Abstract
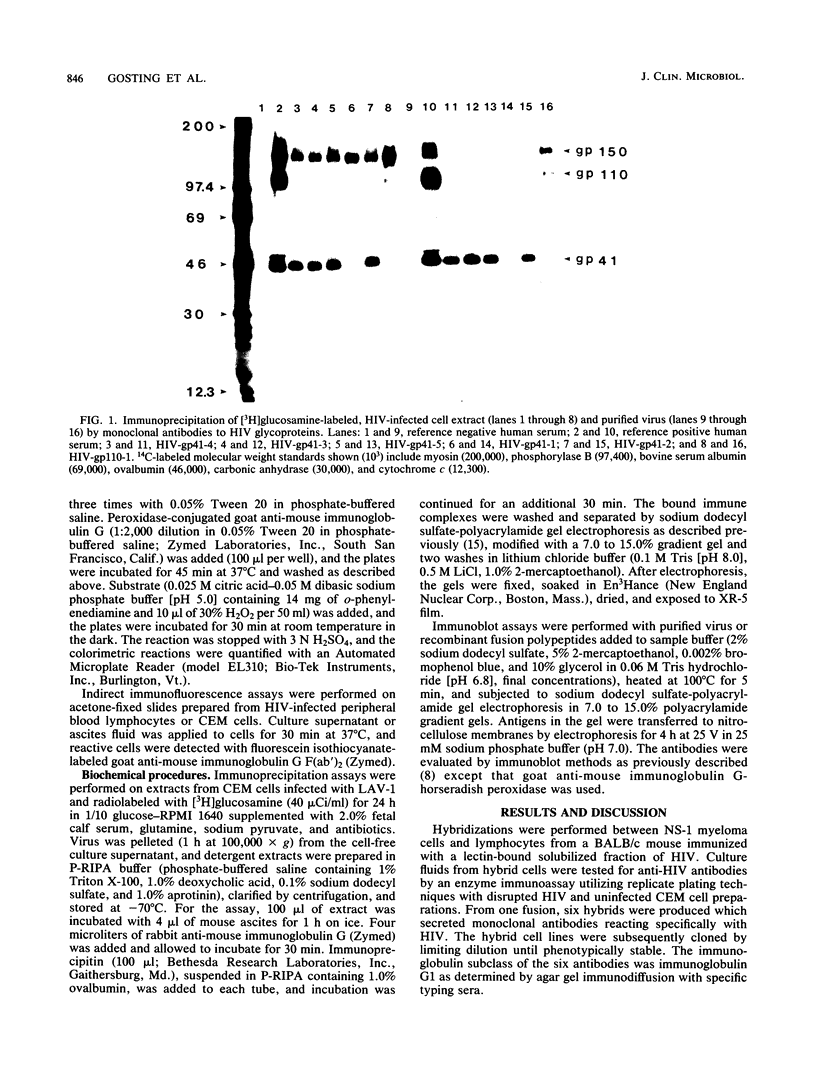
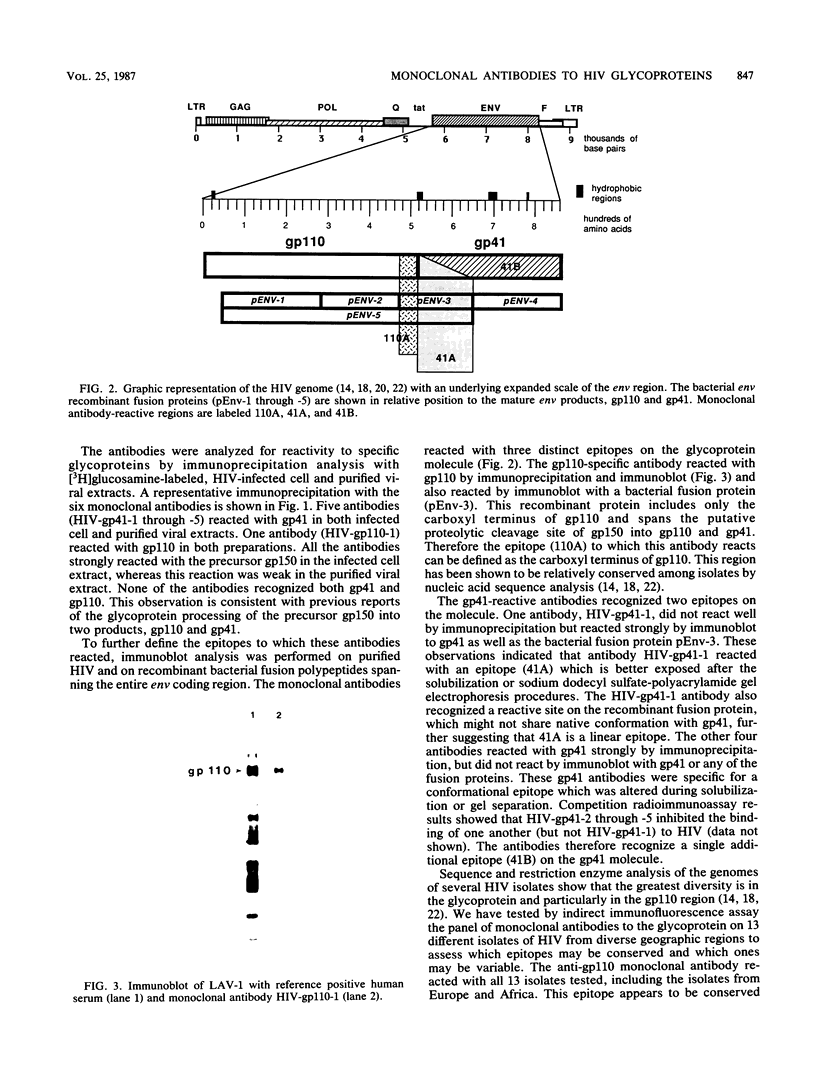
Six mouse hybridomas secreting monoclonal antibodies specific for the glycoproteins of human immunodeficiency virus were developed. All six antibodies reacted by radioimmunoprecipitation with the glycoprotein precursor of 150,000 daltons as well as one of the proteolytic processing products of 110,000 daltons (gp110) or 41,000 daltons (gp41). Recombinant polypeptides spanning the env coding region were used to locate epitopes on the glycoprotein molecule. The panel of antibodies detected two distinct epitopes of gp41 and one epitope of gp110. We used the antibodies in indirect immunofluorescence assays to evaluate 13 clinical isolates of human immunodeficiency virus from diverse geographic regions, and we found that the gp110 epitope was recognized on all tested isolates, whereas the two gp41 epitopes were detected on 10 of 13 and 4 of 13 isolates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Coffin J., Haase A., Levy J. A., Montagnier L., Oroszlan S., Teich N., Temin H., Toyoshima K., Varmus H., Vogt P. Human immunodeficiency viruses. Science. 1986 May 9;232(4751):697–697. doi: 10.1126/science.3008335. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosting L. H., Cabrian K., Sturge J. C., Goldstein L. C. Identification of a species-specific antigen in Legionella pneumophila by a monoclonal antibody. J Clin Microbiol. 