z 461 and 458. Stigmasterol level was calculated from peak area ratios of molecular ion (m/z 494) of d8-sitosterol and molecular ion m/z 484 of endogenous stigmasterol.
Confocal Laser Microscopy Analysis
GFP, YFP, FM4-64, and propidium iodide fluorescence was imaged under a Leica TCS SP2 AOBS confocal laser scanning microscope (Leica Microsystems). For imaging GFP and YFP, the 488- and 514-nm lines of the argon laser were used for excitation, and emission was detected at 510 and 530 nm, respectively. For imaging FM4-64 and propidium iodide, the 543-nm line of the helium/neon laser was used for excitation, and emission was detected at 590 to 620 nm. For semiquantitative measurement of fluorescence intensities, laser, pinhole, and gain settings of the confocal microscope were kept identical among treatments. Digital images were analyzed for fluorescence intensities using Metamorph 6 (Molecular Devices). Images were assembled using Photoshop version 5.0 (Adobe Systems).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: AT5G57090 (PIN2), AT1G05180 (AXR1), AT3G62980 (TIR1), AT4G03190 (AFB1), AT3G26810 (AFB2), AT1G12820 (AFB3), AT4G02570 (AXR6), AT1G73590 (PIN1), AT3G53420 (PIP2A), AT3G05880 (RCI2A), AT2G38120 (AUX1), AT3G52940 (FACKEL), AT1G20330 (CVP1), and AT2G38050 (DET2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Vesicle Trafficking Inhibitor Brefeldin A Slightly but Significantly Reduced the Steady State Level of PIN2.
Supplemental Figure 2. Uptake of FM4-64 Was Not Affected by Auxin in Wild-Type or Auxin Signaling Mutants.
Supplemental Figure 3. Auxin Did Not Inhibit Endocytosis of AUX1:YFP.
Supplemental Figure 4. Induced Endogenous Auxin Inhibited Endocytosis of PIN2:GFP in Wild-Type Plants but Not in Auxin Signaling Mutants.
Supplemental Figure 5. Two-Phase Separation of the Plasma Membrane Fraction from the Total Membrane Fraction and Specificity of Anti-PIN2 Sntibodies.
Supplemental Figure 6. The Sterol Biosynthetic Inhibitor Fenpropimorph Did Not Alter the Steady State Level of PIN2.
Supplemental Figure 7. Brassinosteroids Were Not Involved in Auxin Inhibition of PIN2:GFP Endocytosis.
Supplemental Table 1. Effects of Short-Term Treatments with the Sterol Biosynthesis Inhibitor Fenpropimorph on Membrane Sterol Profiles.
Supplementary Material
Acknowledgments
We thank Mark Estelle, Christian Luschnig, Jeffery Long, Ben Scheres, Jiri Friml, Jyan-Chyun Jang, William Gray, Malcolm Bennett, Natasha Raikhel, Sebastian Bednarek, Klaus Palme, and Suguru Takatsuto for generously sharing published materials and protocols, the ABRC at Ohio State University for seed stocks, Elison Blancaflor, Aline Valster, and Kuihua Zhang for technical assistance, Richard Nelson and Jeremy Murray for critical reading of the manuscript, and the National Science Foundation (DBI-0400580) for an equipment grant. S.F. was supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant 19380069). Financial support for research from the corresponding author's laboratory was provided by the Samuel Roberts Noble Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Rujin Chen (rchen@noble.org).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wisniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., and Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8 249–256. [DOI] [PubMed] [Google Scholar]
- Bar-Peled, M., and Raikhel, N.V. (1997). Characterization of AtSEC12 and AtSAR1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol. 114 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee, J.J., et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres, B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 39–44. [DOI] [PubMed] [Google Scholar]
- Boonsirichai, K., Sedbrook, J.C., Chen, R., Gilroy, S., and Masson, P.H. (2003). ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell 15 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland, F.M., Fujioka, S., Takatsuto, S., Yoshida, S., and Nelson, T. (2002). The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R., and Masson, P.H. (2006). Auxin transport and recycling of PIN proteins in plants. In Plant Cell Monograph: Plant Endocytosis, J. Samaj, F. Baluska, and D. Menzel, eds (Berlin, Heidelberg: Springer-Verlag), pp. 139–157.
