Abstract
Although 75% of endometrial cancers are treated at an early stage, 15% to 20% of these recur. We performed an integrated analysis of genome-wide expression and copy-number data for primary endometrial carcinomas with extensive clinical and histopathological data to detect features predictive of recurrent disease. Unsupervised analysis of the expression data distinguished 2 major clusters with strikingly different phenotypes, including significant differences in disease-free survival. To identify possible mechanisms for these differences, we performed a global genomic survey of amplifications, deletions, and loss of heterozygosity, which identified 11 significantly amplified and 13 significantly deleted regions. Amplifications of 3q26.32 harboring the oncogene PIK3CA were associated with poor prognosis and segregated with the aggressive transcriptional cluster. Moreover, samples with PIK3CA amplification carried signatures associated with in vitro activation of PI3 kinase (PI3K), a signature that was shared by aggressive tumors without PIK3CA amplification. Tumors with loss of PTEN expression or PIK3CA overexpression that did not have PIK3CA amplification also shared the PI3K activation signature, high protein expression of the PI3K pathway member STMN1, and an aggressive phenotype in test and validation datasets. However, mutations of PTEN or PIK3CA were not associated with the same expression profile or aggressive phenotype. STMN1 expression had independent prognostic value. The results affirm the utility of systematic characterization of the cancer genome in clinically annotated specimens and suggest the particular importance of the PI3K pathway in patients who have aggressive endometrial cancer.
Keywords: amplification, endometrial cancer, prognosis, comparative genomic hybridization, stathmin expression
With a 2% to 3% lifetime risk among women, endometrial cancer is the most common pelvic gynecologic malignancy in industrialized countries, and the incidence is increasing (1). Approximately 75% of cases are diagnosed with the tumor confined to the uterine corpus (1, 2), but after primary surgery 15% to 20% of these tumors recur and have limited response to systemic therapy. In light of these recurrences, patients who have localized endometrial cancer have 2 major needs: (1) adjuvant therapies that will reduce the recurrence rate, and (2) the ability to target these therapies to the patients in whom disease is most likely to recur. In addition, women who have metastatic disease require effective systemic therapy.
The needs for effective systemic therapies and for reliable prognostic markers have been addressed only partly. The most common basis for determining risk of recurrent disease has been the categorization of endometrial cancer into 2 subtypes. The majority are type I, associated with good prognosis, low stage and grade, and endometrioid histology. In contrast, type II cancers are characterized by high stage and grade, non-endometrioid histology, and poor prognosis. The prognostic value of this distinction is limited, however, because up to 20% of type I cancers recur, and half of type II cancers do not (2).
The molecular basis of the distinction between type I and II cancer is understood only partially. Type I cancer is associated with hyperestrogenic risk factors, is more often estrogen and progesterone receptor positive, diploid, microsatellite unstable, and KRAS or PTEN mutant. Type II cancer is more often aneuploid and harbors alterations in CDKN2A, TP53, and ERBB2 (3). Such molecular alterations are of prognostic value (4) but have not provided a basis for improved therapy (3). Hormone receptor status influences the choice of treatment in metastatic disease, but most aggressive tumors are receptor negative (1).
We hypothesized that tumors with an aggressive phenotype are likely to be distinguished by underlying genetic alterations reflected in distinct transcriptional signatures, and we investigated whether tumors that recur share transcriptional signatures that suggest shared underlying genetic alterations.
Results and Discussion
Unsupervised Analysis of Expression Profiles Distinguishes Aggressive Tumors.
We collected genome-wide expression and clinical and histopathologic data from a random sampling of 57 endometrial carcinomas in a population-based tissue bank of gynecologic cancer in Hordaland County, Norway. The characteristics of these patients were not significantly different from those of all patients diagnosed with endometrial carcinoma in a 10-year period from the same region (4) [supporting information (SI) Table S1].
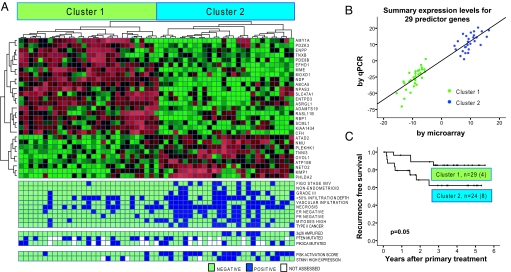
An unsupervised analysis of these data distinguished 2 major groups of tumors (clusters 1 and 2, Fig. 1A). Significant analysis of microarrays (SAM) (5) between these clusters identified a 2-fold or higher change for 138 significant genes, of which 64 were up-regulated and 74 downregulated in cluster 2 (data not shown). A set of 29 genes, validated by quantitative RT-PCR, predicted the clusters with 100% accuracy (Fig. 1 A and B).