1984 Dec;20(6):1031–1035. doi: 10.1128/jcm.20.6.1031-1035.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Popovic M., Patel A., Veren K., Fladager A., Orndorff S., Gallo R. C. Advances in the isolation of HTLV-III from patients with AIDS and AIDS-related complex and from donors at risk. Cancer Res. 1985 Sep;45(9 Suppl):4588s–4591s. [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Montagnier L., Clavel F., Krust B., Chamaret S., Rey F., Barré-Sinoussi F., Chermann J. C. Identification and antigenicity of the major envelope glycoprotein of lymphadenopathy-associated virus. Virology. 1985 Jul 15;144(1):283–289. doi: 10.1016/0042-6822(85)90326-5. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Groopman J. E., Markham P. D., Sarngadharan M. G., Redfield R. R., McLane M. F., Essex M., Sliski A., Gallo R. C. HTLV-III in symptom-free seronegative persons. Lancet. 1984 Dec 22;2(8417-8418):1418–1420. doi: 10.1016/s0140-6736(84)91619-2. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Popovic M., Sarngadharan M. G., Orndorff S., Fladagar A., Patel A., Gold J., Gallo R. C. Isolation of infectious human T-cell leukemia/lymphotropic virus type III (HTLV-III) from patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related complex (ARC) and from healthy carriers: a study of risk groups and tissue sources. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5530–5534. doi: 10.1073/pnas.82.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]