- Cutler, S.R., Ehrhardt, D.W., Griffitts, J.S., and Somerville, C.R. (2000). Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt, N.D., Hong, B., Sussman, M.R., and Harper, J.F. (1996). Targeting of two Arabidopsis H(+)-ATPase isoforms to the plasma membrane. Plant Physiol. 112 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., and Estelle, M. (2005. a). The F-box protein TIR1 is an auxin receptor. Nature 435 441–445. [DOI] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., Weijers, D., Lechner, E., Yamada, M., Hobbie, L., Ehrismann, J.S., Jürgens, G., and Estelle, M. (2005. b). Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9 109–119. [DOI] [PubMed] [Google Scholar]
- Dhonukshe, P., Aniento, F., Hwang, I., Robinson, D.G., Mravec, J., Stierhof, Y.D., and Friml, J. (2007). Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17 520–527. [DOI] [PubMed] [Google Scholar]
- Dhonukshe, P., et al. (2008). Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 105 4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R., and Jürgens, G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 147–153. [DOI] [PubMed] [Google Scholar]
- Fujioka, S., Takatsuto, S., and Yoshida, S. (2002). An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiol. 130 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Garbers, C., DeLong, A., Deruere, J., Bernasconi, P., and Soll, D. (1996). A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 15 2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Jürgens, G., and Palme, K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413 425–428. [DOI] [PubMed] [Google Scholar]
- Gray, W.M., Kepinski, S., Rouse, D., Leyser, O., and Estelle, M. (2001). Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414 271–276. [DOI] [PubMed] [Google Scholar]
- He, J.X., Fujioka, S., Li, T.C., Kang, S.G., Seto, H., Takatsuto, S., Yoshida, S., and Jang, J.C. (2003). Sterols regulate development and gene expression in Arabidopsis. Plant Physiol. 131 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler, M.G., Ohno, C., Das, P., Sieber, P., Reddy, G.V., Long, J.A., and Meyerowitz, E.M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15 1899–1911. [DOI] [PubMed] [Google Scholar]
- Jang, J.C., Fujioka, S., Tasaka, M., Seto, H., Takatsuto, S., Ishii, A., Aida, M., Yoshida, S., and Sheen, J. (2000). A critical role of sterols in embryonic patterning and meristem programming revealed by the fackel mutants of Arabidopsis thaliana. Genes Dev. 14 1485–1497. [PMC free article] [PubMed] [Google Scholar]
- Kepinski, S., and Leyser, O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn, J., Dhonukshe, P., Sauer, M., Brewer, P.B., Wisniewska, J., Paciorek, T., Benkova, E., and Friml, J. (2008). ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 18 526–531. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn, J., Dhonukshe, P., Swarup, R., Bennett, M., and Friml, J. (2006). Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18 3171–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, C., Sommarin, M., and Widell, S. (1994). Isolation of highly purified plant plasma membranes and separation of inside-out and right-side-out vesicles. Methods Enzymol. 228 451–462. [Google Scholar]
- Laxmi, A., Pan, J., Morsy, M., and Chen, R. (2008). Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One 3 e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, O. (2006). Dynamic integration of auxin transport and signalling. Curr. Biol. 16 R424–R433. [DOI] [PubMed] [Google Scholar]
- Men, S., Boutte, Y., Ikeda, Y., Li, X., Palme, K., Stierhof, Y.D., Hartmann, M.A., Moritz, T., and Grebe, M. (2008). Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 10 237–244. [DOI] [PubMed] [Google Scholar]
- Michniewicz, M., et al. (2007). Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130 1044–1056. [DOI] [PubMed] [Google Scholar]
- Mravec, J., Kubes, M., Bielach, A., Gaykova, V., Petrasek, J., Skupa, P., Chand, S., Benkova, E., Zazimalova, E., and Friml, J. (2008). Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135 3345–3354. [DOI] [PubMed] [Google Scholar]
- Muday, G.K., Peer, W.A., and Murphy, A.S. (2003). Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci. 8 301–304. [DOI] [PubMed] [Google Scholar]
- Müller, A., Guan, C., Gälweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998). AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, A.S., Bandyopadhyay, A., Holstein, S.E., and Peer, W.A. (2005). Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol. 56 221–251. [DOI] [PubMed] [Google Scholar]