Fig. 1.
Results of unsupervised clustering of expression data from 57 endometrial carcinomas. (A) Two major clusters are identified and recapitulated using the displayed predictor set of 29 genes. Features associated with each tumor are displayed in the bottom 3 panels. PI3K scores in the top tertile are called positive. (B) The ability of the 29 predictor genes to distinguish cluster 1 and 2 tumors was validated in these tumors by quantitative RT-PCR. (C) Recurrence-free survival was significantly lower for patients in cluster 2. Four tumors (all in cluster 2) were metastatic at presentation and omitted from this analysis.
The 2 clusters had strikingly different clinical and histopathologic characteristics. Cluster 2 contained more aggressive tumors, with higher International Federation of Gynecology and Obstetrics (FIGO) stage, histologic grade, number of mitoses, presence of non-endometrioid histologic subtype, tumor necrosis, and vascular invasion, (P < 0.001 for the presence of any of these characteristics; Fig. 1A, Table S2). Cluster 2 contains almost all the type II tumors (P < 0.001) (Fig. 1A, Table S2) but it also contains almost 1/3 of the type I tumors, and these tumors have more vascular invasion, necrosis, and frequent mitoses than the type I tumors in cluster 1 (P = 0.01). The 29-gene summary set also was significantly correlated with aggressive cancer (Table S2).
Most prominently, patients who had tumors in cluster 2 had significantly poorer recurrence-free survival (P = 0.05, Fig. 1C). Segregation into cluster 2 predicted recurrence better than FIGO stage, histologic subtype, or receptor status and slightly less well than grade (Table S3) but did not exhibit independent prognostic impact, probably because of the limited number of cases and events.
Regions of Significant Amplification, Deletion, and Loss of Heterozygosity.
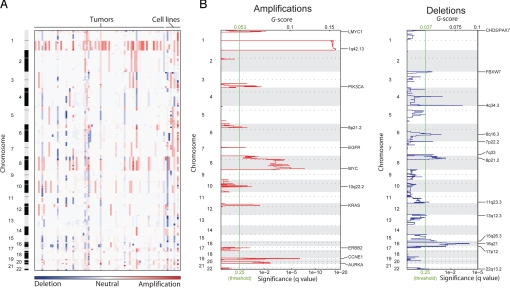
To identify the underlying somatic changes distinguishing aggressive tumors with the cluster 2 signature, we performed a genome-wide survey of copy-number changes and loss of heterozygosity (LOH) among 84 tumors. The majority of tumors exhibit a small number of amplifications (median of 4 in each tumor) and even fewer deletions (median of 1). Nevertheless, virtually every region of the genome is amplified or deleted in at least 1 tumor (Fig. 2A).
Fig. 2.
Significant copy-number alterations in endometrial cancer. (A) Amplifications (red) and deletions (blue), determined by segmentation analysis of normalized signal intensities from 100K SNP arrays (see Methods), are displayed across the genome (chromosome positions, indicated along the y axis, are proportional to marker density) for 76 tumors and 9 cell lines. (B) GISTIC analysis of copy-number changes. The G score represents the frequency times average amplitude of the aberrations identified in (A). The false discovery rate q-values, representing the statistical significance associated with these scores (7), are displayed along the bottom. Regions with q-values < 0.25 (green lines) were considered significantly altered. The locations of the peak regions of maximal copy-number change and the known cancer-related genes within those peaks are indicated to the right of each panel.
To distinguish copy-number changes associated with endometrial cancer from potentially random events, we applied the statistical method Genomic Identification of Significant Targets in Cancer (GISTIC) (6). GISTIC assigns each region of the genome 2 G-scores (Fig. 2B), each representing the combined frequency and amplitude of either local amplifications or deletions. It then compares these scores with similar scores generated from random permutations of the data to determine false discovery rate q-values (7), representing the likelihood of obtaining the observed G-scores from chance events alone. The G-scores tend to be larger for amplifications than deletions (Fig. 2B) because of the greater prevalence of amplifications (Fig. 2A). Conversely, deletions attain statistical significance (using a q-value threshold of 0.25, green lines in Fig. 2B) at lower prevalence because of their overall infrequency.
