Abstract
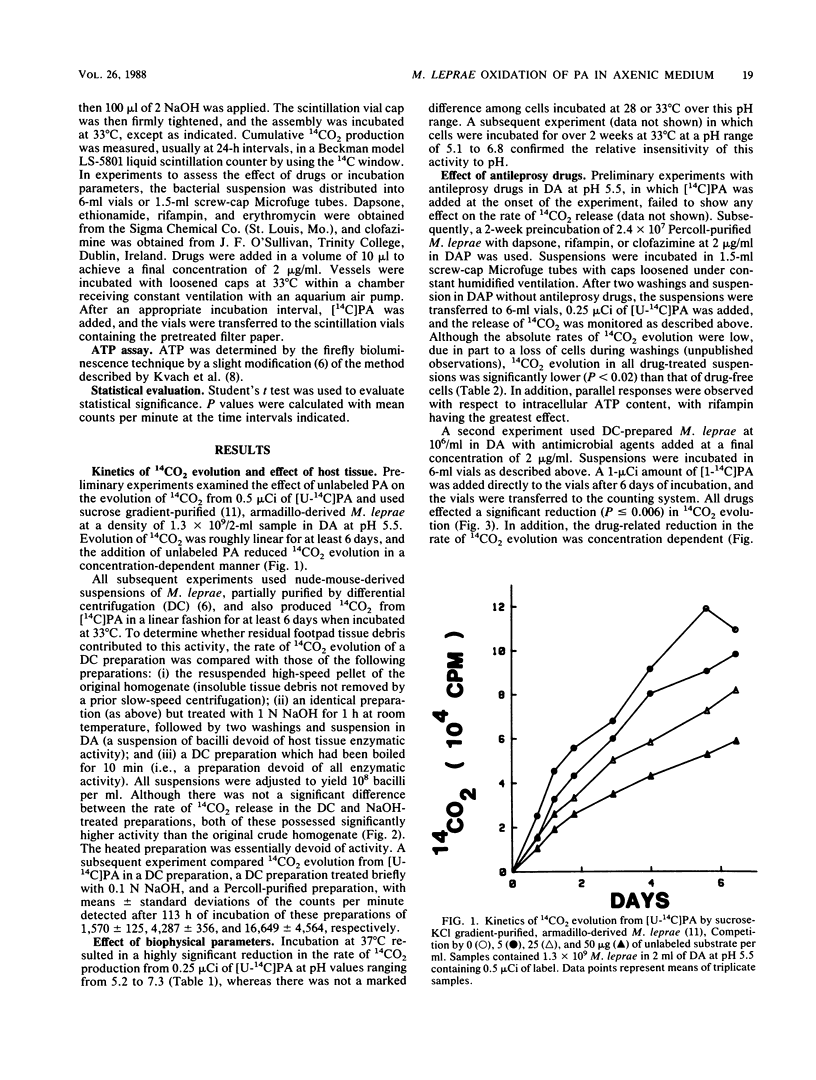
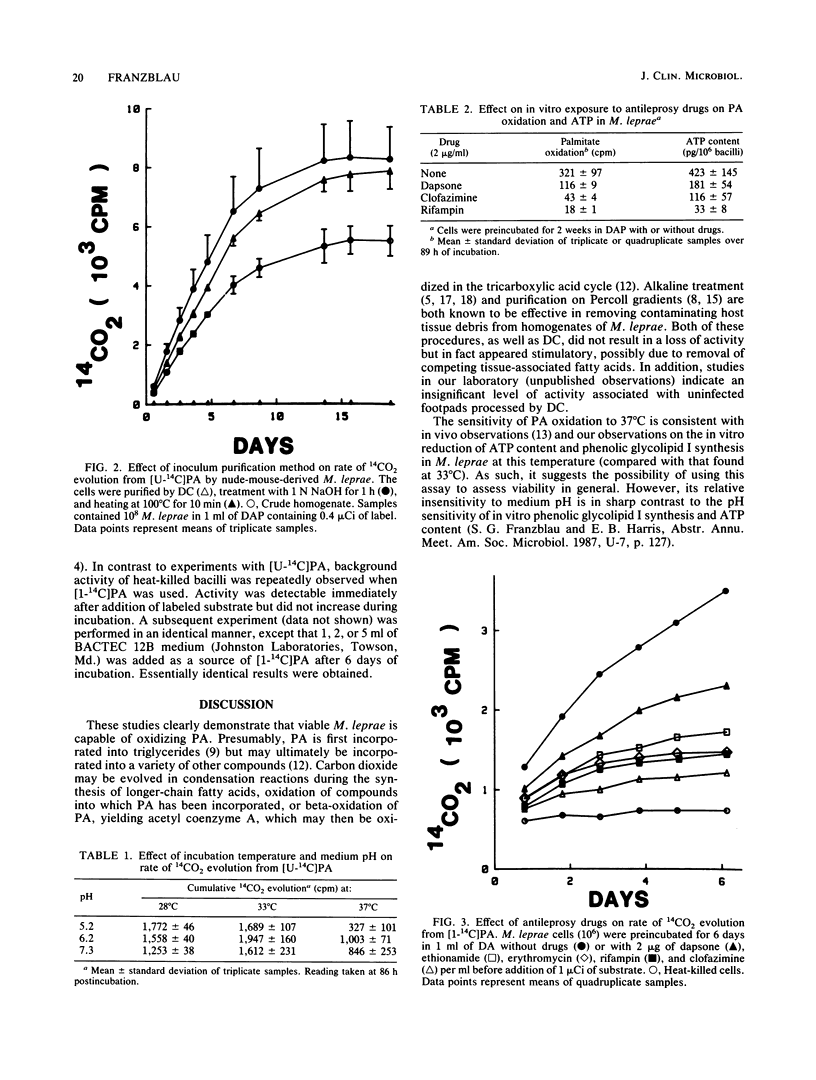
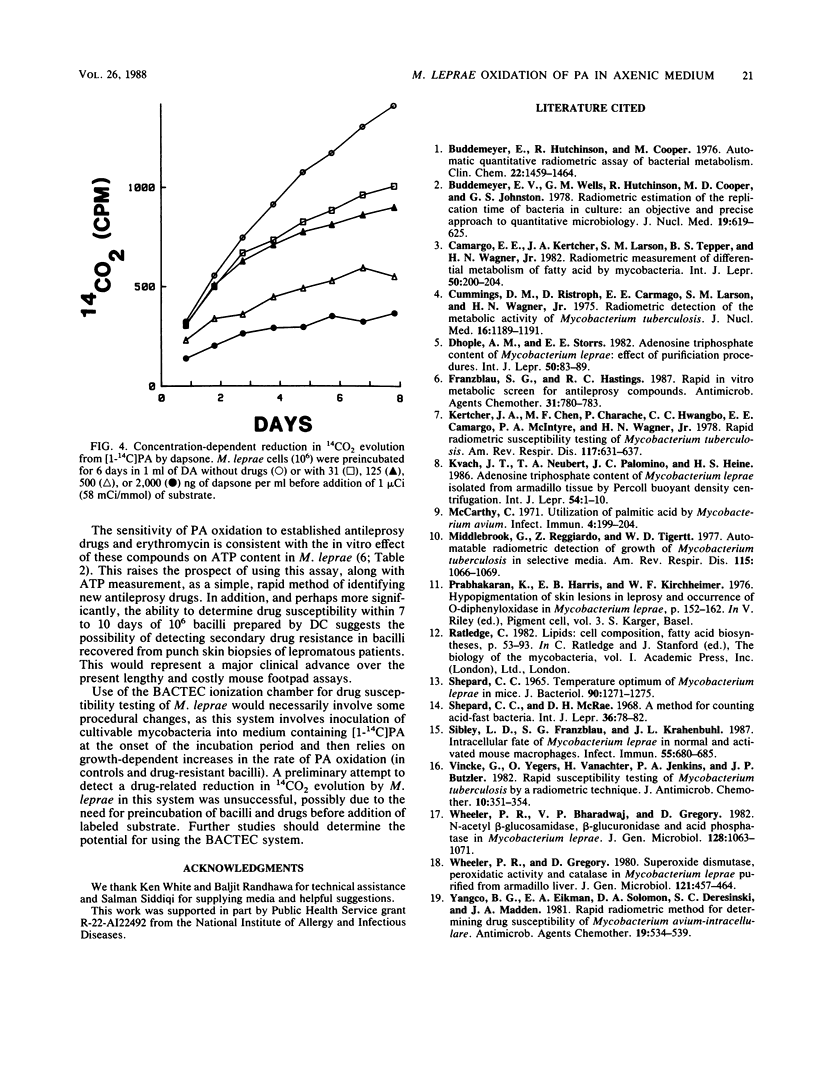
The ability of Mycobacterium leprae to oxidize palmitic acid during incubation in an axenic medium was studied. By using a Buddemeyer-type detection system, partially purified nude-mouse-derived M. leprae was found to produce 14CO2 from 14C-labeled palmitic acid in a linear fashion for at least 1 week. Procedures known to remove residual host tissue did not diminish the rate of 14CO2 evolution, indicating that bacterial metabolism was being measured. Palmitate oxidation was temperature sensitive, with an apparent optimum of 33 degrees C, but pH insensitive. Bacilli exposed to a variety of antileprosy drugs for 1 or 2 weeks displayed significantly reduced rates of 14CO2 evolution upon subsequent addition of 14C-labeled palmitic acid. This activity could be readily detected with 10(6) bacilli, thus indicating its potential for use in clinical susceptibility testing.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buddemeyer E. U., Wells G. M., Hutchinson R., Cooper M. D., Johnston G. S. Radiometric estimation of the replication time of bacteria in culture: an objective and precise approach to quantitative microbiology. J Nucl Med. 1978 Jun;19(6):619–625. [PubMed] [Google Scholar]
- Camargo E. E., Kertcher J. A., Larson S. M., Tepper B. S., Wagner H. N., Jr Radiometric measurement of differential metabolism of fatty acid by mycobacteria. Int J Lepr Other Mycobact Dis. 1982 Jun;50(2):200–204. [PubMed] [Google Scholar]
