Abstract
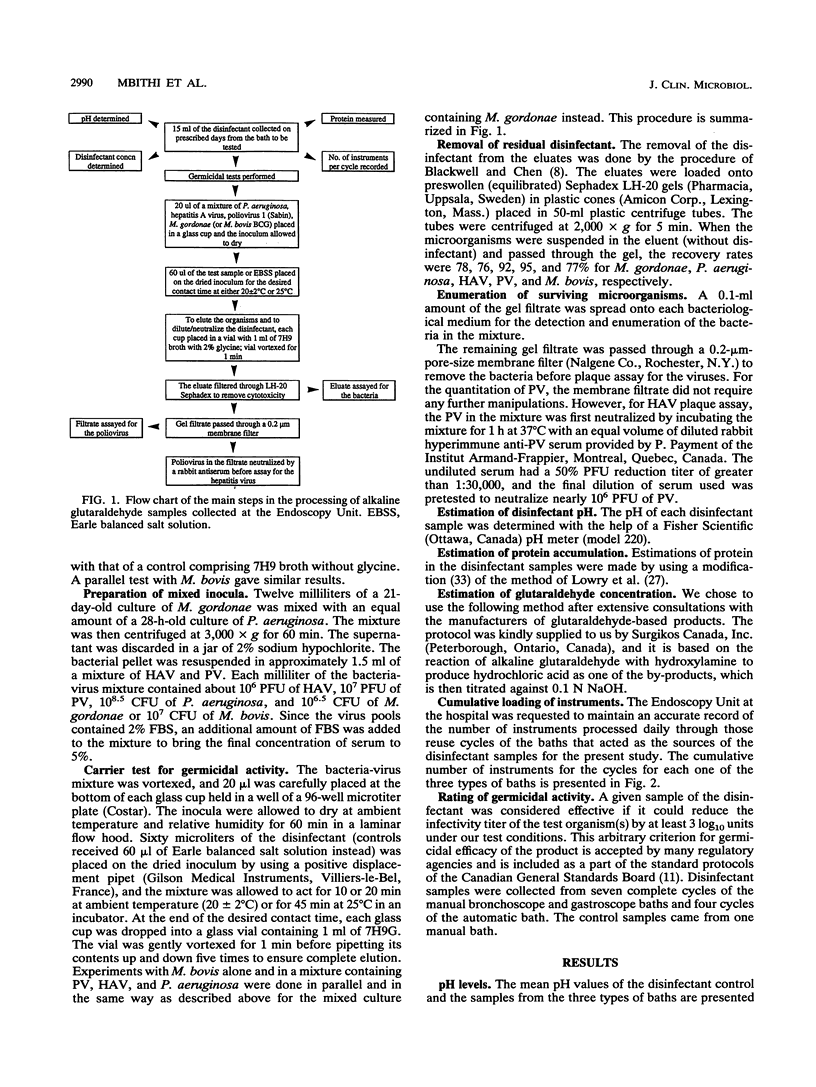
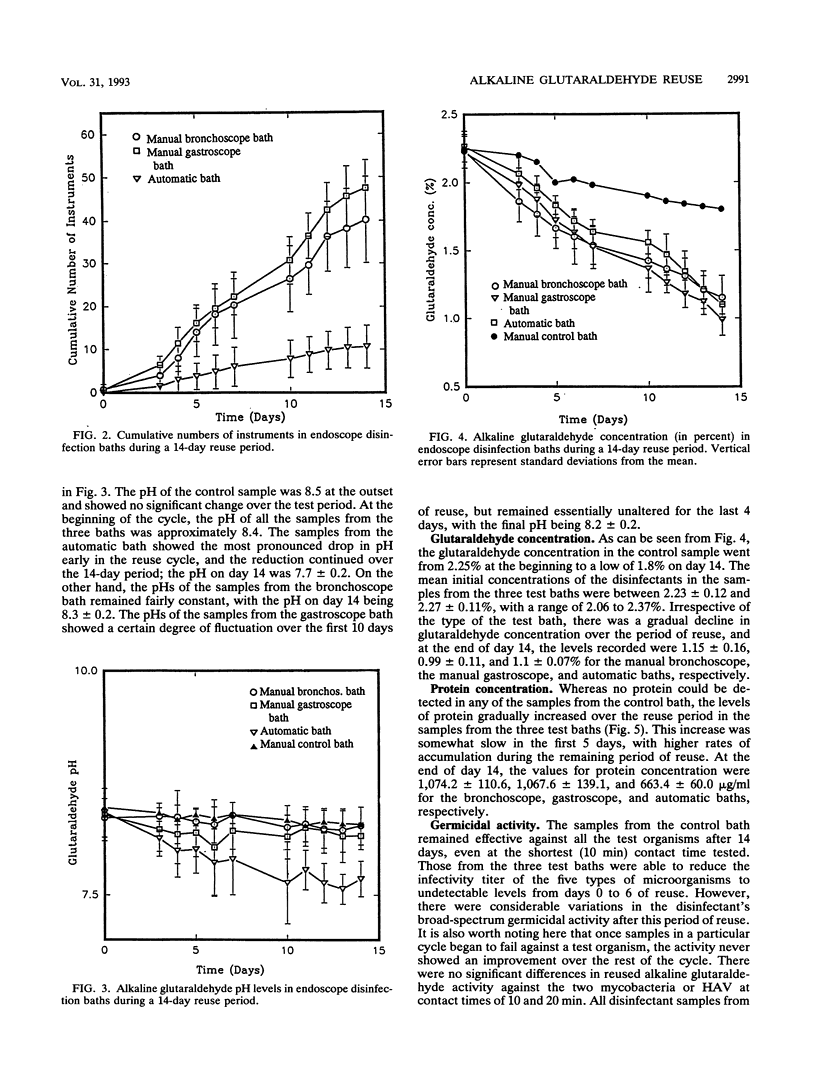
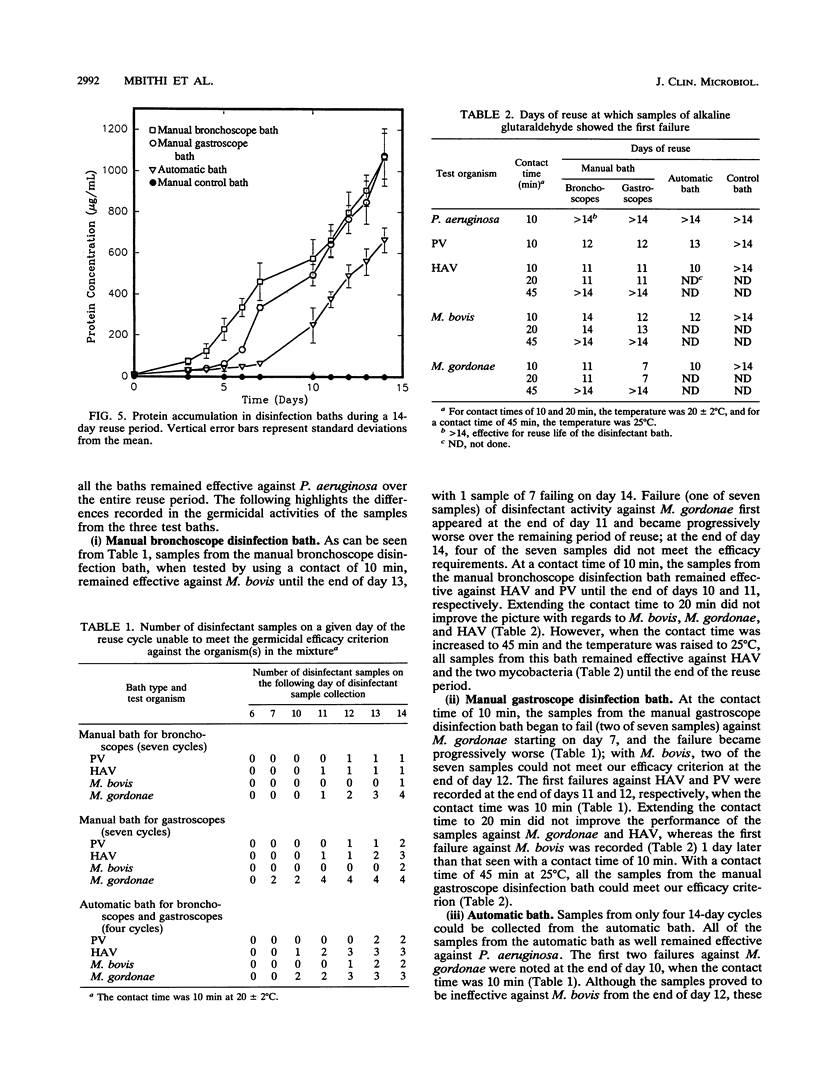
Baths with 2% alkaline glutaraldehyde are often reused for 14 days to decontaminate flexible fiberoptic endoscopes (FFEs) between patients, but the effect of such reuse on the disinfectant's activity has not been known. Many busy endoscopy units also disinfect FFEs with contact times shorter than those recommended by the disinfectant manufacturer. We therefore collected samples of the disinfectant over the 14-day reuse period from two manual and one automatic bath used for bronchoscopes and gastroscopes at a local hospital. Control samples were also collected from a manual bath of 2% alkaline glutaraldehyde which did not receive any endoscopes. The germicidal activities of the samples were assessed in a carrier test against a mixture of hepatitis A virus, poliovirus 1 (Sabin), and Pseudomonas aeruginosa; the mixture also contained either Mycobacterium bovis or Mycobacterium gordonae. Bovine serum (5%) was the organic load. The criterion of efficacy was a minimum of a 3-log10-unit reduction in the infectivity titers of the organisms tested. The initial disinfectant concentration in all the baths was nearly 2.25%; it became about 1.8% in the control bath and fell to approximately 1% in the three test baths after 14 days. No protein was detected in the control bath, while its concentration rose gradually in the test baths to a maximum of 1,267 micrograms/ml after 14 days. With a contact time of 10 min at 20 +/- 2 degrees C, all the samples from the control bath were effective against all the test organisms and all the samples from all the test baths were also effective against P. aeruginosa. With a contact time of 10 or 20 min at 20+/-2 degrees C, the virucidal and mycobactericidal activities of the samples from the test baths showed broad-spectrum germicidal activity when the contact time was increased to 45 min and the temperature was raised to 25 degrees C. These findings emphasize the care needed in