We found 11 significantly amplified and 13 significantly deleted regions of the genome (Table S4), including several that had been noted previously (8, 9). For each we selected the peak region (ie, the highest frequency and amplitude of events) as the region most likely to contain a cancer gene target. Known oncogenes are located within these peaks for 8 amplified regions, and known tumor suppressors are located within deletion peaks on chromosomes 1 and 3 (Table S4), but functional data tying any of these genes to endometrial carcinogenesis are lacking. Also, 14 regions contain no known cancer genes. These peaks usually represent infrequent events (< 17% of tumors), with the exception of 1q amplification, in which the gene target is unclear because of the large size of the amplicon. The consistent breadth of this amplicon, in fact, may suggest more than 1 target (6). LOH generally reflects deletions, with the exception of prevalent copy-neutral LOH on 10q (Fig. S1) containing the known endometrial tumor suppressor PTEN (10).
Amplifications of KRAS and PIK3CA Associate with Poor Prognosis.
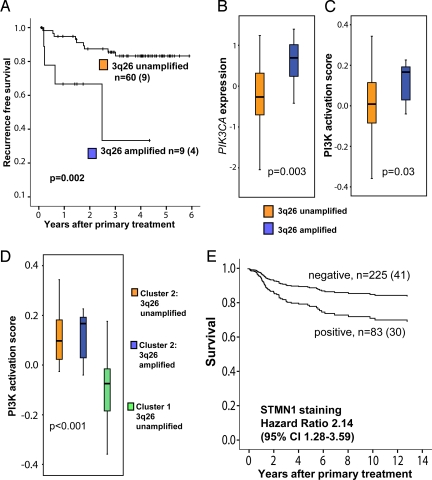
Among the 11 significant amplifications, only 2 (3q26.32 and 12p12.1) are associated with recurrence-free survival (in both cases poor survival) after correction for multiple hypotheses (Table S5, Fig. 3A). We considered only amplifications because of the low prevalence of deletions. Amplification of 3q26.32 (3qamp) also is associated with non-endometrioid (44% vs. 11% prevalence; P = 0.02) and high-grade (P < 0.001) histology. The association between 12p12.1 amplification and poor survival is surprising, because mutations of KRAS, which is within the peak region, are known to associate with better survival. However, we sequenced KRAS in 64 tumors and found mutant KRAS in none of the 12p12.1-amplified samples, although mutations were seen in 4 unamplified samples. Amplification of 12p12.1 is also associated with high grade (P = 0.02) and FIGO stage (P = 0.04). Although 3q26.32 and 12p12.1 tended to be amplified in the same tumors (P = 0.03), they usually did not coincide. We directed further analyses at 3qamp because all the samples with this amplification segregated into expression cluster 2 (P = 0.01) (Fig. 1A), suggesting that the amplification could be associated with the cluster 2 transcriptional profile (SI Text Note 1).
Fig. 3.
Relations between PIK3CA amplification, PI3K activation, and survival. (A) Amplification of a region in 3q26 that includes PIK3CA is significantly associated with poor recurrence-free survival. These amplifications are associated with (B) overexpression of PIK3CA and (C) increased PI3K scores. (D) Among the broader set of poor-prognosis tumors in expression cluster 2, PI3K scores are equally high among tumors without 3q26 amplification and with 3q26 amplification, suggesting alternative methods of pathway activation. (E) Tumors with elevated protein expression of the PI3K pathway member STMN1 had significantly poorer survival, after controlling for age, FIGO stage, histologic subtype, and grade.
Integrated Analyses Associate Markers of PI3 Kinase Activation with Aggressive Cancer.
We wanted to investigate whether 3qamp leads to an aggressive phenotype through activation of PIK3CA. Although PIK3CA has not been shown to be the 3qamp target, we suspected it for 4 reasons: (i) PIK3CA is 1 of 36 genes within the peak region (indeed, PIK3CA amplifications were noted previously; see refs. 11, 12); (ii) tumors with 3qamp overexpress PIK3CA compared with unamplified tumors (P = 0.003; Fig. 3B); (iii) similar amplifications in ovarian cancer act through PIK3CA (13); and (iv) the PI3 kinase (PI3K) pathway frequently is aberrant in endometrial cancer (11), including point mutations in PIK3CA (14).