- Nagata, N., Asami, T., and Yoshida, S. (2001). Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol. 42 1006–1011. [DOI] [PubMed] [Google Scholar]
- Noguchi, T., Fujioka, S., Takatsuto, S., Sakurai, A., Yoshida, S., Li, J., and Chory, J. (1999). Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5alpha-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 120 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek, T., Zazimalova, E., Ruthardt, N., Petrasek, J., Stierhof, Y.D., Kleine-Vehn, J., Morris, D.A., Emans, N., Jürgens, G., Geldner, N., and Friml, J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 1251–1256. [DOI] [PubMed] [Google Scholar]
- Petrasek, J., et al. (2006). PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918. [DOI] [PubMed] [Google Scholar]
- Pichler, H., and Riezman, H. (2004). Where sterols are required for endocytosis. Biochim. Biophys. Acta 1666 51–61. [DOI] [PubMed] [Google Scholar]
- Quint, M., Ito, H., Zhang, W., and Gray, W.M. (2005). Characterization of a novel temperature-sensitive allele of the CUL1/AXR6 subunit of SCF ubiquitin-ligases. Plant J. 43 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancour, D.M., Dickey, C.E., Park, S., and Bednarek, S.Y. (2002). Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol. 130 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and Russell, D. (2001). Molecular Cloning: A Laboratory Manual, 3rd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sauer, M., Balla, J., Luschnig, C., Wisniewska, J., Reinohl, V., Friml, J., and Benkova, E. (2006). Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 20 2902–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick, K., Mayer, U., Horrichs, A., Kuhnt, C., Bellini, C., Dangl, J., Schmidt, J., and Jürgens, G. (2000). FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev. 14 1471–1484. [PMC free article] [PubMed] [Google Scholar]
- Shin, H., Shin, H.S., Guo, Z., Blancaflor, E.B., Masson, P.H., and Chen, R. (2005). Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J. 42 188–200. [DOI] [PubMed] [Google Scholar]
- Souter, M., Topping, J., Pullen, M., Friml, J., Palme, K., Hackett, R., Grierson, D., and Lindsey, K. (2002). hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14 1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, C.M., and Pichler, H. (2007). Lipid requirements for endocytosis in yeast. Biochim. Biophys. Acta 1771 442–454. [DOI] [PubMed] [Google Scholar]
- Swarup, R., et al. (2004). Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16 3069–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, X., Calderon-Villalobos, L.I., Sharon, M., Zheng, C., Robinson, C.V., Estelle, M., and Zheng, N. (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446 640–645. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Dhonukshe, P., Brewer, P.B., and Friml, J. (2006). Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell. Mol. Life Sci. 63 2738–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale, W.D., Paponov, I.A., and Palme, K. (2006). Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 7 847–859. [DOI] [PubMed] [Google Scholar]
- Timpte, C., Lincoln, C., Pickett, F.B., Turner, J., and Estelle, M. (1995). The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 8 561–569. [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun, B., et al. (2008). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57 27–44 [DOI] [PubMed] [Google Scholar]
- Vieten, A., Sauer, M., Brewer, P.B., and Friml, J. (2007). Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 12 160–168. [DOI] [PubMed] [Google Scholar]
- Willemsen, V., Friml, J., Grebe, M., van den Toorn, A., Palme, K., and Scheres, B. (2003). Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska, J., Xu, J., Seifertova, D., Brewer, P.B., Ruzicka, K., Blilou, I., Rouquie, D., Benkova, E., Scheres, B., and Friml, J. (2006). Polar PIN localization directs auxin flow in plants. Science 312 883. [DOI] [PubMed] [Google Scholar]
- Woodward, A.W., and Bartel, B. (2005). Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 95 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Hofhuis, H., Heidstra, R., Sauer, M., Friml, J., and Scheres, B. (2006). A molecular framework for plant regeneration. Science 311 385–388. [DOI] [PubMed] [Google Scholar]
- Xu, J., and Scheres, B. (2005). Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.