Abstract
The human papillomavirus (HPV) E7 oncoprotein shares functional similarities with such proteins as adenovirus E1A and SV40 large tumor antigen. As one of only two viral proteins always expressed in HPV-associated cancers, E7 plays a central role in both the viral life cycle and carcinogenic transformation. In the HPV viral life cycle, E7 disrupts the intimate association between cellular differentiation and proliferation in normal epithelium, allowing for viral replication in cells that would no longer be in the dividing population. This function is directly reflected in the transforming activities of E7, including tumor initiation and induction of genomic instability.
Keywords: Human papillomavirus, E7 oncoprotein, cellular transformation, retinoblastoma tumor suppressor, p53, genomic instability, centrosome duplication, ubiquitin ligase, cell death, cellular metabolism
Introduction
Human papillomaviruses (HPVs) are a DNA virus family of approximately 200 types that display a marked tropism for squamous epithelium. Despite a similar genomic make-up, different HPVs infect epithelia at distinct anatomic locations. Approximately 30 HPV types infect the anogenital and oral mucosa and can be further classified as “low-risk” and “high-risk” based on the clinical prognosis of their associated lesions. Low-risk HPVs cause benign epithelial hyperplasias (warts), while high-risk HPVs cause lesions that have a propensity for malignant progression. Virtually all cervical carcinoma cases are associated with high-risk HPV infection, and two viral proteins, E6 and E7, which are consistently expressed in the tumors, are required for both the induction and maintenance of the transformed phenotype (Munger et al., 2004).
Cellular transformation activities of HPV E7 proteins
In the mid 1980s it was recognized that high-risk HPV genomes encode cellular transformation activities when assayed in rodent fibroblast lines such as NIH3T3 cells (Yasumoto et al., 1986) or in the classical ras oncogene cooperation assay in baby rat kidney cells (Matlashewski et al., 1987). Subsequent mutational analyses revealed that the E7 protein scored as the major transforming activity of high-risk HPVs in these assay systems (Bedell et al., 1989; Kanda et al., 1988; Phelps et al., 1988; Tanaka et al., 1989; Vousden et al., 1988; Watanabe et al., 1988; Yutsudo et al., 1988).
Later studies revealed that expression of cloned high-risk HPV genomes in the natural host cell type of HPVs, primary human genital epithelial cells, causes life span extension and cellular immortalization and inhibits keratinocyte differentiation and facilitates immortalization (Schlegel et al., 1988; Woodworth et al., 1988; Woodworth et al., 1989). High-risk HPV genome expressing keratinocytes grown in organotypic raft cultures display cellular alterations and abnormalities in tissue architecture that resemble high-grade HPV associated clinical lesions (McCance et al., 1988; Woodworth et al., 1990). The resulting cell lines were non-tumorigenic in nude mice but tumorigenic clones were obtained when cultured over extended periods of time or when additional oncogenes such the ras or fos oncogenes were co-expressed (DiPaolo et al., 1989; Durst et al., 1989; Hurlin et al., 1991; Pei et al., 1993). Mutational analyses revealed that the E7 protein in cooperation with E6 is necessary for these transforming activities in human epithelial cells (Bedell et al., 1989; Hawley-Nelson et al., 1989; Hudson et al., 1990; Munger et al., 1989a). The transforming activities of HPV E7 proteins correlate with the low-risk/high-risk classification, as low-risk HPV E7 proteins, such as HPV6 and 11 E7, have greatly decreased transforming and immortalizing activities as compared to high-risk HPV E7 proteins (Barbosa et al., 1991; Halbert et al., 1992).