We therefore looked for wider effects of PIK3CA activation in the transcriptome of tumors with 3qamp. We used published data (15) from cell lines transfected with mutationally activated PIK3CA to define a PI3K activation score (PI3K score), representing the expression levels of genes that correlate with activated PIK3CA (see Methods). Tumors with 3qamp scored higher than unamplified samples (P = 0.05; Fig. 3C). However, the impact of this finding is limited by its borderline statistical significance and by the possibility that the PI3K score may reflect PI3K activation only in the model systems in which it was measured but not generally .
We therefore sought to corroborate this finding by seeing whether samples with 3qamp have an expression profile opposite that induced by PI3K pathway inhibition. To that end, we queried the 50 most overexpressed and underexpressed genes in samples with 3qamp relative to unamplified samples (Fig. S2A) using the Connectivity Map (16). Among 164 small molecules represented in the Connectivity Map, the PI3K inhibitor LY-294002 (17) had an expression signature most significantly anticorrelated with the 3qamp signature (Fig. S2 B and C, P = 0.003). LY-294002 is known to bind to additional kinases (18), raising the possibility that this anticorrelation results from nonspecific effects. The anticorrelation between the 3qamp signature and inhibitors of adenylate cyclase and Hsp90 (Fig. S2B) also suggests potentially complex effects of the amplicon. Nevertheless, the findings that the 3qamp signature correlates with a PI3K activation signature and anticorrelates with the signature of a PI3K inhibitor support the hypothesis that an effect of 3qamp may be to increase PI3K activity.
We also validated the correlation between PIK3CA amplification and the PI3K score in an independent expression dataset. We first inferred amplification of 3q26–27 from local gene expression levels, as reflected in a “functional amplification” (FA) score (19). As expected, samples determined to have 3qamp by SNP array analysis also had high 3q26–27 FA scores (P < 10−5, Fig. S3A), confirming the score as a meaningful assessment of amplification status. We then inferred 3q26–27 amplification levels in a publicly available expression dataset of 134 endometrial tumors (http://expo.intgen.org/geo/home.do). The correlations between 3qamp and both PIK3CA overexpression and the PI3K score validated (P = 2 × 10−10 and 7 × 10−5, respectively; Fig. S3 B and C).
In addition, we validated the correlations between aggressive phenotype and both PIK3CA amplification and the cluster 2 signature in this independent dataset. Although survival data were unavailable, both available markers of poor survival, high-grade and non-endometrioid subtype (20) correlated with high 3q26–27 FA scores (P = 0.001 and 0.005, respectively; Fig. S3 D and E) and with high values of the 29-gene summary predictor for membership in cluster 2 (P = 3 × 10−4 and P = 0.004, respectively; Fig. S3 F and G).
The finding that both PIK3CA amplification and the cluster 2 expression profile indicate aggressive tumors, coupled with the association between PIK3CA amplification and the in vitro PI3K activation signature, suggested that the broader set of aggressive tumors in cluster 2 might share the in vitro PI3K activation signature. This conjecture seems to be true: tumors in cluster 2 without PIK3CA amplification have PI3K scores significantly higher than the scores of tumors in cluster 1 (P < 0.001) and equal to those in tumors with amplification of PIK3CA (Fig. 3D). Moreover, the cluster 2 signature is highly anticorrelated with the signature of treatment with LY-294002 (P = 0.02; Fig. S4 A and B). Furthermore, tumors with high PI3K scores are associated with poor survival (P = 0.03, Fig. S4C) and other markers of aggressive phenotype in both the test and validation datasets (P = 0.01 and P = 0.001, respectively).
A possible cause of overexpression of the PI3K activation signature among tumors without PIK3CA amplification is decreased expression of the downstream PI3K pathway member PTEN. Decreased PTEN expression was associated with increased PI3K scores in both our test and validation datasets (P < 0.001 and P = 0.03, respectively), regardless of PIK3CA amplification status. Decreased PTEN expression also was associated with markers of aggressive disease (P = 0.02), in agreement with prior studies (21).