the disinfection of FFEs, especially in view of the increasing threat of AIDS and the resurgence of tuberculosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. I., Allen M. O., Olson M. M., Gerding D. N., Shanholtzer C. J., Meier P. B., Vennes J. A., Silvis S. E. Pseudomonas infection of the biliary system resulting from use of a contaminated endoscope. Gastroenterology. 1987 Mar;92(3):759–763. doi: 10.1016/0016-5085(87)90029-1. [DOI] [PubMed] [Google Scholar]
- Anderson P. J. Purification and quantitation of glutaraldehyde and its effect on several enzyme activities in skeletal muscle. J Histochem Cytochem. 1967 Aug;15(11):652–661. doi: 10.1177/15.11.652. [DOI] [PubMed] [Google Scholar]
- Axon A. T. Disinfection of endoscopic equipment. Baillieres Clin Gastroenterol. 1991 Mar;5(1):61–77. doi: 10.1016/0950-3528(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Bageant R. A., Marsik F. J., Kellogg V. A., Hyler D. L., Gröschel D. H. In-use testing of four glutaraldehyde disinfectants in the Cidematic washer. Respir Care. 1981 Dec;26(12):1255–1261. [PubMed] [Google Scholar]
- Bailly J. L., Chambon M., Peigue-Lafeuille H., Laveran H., De Champs C., Beytout D. Activity of glutaraldehyde at low concentrations (less than 2%) against poliovirus and its relevance to gastrointestinal endoscope disinfection procedures. Appl Environ Microbiol. 1991 Apr;57(4):1156–1160. doi: 10.1128/aem.57.4.1156-1160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Carr-Locke D. L., Clayton P. Disinfection of upper gastrointestinal fibreoptic endoscopy equipment: an evaluation of a cetrimide chlorhexidine solution and glutaraldehyde. Gut. 1978 Oct;19(10):916–922. doi: 10.1136/gut.19.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E. C., Rutala W. A., Nessen L., Wannamaker N. S., Weber D. J. Effect of methodology, dilution, and exposure time on the tuberculocidal activity of glutaraldehyde-based disinfectants. Appl Environ Microbiol. 1990 Jun;56(6):1813–1817. doi: 10.1128/aem.56.6.1813-1817.