Biochemical characterization of E7 proteins
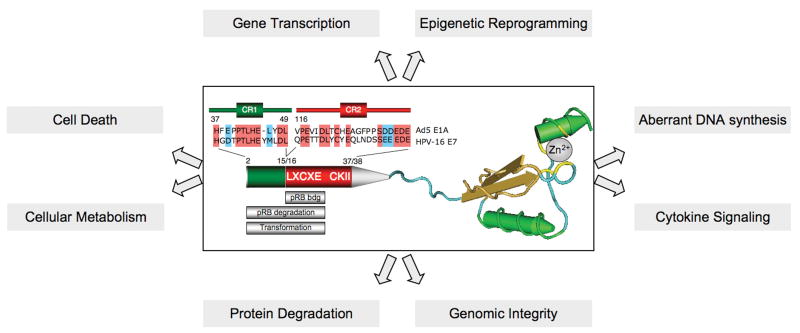
The E7 proteins are small, acidic polypeptides composed of approximately 100 amino acids (Figure 1). The amino terminus of E7 contains a region of sequence similarity to a portion of conserved region (CR) 1 and the entire CR2 of adenovirus (Ad) E1A and related sequences in simian vacuolating virus 40 (SV40) large tumor antigen (T Ag) (Figge et al., 1988; Phelps et al., 1988; Vousden et al., 1989). As with SV40 T Ag and Ad E1A, these two conserved regions significantly contribute to the transforming activities of high-risk HPV E7 oncoproteins (Edmonds et al., 1989; Jewers et al., 1992; Phelps et al., 1992; Storey et al., 1990; Watanabe et al., 1990). A conserved Leu-X-Cys-X-Glu (LXCXE) motif in the CR2 homology domain is necessary and sufficient for the association of E7 protein with the retinoblastoma tumor suppressor protein, pRB (Munger et al., 1989b). Some studies claim that the C-terminal E7 domain contains an independent, low-affinity pRB binding site (Liu et al., 2006; Patrick et al., 1994), but HPV16 E7 mutants with a deletion of the pRB core binding site in CR2 fail to associate with pRB family members as determined by Western blotting and extensive proteomic analyses of associated cellular protein complexes (K.W. Huh, M. Grace, C. L. Nguyen and K. Munger, unpublished data). Adjacent to the LXCXE motif is a consensus casein kinase II (CK II) phosphorylation site (Barbosa et al., 1990; Firzlaff et al., 1989). The carboxyl terminus is phosphorylated by an as yet unidentified kinase (Massimi et al., 2000) and importantly contributes the transforming activities of E7 (Helt et al., 2002; Helt et al., 2001). The E7 carboxyl terminus contains a zinc-binding domain that is composed of two Cys-X-X-Cys motifs (Barbosa et al., 1989) and functions as a dimerization domain (Clemens et al., 1995; Liu et al., 2006; McIntyre et al., 1993; Ohlenschlager et al., 2006), although there is no compelling evidence that E7 exists as a dimer in vivo and/or that dimerization is necessary for the biological activities of E7. Interestingly, HPV E6 proteins consist of two tandem copies of a Cys-X-X-Cys motif that shares sequence similarity to the E7 carboxyl terminus, indicating that E6 and E7 may have a common ancestral precursor (Cole et al., 1987). The 3-dimensional structure of HPV E7 proteins has been solved by NMR (Liu et al., 2006) and X-ray crystallography (Ohlenschlager et al., 2006). These studies have revealed that the amino terminal domain is unfolded whereas the C-terminal domain forms a unique, tightly packed zinc-binding fold (Liu et al., 2006; Ohlenschlager et al., 2006) (Figure 1).
Figure 1. Schematic representation of the HPV E7 oncoprotein and affected cellular processes.
The unstructured amino terminal 37 amino acid residues of HPV16 E7 have sequence similarity to a portion of CR1 (green) and the entire CR2 (red) of Ad E1A, with specific identical and chemically similar amino acid residues indicated by red and blue boxes, respectively. CR1 sequences are required for cellular transformation and pRB degradation but do not directly contribute to pRB binding. The core pRB binding site (LXCXE), also required for transformation, is located within CR2, adjacent to a casein kinase II consensus phosphorylation site (CKII). The carboxyl terminal E7 domain shown here is from HPV45 E7 as determined by X-ray crystallography (Ohlenschlager et al., 2006). This portion of the E7 sequence has a compact β1β2αIβ3α2 topology that represents a unique zinc-binding fold. The cysteine residues involved in zinc binding are indicated in yellow. They are arranged as two Cys-X-X-Cys motifs separated by 29 amino acid residues. Cellular processes affected by E7 and discussed in this chapter are indicated in the grey boxes. See text for details and references.