Conversely, among the 45 tumors with expression data that we sequenced for PTEN, mutations did not associate with high PI3K scores (P = 0.6; Fig. S4D). On the contrary, we observed more mutations in the nonaggressive cluster 1 than in cluster 2 (P = 0.04; Fig. 1A), in line with prior studies associating PTEN mutations with indolent disease (22, 23).
Overexpression and mutation of PIK3CA also seem to have different implications. We found significantly higher PIK3CA expression in tumors with aggressive features, including those without PIK3CA amplification (P = 0.05 and P = 0.0009 among test and validation data, respectively). However, among the 41 tumors with expression data that we sequenced for PIK3CA, mutations did not associate with high PI3K scores (P = 0.8; Fig. S4E) or features of aggressive disease (P = 0.5). We also cannot confirm the finding that exon 20 mutations correspond to aggressive tumors (24) (data not shown). These results were surprising in light of evidence that overexpression of mutated, but not wild-type, PIK3CA leads to transformation (25, 26) and suggest either that PIK3CA suffers from prevalent cryptic mutations or that transformation assays did not capture the effects of wild-type PIK3CA overexpression in human tumors. Although PIK3CA mutations previously have been noted primarily in endometrioid cancers (11, 12), we also find no correlation with histologic subtype (P = 1).
Expression of the PI3 Kinase Pathway Member STMN1 is an Independent Prognostic Indicator.
The suggestion that PI3K activation associates with poor prognosis suggested that measuring PI3K activity might improve prognostication of localized endometrial cancer. Expression of STMN1 previously has been shown to correlate with PI3K activity in breast cancer (27) and can be measured by immunohistochemistry in paraffin-embedded tissue. We therefore measured STMN1 expression by immunohistochemistry (SI Methods and Fig. S4 F and G) in 72 tumors, including 66 with SNP array and 53 with expression data (Fig. 1A). Although STMN1 is not a member of our PI3K activation signature, STMN1 expression correlated with PI3K scores (P = 0.05). High STMN1 expression also correlated with PIK3CA amplification (P = 0.04) and overexpression (P = 0.04) and with segregation in cluster 2 (P = 0.03), supporting our prior associations between these features and PI3K pathway activation.
High STMN1 expression also was associated with poor recurrence-free survival in our original tumor set (P = 0.006) and with poor recurrence-free (P = 0.01) and overall (P = 0.01) survival in a validation set of 241 tumors from a population-based series of all endometrial carcinomas in Hordaland County from 1981 to 1990 (4, 21). In both tumor sets, STMN1 expression correlated with grade, mitotic rate, presence of necrosis or vascular invasion, and type II status (Table S6). Nevertheless, across all 313 cases (except for 5 cases with missing clinical data), high STMN1 expression was a prognostic indicator independent of FIGO stage, histologic subtype, grade, and age (P = 0.004; Fig. 3G, Table S7). In particular, high STMN1 expression was associated with poor prognosis in the otherwise low-risk endometrioid subgroup (P = 0.007, data not shown).
Ultimately, the goals of integrated genomic analyses of localized tumors are to enable development of clinical assays to distinguish aggressive tumors requiring therapy beyond resection and of effective therapeutics for such tumors. We have shown that both transcriptional and copy-number profiles of endometrial tumors contain prognostic information that is partly reflected in expression levels of PIK3CA, in vitro PI3K activation signatures, PTEN, and STMN1. We also find that PTEN and PIK3CA mutations seem to have different transcriptional and phenotypic correlates than changes in expression of these genes. These results suggest that further investigation of the specific consequences of mutation and altered expression is warranted. They also emphasize the potential utility of clinical assays for PI3K pathway activation to identify patients who have aggressive disease and the particular relevance of therapeutics that inhibit this pathway.
Aside from the PI3K pathway, our general survey of chromosomal changes in endometrial carcinoma also identified approximately 20 other regions of significant copy-number change. Most of these copy-number changes involve tens to hundreds of genes, so even when known oncogenes or tumor suppressors are within the regions most affected by these copy-number changes, the genomic data are ambiguous as to the actual target. In many cases, including amplification of 3q26.32, the size of these events suggests the possibility of multiple targets. Moreover, functional data tying even known oncogenes and tumor suppressors to carcinogenesis in model systems of endometrial cancer are lacking, for the most part. The limited number of significant regions of copy-number change suggests that comprehensive, systematic experiments to identify these oncogenes and tumor suppressors in endometrial cancer are feasible. Such experiments are likely to point to therapeutic targets for women with all stages of endometrial carcinoma.