- Cummings D. M., Ristroph D., Camargo E. E., Larson S. M., Wagner H. N., Jr Radiometric detection of the metabolic activity of Mycobacterium tuberculosis. J Nucl Med. 1975 Dec;16(12):1189–1191. [PubMed] [Google Scholar]
- Dhople A. M., Storrs E. E. Adenosine triphosphate content of Mycobacterium leprae: effect of purification procedures. Int J Lepr Other Mycobact Dis. 1982 Mar;50(1):83–89. [PubMed] [Google Scholar]
- Franzblau S. G., Hastings R. C. Rapid in vitro metabolic screen for antileprosy compounds. Antimicrob Agents Chemother. 1987 May;31(5):780–783. doi: 10.1128/aac.31.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertcher J. A., Chen M. F., Charache P., Hwangbo C. C., Camargo E. E., McIntyre P. A., Wagner H. N., Jr Rapid radiometric susceptibility testing of Mycobacterium tuberculosis. Am Rev Respir Dis. 1978 Apr;117(4):631–637. doi: 10.1164/arrd.1978.117.4.631. [DOI] [PubMed] [Google Scholar]
- Kvach J. T., Neubert T. A., Palomino J. C., Heine H. S. Adenosine triphosphate content of Mycobacterium leprae isolated from armadillo tissue by Percoll buoyant density centrifugation. Int J Lepr Other Mycobact Dis. 1986 Mar;54(1):1–10. [PubMed] [Google Scholar]
