Abstract
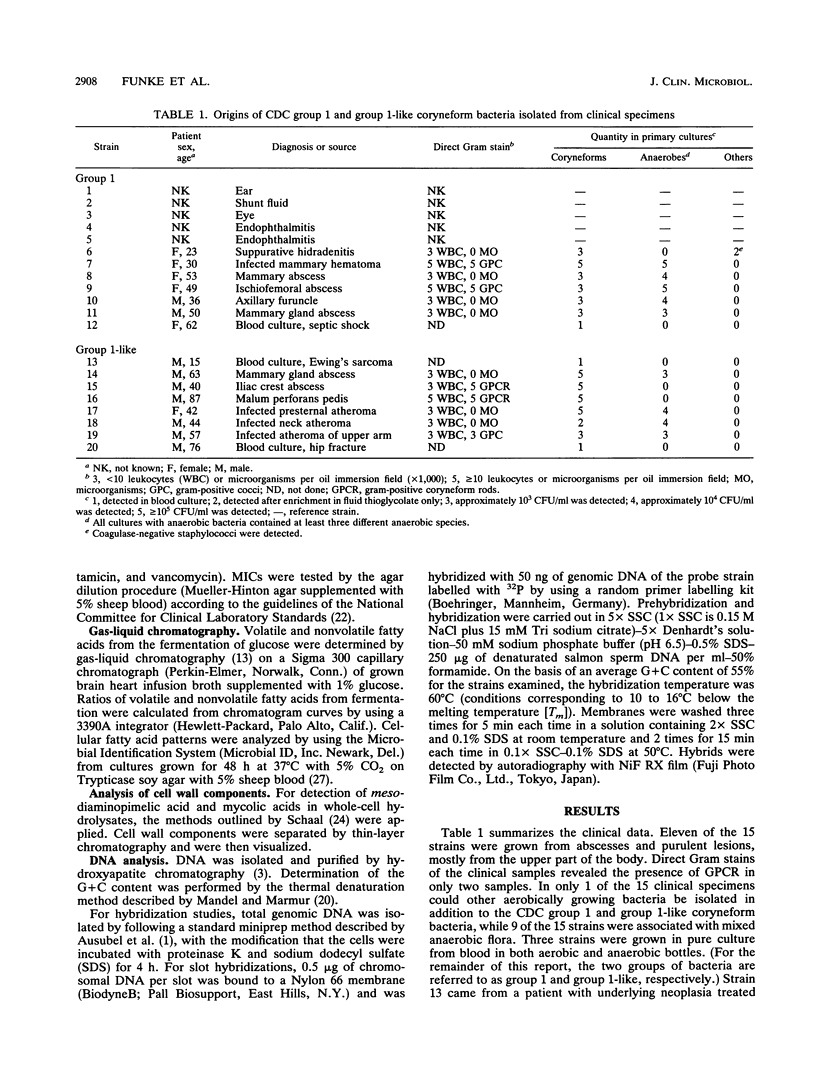
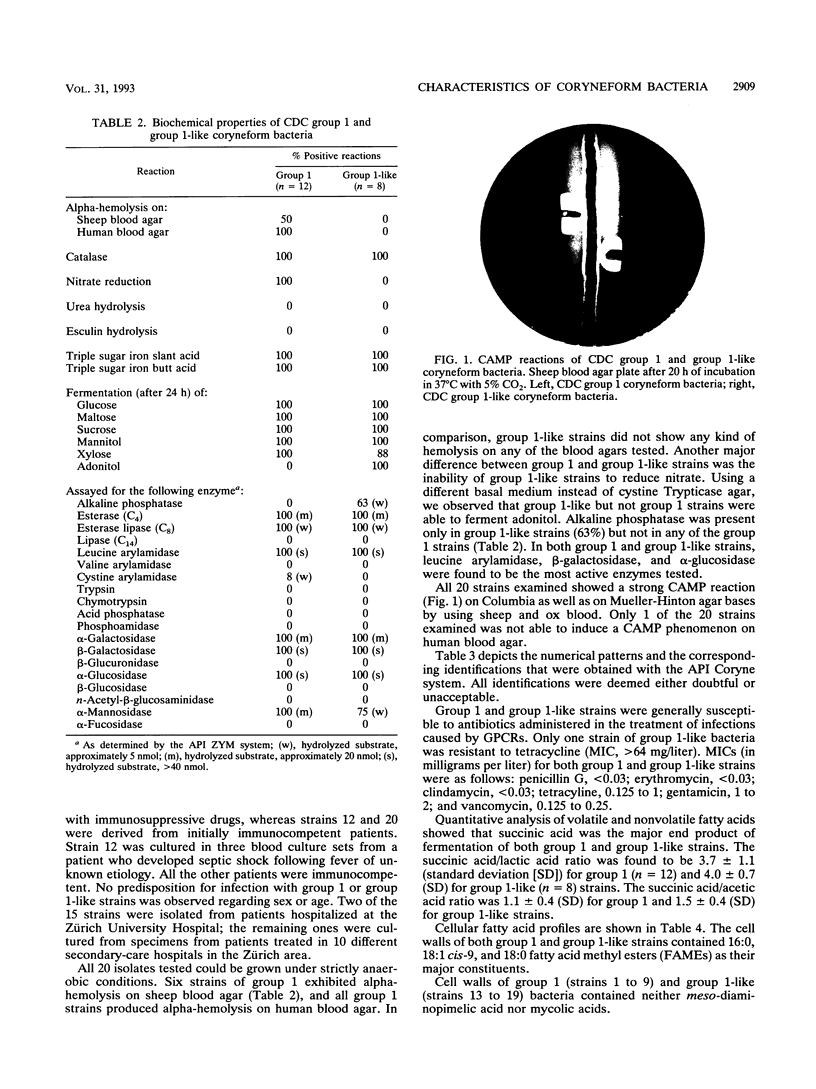
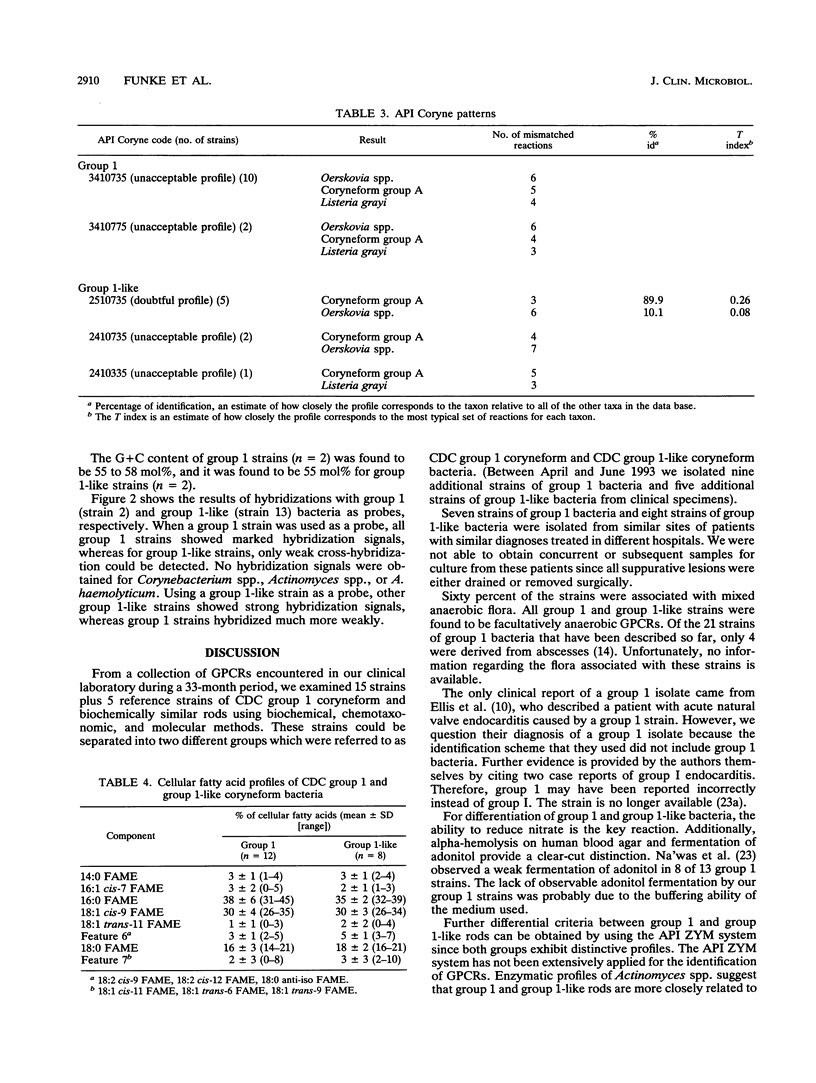
Fifteen strains of CDC group 1 coryneform and biochemically similar bacteria were isolated from clinical specimens. Of the 15 strains isolated, 11 were derived from abscesses and purulent lesions, mostly from the upper part of the body, and 3 were grown from blood cultures. Nine strains were associated with mixed anaerobic but no other aerobic flora. Seven strains exhibited the classical biochemical profile of CDC coryneform group 1; however, eight strains were unable to reduce nitrate and were called "group 1-like." Other reactions to differentiate CDC group 1 and group 1-like coryneform rods include alpha-hemolysis on human blood agar, fermentation of adonitol, and the presence of alkaline phosphatase. Fifteen strains showed marked CAMP reactions on different erythrocyte agars. Gas-liquid chromatography of volatile and nonvolatile fatty acids as well as cellular fatty acid patterns and the composition of cell wall components suggest that CDC group 1 and group 1-like coryneform bacteria do not belong to the genus Corynebacterium but possibly to the genus Actinomyces or Arcanobacterium. DNA-DNA hybridization studies revealed that group 1 and group 1-like strains represent different species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard K. A., Bellefeuille M., Ewan E. P. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991 Jan;29(1):83–89. doi: 10.1128/jcm.29.1.83-89.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion P., Holder-Franklin M. A., McCully J., Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977 Aug;81(2):461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- Choudhury T. K. Synergistic lysis of erythrocytes by Propionibacterium acnes. J Clin Microbiol. 1978 Aug;8(2):238–241. doi: 10.1128/jcm.8.2.238-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Harris R. C., Vaughan M. G., Dalton H. P. Two similar but atypical strains of coryneform group A-4 isolated from patients with endophthalmitis. J Clin Microbiol. 1985 Oct;22(4):475–477. doi: 10.1128/jcm.22.4.475-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle M. B., Lipsky B. A. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev. 1990 Jul;3(3):227–246. doi: 10.1128/cmr.3.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Briel D., Couderc F., Riegel P., Jehl F., Minck R. High-performance liquid chromatography of corynomycolic acids as a tool in identification of Corynebacterium species and related organisms. J Clin Microbiol. 1992 Jun;30(6):1407–1417. doi: 10.1128/jcm.30.6.1407-1417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. E., Mullangi C., Hokail A., Qadri S. M., Akhtar M. Acute natural valve endocarditis due to corynebacterium Group 1 in a normal competent host. Eur Heart J. 1991 Jul;12(7):842–843. [PubMed] [Google Scholar]
- Gavin S. E., Leonard R. B., Briselden A. M., Coyle M. B. Evaluation of the rapid CORYNE identification system for Corynebacterium species and other coryneforms. J Clin Microbiol. 1992 Jul;30(7):1692–1695. doi: 10.1128/jcm.30.7.1692-1695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Moore L. V., Kaneko B., Moore W. E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990 Jul;40(3):273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- Na'Was T. E., Hollis D. G., Moss C. W., Weaver R. E. Comparison of biochemical, morphologic, and chemical characteristics of Centers for Disease Control fermentative coryneform groups 1, 2, and A-4. J Clin Microbiol. 1987 Aug;25(8):1354–1358. doi: 10.1128/jcm.25.8.1354-1358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Graevenitz A., Osterhout G., Dick J. Grouping of some clinically relevant gram-positive rods by automated fatty acid analysis. Diagnostic implications. APMIS. 1991 Feb;99(2):147–154. doi: 10.1111/j.1699-0463.1991.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Zaki M. M. Relation between staphylococcal beta-lysin and different Corynebacteria. Vet Rec. 1965 Aug 7;77(32):941–941. doi: 10.1136/vr.77.32.941. [DOI] [PubMed] [Google Scholar]