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon P. J., Graham E. Cleaning and disinfection of endoscopes: have there been recent improvements? Med J Aust. 1991 Mar 18;154(6):391-2, 394. doi: 10.5694/j.1326-5377.1991.tb121129.x. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Bactericidal activity of alkaline glutaraldehyde solution against a number of atypical mycobacterial species. J Appl Bacteriol. 1986 Sep;61(3):247–251. doi: 10.1111/j.1365-2672.1986.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Gorman S. P., Scott E. M., Russell A. D. Antimicrobial activity, uses and mechanism of action of glutaraldehyde. J Appl Bacteriol. 1980 Apr;48(2):161–190. doi: 10.1111/j.1365-2672.1980.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Messner R. L. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Infect Control Hosp Epidemiol. 1991 May;12(5):289–296. doi: 10.1086/646341. [DOI] [PubMed] [Google Scholar]
- Gélinas P., Goulet J. Neutralization of the activity of eight disinfectants by organic matter. J Appl Bacteriol. 1983 Apr;54(2):243–247. doi: 10.1111/j.1365-2672.1983.tb02613.x. [DOI] [PubMed] [Google Scholar]
- Hajdu J., Friedrich P. Reaction of glutaraldehyde with NH2 compounds. A spectrophotometric method for the determination of glutaraldehyde concentration. Anal Biochem. 1975 May 12;65(1-2):273–280. doi: 10.1016/0003-2697(75)90510-2. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Gor D., Clarke J. R., Chadwick M. V., Nicholson G., Shah N., Gazzard B., Jeffries D. J., Gaya H., Collins J. V. Contamination of endoscopes used in AIDS patients. Lancet. 1989 Jul 8;2(8654):86–88. doi: 10.1016/s0140-6736(89)90323-1. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., Giugliano E. R., France K., Alperstein P. Evaluation of three disinfectants after in-use stress. J Hosp Infect. 1988 Apr;11(3):278–285. doi: 10.1016/0195-6701(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Jarikre L. N. Case report: disseminated Mycobacterium gordonae infection in a nonimmunocompromised host. Am J Med Sci. 1991 Dec;302(6):382–384. doi: 10.1097/00000441-199112000-00012. [DOI] [PubMed] [Google Scholar]