- McCarthy C. Utilization of palmitic acid by Mycobacterium avium. Infect Immun. 1971 Sep;4(3):199–204. doi: 10.1128/iai.4.3.199-204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook G., Reggiardo Z., Tigertt W. D. Automatable radiometric detection of growth of Mycobacterium tuberculosis in selective media. Am Rev Respir Dis. 1977 Jun;115(6):1066–1069. doi: 10.1164/arrd.1977.115.6.1066. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C. Temperature optimum of Mycobacterium leprae in mice. J Bacteriol. 1965 Nov;90(5):1271–1275. doi: 10.1128/jb.90.5.1271-1275.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Franzblau S. G., Krahenbuhl J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987 Mar;55(3):680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincké G., Yegers O., Vanachter H., Jenkins P. A., Butzler J. P. Rapid susceptibility testing of Mycobacterium tuberculosis by a radiometric technique. J Antimicrob Chemother. 1982 Oct;10(4):351–354. doi: 10.1093/jac/10.4.351. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R., Bharadwaj V. P., Gregory D. N-acetyl-beta-glucosaminidase, beta-glucuronidase and acid phosphatase in Mycobacterium leprae. J Gen Microbiol. 1982 May;128(5):1063–1071. doi: 10.1099/00221287-128-5-1063. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Yangco B. G., Eikman E. A., Solomon D. A., Deresinski S. C., Madden J. A. Rapid radiometric method for determining drug susceptibility of Mycobacterium avium-intracellulare. Antimicrob Agents Chemother. 1981 Apr;19(4):534–539. doi: 10.1128/aac.19.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]