Materials and Methods
Patient Series.
For the primary investigation series, primary endometrial carcinomas were frozen immediately during hysterectomies conducted from 2001 to 2003. All samples were reviewed by a pathologist according to published criteria (28). Treatment included bilateral salpingo-oophorectomy and pelvic lymphadenectomy. Adjuvant therapy was recommended for patients who had disease of FIGO surgical stage IIB or higher or non-endometrioid histology. Patients were followed from primary surgery until June, 2007 or death, with a median follow-up for survivors of 3.6 years (range, 0.8–5.5). Deaths not attributable to endometrial cancer were censored. No patient was lost to follow-up.
RNA Analysis.
RNA was extracted from biopsies with at least 50% (usually > 80%) tumor content using the RNeasy kit (Qiagen). Quality and yield were assessed by agarose electrophoresis, the Agilent Bioanalyser 2100, and spectrophotometry. RNA was prepared in 2 batches and hybridized to Agilent 21K and 22K arrays, respectively, according to manufacturer's instructions (www.agilent.com). Arrays were scanned using the Agilent Microarray Scanner Bundle.
Signal intensities were determined using J-Express (www.molmine.com) and filtered to remove genes with signal intensities below 2 SD over background in either channel (Cy5, Cy3) in more than 30% of samples. Batch adjustment was performed as described previously (29). Genes were mean-centered across the tumor set.
Hierarchical clustering was performed using the 3500 genes with highest variance using weighted average linkage and Pearson correlation as similarity measures. Clustering with more or fewer genes gave stable results (data not shown). A SAM analysis using these clusters as class labels identified 138 significantly changed genes, 29 of which were selected for their combined discriminatory power as described in SI Methods. Messenger RNA levels for these 29 genes and PTEN were validated by quantitative PCR using the TaqMan Low Density Array (Applied Biosystems) according to manufacturer's instructions (29).
For the external dataset (Affymetrix U133 + 2 arrays), individual probes were sequence-matched against Aceview (NCBI35) (19) to construct transcript-level probe sets. Summary expression levels then were derived by batch normalization across samples via RMA (30).
The PI3K score was obtained by comparing previously published expression data of 9 replicate transfections of activated PIK3CA with 5 GFP controls (15) and includes the 495 genes surpassing a Bonferroni-corrected 2-sided t test p-value of 0.05. To evaluate this signature, expression data for each gene were normalized to a common mean and scaled to the same SD. For each sample, the activation score is the sum of genes significantly up-regulated in the cells with activated PIK3CA (relative to the cells with GFP control) minus genes significantly down-regulated in those cells.
DNA Analysis.
Genomic DNA was extracted from surgically dissected, fresh-frozen primary tumors and from 9 cell lines: Ishicawa, Hec1A, KLE, AN3-CA, EFE184, MFE-280, MFE-296, MFE-319, and RL-95–2. Tumors were needle dissected to ensure 80% purity.
PIK3CA, KRAS, and PTEN were sequenced as previously described (31). Genomic DNA was analyzed by SNP arrays interrogating 116,204 SNP loci (Affymetrix) and the GISTIC algorithm, as previously described (6). SNP, gene, and cytogenetic band locations are based on the hg16 (July 2003) genome build (genome.ucsc.edu).
Statistics.
For relations of molecular data to clinical phenotype, Pearson's χ2- (χ2), Fisher's exact-, Mann-Whitney-, or Kruskal-Wallis tests were used as appropriate. P-values represent 2-sided tests except when testing the 1-sided hypothesis that 3qamp correlates with measures of PI3K activation. Univariate survival analyses were performed by the Kaplan-Meier method. The log-rank (Mantel-Cox) test with Bonferroni correction was used to compare survival curves for different categories. Variables with significant impact on survival (P ≤ 0.05) were examined further by log-minus-log plot before incorporation in the Cox proportional hazards regression model.
Supplementary Material
Acknowledgments.