- Kaczmarek R. G., Moore R. M., Jr, McCrohan J., Goldmann D. A., Reynolds C., Caquelin C., Israel E. Multi-state investigation of the actual disinfection/sterilization of endoscopes in health care facilities. Am J Med. 1992 Mar;92(3):257–261. doi: 10.1016/0002-9343(92)90074-l. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall B. C., Carroll K. C. Interaction between Pseudomonas aeruginosa and host defenses in cystic fibrosis. Semin Respir Infect. 1991 Mar;6(1):11–18. [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Boulet J. R., Sattar S. A. Survival of hepatitis A virus on human hands and its transfer on contact with animate and inanimate surfaces. J Clin Microbiol. 1992 Apr;30(4):757–763. doi: 10.1128/jcm.30.4.757-763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Sattar S. A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1990 Nov;56(11):3601–3604. doi: 10.1128/aem.56.11.3601-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Sattar S. A. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1991 May;57(5):1394–1399. doi: 10.1128/aem.57.5.1394-1399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E. J., Morice R. Miliary pulmonary infection caused by Mycobacterium terrae in an autologous bone marrow transplant patient. Chest. 1991 Nov;100(5):1449–1450. doi: 10.1378/chest.100.5.1449. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Power E. G., Russell A. D. Assessment of 'cold Sterilog glutaraldehyde monitor'. J Hosp Infect. 1988 May;11(4):376–380. doi: 10.1016/0195-6701(88)90092-8. [DOI] [PubMed] [Google Scholar]
- Robison R. A., Bodily H. L., Robinson D. F., Christensen R. P. A suspension method to determine reuse life of chemical disinfectants during clinical use. Appl Environ Microbiol. 1988 Jan;54(1):158–164. doi: 10.1128/aem.54.1.158-164.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum L. S., Villarino M. E., Nainan O. V., Melish M. E., Hadler S. C., Pinsky P. P., Jarvis W. R., Ott C. E., Margolis H. S. Hepatitis A outbreak in a neonatal intensive care unit: risk factors for transmission and evidence of prolonged viral excretion among preterm infants. J Infect Dis. 1991 Sep;164(3):476–482. doi: 10.1093/infdis/164.3.476. [DOI] [PubMed] [Google Scholar]