The authors thank Gerd Lillian Hallseth, Bendik Nordanger, Britt Edvardsen, Randi Nygaard, Beth Johannessen, and Hua My Hoang for technical assistance. This study was supported by Helse Vest Grants 911005, 911351, and 911069, the University of Bergen, the Norwegian Cancer Society Grants D94070/04, D00019/01, HS01–2006-0446 (Harald Andersens legat), the Research Council of Norway Grants 154942/V40 and 163920/V50, Department of Defense Grants PC040638 and PC061642, National Institutes of Health Grant K08CA122833, and the Dana-Farber Harvard Cancer Center Prostate Specialized Program in Research Excellence. This research was approved by the Norwegian Data Inspectorate (961478–2), Norwegian Social Sciences Data Services (15501), and local ethics committee (REKIII nr. 052.01).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.W.G. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.gov/projects/geo (accession no. GSE14860).
This article contains supporting information online at www.pnas.org/cgi/content/full/0806514106/DCSupplemental.
References
- 1.Amant F, et al. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG. Endometrial carcinoma. N Eng J Med. 1996;335:640–649. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 3.Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: From a phenotypical to a molecular-based classification. Virchows Archiv. 2004;444:213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- 4.Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53 and p21 expression: A population-based endometrial carcinoma study. J Clin Oncol. 1999;17:1382–1390. doi: 10.1200/JCO.1999.17.5.1382. [DOI] [PubMed] [Google Scholar]
- 5.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beroukhim R, et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. JR Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 8.Levan K, et al. Chromosomal alterations in 98 endometrioid adenocarcinomas analyzed with comparative genomic hybridization. Cytogenet Genome Res. 2006;115:16–22. doi: 10.1159/000094796. [DOI] [PubMed] [Google Scholar]
- 9.Micci F, et al. Genomic aberrations in carcinomas of the uterine corpus. Genes Chromosomes Cancer. 2004;40:229–246. doi: 10.1002/gcc.20038. [DOI] [PubMed] [Google Scholar]
- 10.Risinger JI, et al. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–4738. [PubMed] [Google Scholar]
- 11.Ollikainen M, et al. Patterns of PIK3CA alterations in familial colorectal and endometrial carcinoma. Int J Cancer. 2007;121:915–920. doi: 10.1002/ijc.22768. [DOI] [PubMed] [Google Scholar]
- 12.Miyake T, et al. PIK3CA gene mutations and amplifications in uterine cancers, identified by methods that avoid confounding by PIK3CA pseudogene sequences. Cancer Lett (Shannon, Irel) 2008;261:120–126. doi: 10.1016/j.canlet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Shayesteh L, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 14.Oda K, et al. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 15.Potti A, et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006;12:1294–1300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- 16.Lamb J, et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 17.Vlahos CJ, et al. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 18.Gharbi SI, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter SL, et al. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 20.Abeler VM, Kjørstad KE, Berle E. Carcinoma of the endometrium in Norway: A histopathological and prognostic survey of a total population. Int J Gynecol Cancer. 1992;2:9–22. doi: 10.1046/j.1525-1438.1992.02010009.x. [DOI] [PubMed] [Google Scholar]
- 21.Salvesen HB, et al. Loss of PTEN expression is associated with metastatic disease in patients with endometrial carcinoma. Cancer. 2002;94:2185–2191. doi: 10.1002/cncr.10434. [DOI] [PubMed] [Google Scholar]
- 22.Salvesen HB, et al. Significance of PTEN alterations in endometrial carcinoma: A population-based study of mutations, promoter methylation and PTEN protein expression. Int J Oncol. 2004;25:1615–1623. [PubMed] [Google Scholar]
- 23.Risinger JI, et al. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res. 1998;4:3005–3010. [PubMed] [Google Scholar]
- 24.Catasus L, et al. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–139. doi: 10.1038/modpathol.3800992. [DOI] [PubMed] [Google Scholar]
- 25.Kang S, et al. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao JJ, et al. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA. 2005;102:18443–18448. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saal LH, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scully RE, et al. World Health Organization. Berlin/Heidelberg: Springer-Verlag; 1994. Histological Typing of Female Genital Tract Tumours. International Histological Classification of Tumours. [Google Scholar]
- 29.Engelsen IB, et al. Low BMI-1 expression is associated with an activated BMI-1-driven signature, vascular invasion, and hormone receptor loss in endometrial carcinoma. Br J Cancer. 2008;98:1662–1669. doi: 10.1038/sj.bjc.6604360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:1–8. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutt A, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA. 2008;105:8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.