- Rutala W. A. APIC guideline for selection and use of disinfectants. Am J Infect Control. 1990 Apr;18(2):99–117. doi: 10.1016/0196-6553(90)90089-b. [DOI] [PubMed] [Google Scholar]
- Rutala W. A., Clontz E. P., Weber D. J., Hoffmann K. K. Disinfection practices for endoscopes and other semicritical items. Infect Control Hosp Epidemiol. 1991 May;12(5):282–288. doi: 10.1086/646340. [DOI] [PubMed] [Google Scholar]
- Seto W. H., Ching T. Y., Yuen K. Y., Chu Y. B., Seto W. L. The enhancement of infection control in-service education by ward opinion leaders. Am J Infect Control. 1991 Apr;19(2):86–91. doi: 10.1016/0196-6553(91)90044-d. [DOI] [PubMed] [Google Scholar]
- Spach D. H., Silverstein F. E., Stamm W. E. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993 Jan 15;118(2):117–128. doi: 10.7326/0003-4819-118-2-199301150-00008. [DOI] [PubMed] [Google Scholar]
- Tandon R. K. Endoscopic disinfection: practices and recommendations. J Gastroenterol Hepatol. 1991 Jan-Feb;6(1):37–39. doi: 10.1111/j.1440-1746.1991.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Tyler R., Ayliffe G. A., Bradley C. Virucidal activity of disinfectants: studies with the poliovirus. J Hosp Infect. 1990 May;15(4):339–345. doi: 10.1016/0195-6701(90)90090-b. [DOI] [PubMed] [Google Scholar]
- Van Gossum A., Loriers M., Serruys E., Cremer M. Methods of disinfecting endoscopic material: results of an international survey. Endoscopy. 1989 Nov;21(6):247–250. doi: 10.1055/s-2007-1012962. [DOI] [PubMed] [Google Scholar]
- Whyman C. A., McDonald S. A., Zoutman D. Unsuspected dilution of glutaraldehyde in an automatic washer for flexible fibreoptic endoscopes. Can J Infect Control. 1991 Winter;6(4):91–93. [PubMed] [Google Scholar]
- Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin Infect Dis. 1992 Jul;15(1):1–10. doi: 10.1093/clinids/15.1.1. [DOI] [PubMed] [Google Scholar]