HPV16 E7 migrates on SDS polyacrylamide gels with an apparent molecular size of 18 to 20 kDa, rather than according to its predicted molecular weight of ∼11 kDa (Smotkin et al., 1986; Smotkin et al., 1987); this aberrant migration is mediated by the amino terminal CR1 homology domain (Heck et al., 1992; Münger et al., 1991) and may be caused by the high content of acidic residues (Armstrong et al., 1993). HPV16 E7 is located largely in the cytoplasm (Huh et al., 2005; Nguyen et al., 2007; Ressler et al., 2007; Smotkin et al., 1987) but also exists in nuclear pools (Greenfield et al., 1991; Sato et al., 1989; Smith-McCune et al., 1999). Although HPV16 E7 does not have a prototypical nuclear targeting sequence, it is actively transported into the nucleus through a novel Ran-dependent that does not involve Kap β import receptors (Angeline et al., 2003). More recent studies have shown that E7 also contains a nuclear export sequence and can shuttle between the cytoplasm and nucleus (Knapp et al., 2008). In addition to nuclear and cytoplasmic pools of E7, nucleolar HPV16 E7 localization has also been reported (Zatsepina et al., 1997), but in most immunofluorescence studies nuclear HPV16 E7 staining seems to specifically exclude nucleolar structures. The half-life of HPV16 E7 is less than 2 hours (Smotkin et al., 1987). E7 is degraded via a ubiquitin-mediated proteasomal mechanism that involves conjugation of ubiquitin to the amino terminus of E7 (Reinstein et al., 2000). A cullin 1 containing ubiquitin ligase complex has been implicated in HPV16 E7 degradation (Oh et al., 2004a) although association of HPV16 E7 with cullin 1 was not detected in other studies (Huh et al., 2007).
Glycerol gradient centrifugation experiments demonstrated that HPV16 E7 is a component of higher molecular weight complexes, indicating that it may associate with cellular protein complexes (Smotkin et al., 1987). E7 lacks intrinsic enzymatic activities and specific DNA binding activities and it is now widely accepted that its biological activities are linked to its ability to associate with and subvert the normal activities of cellular regulatory complexes (Figure 1).
HPV E7 and the viral life cycle
During carcinogenic progression the HPV genome frequently integrates into a host cell chromosome and, as a result, the viral oncoproteins, E6 and E7, are the only viral proteins that are consistently expressed in HPV positive cervical carcinomas. Viral genome integration is a terminal event and is thus not a manifestation of the normal viral life cycle. Hence, the oncogenic activities of high-risk HPV E7 proteins reflect their functions during the viral life cycle. The HPV life cycle is intimately associated with the differentiation process of the infected epithelial cell. The skin is the largest organ of the human body and is subject to continuous turnover. It contains a single layer of dividing cells, the basal cells. Basal cells undergo asymmetric mitosis; one daughter cell remains an undifferentiated, proliferating basal cell whereas the other daughter becomes a differentiating suprabasal cell. The suprabasal daughter cell withdraws from the cell division cycle and undergoes a program of terminal differentiation. This ensures the mechanical stability of the skin and provides protection for the dividing basal cells from direct exposure to environmental mutagens. In order to establish a persistent infection, papillomaviruses need to infect basal cells where the viral genomes are maintained at a low copy number. The production of infectious progeny virus, however, exclusively occurs in the terminally differentiated layers of the epithelium (reviewed in Lee et al., 2007).
With the exception of the E1/E2 complex, papillomaviruses lack essential enzymes for viral genome replication; the host cell's DNA synthesis machinery must be utilized for this purpose (reviewed in Stubenrauch et al., 1999). In normal epithelia, cellular DNA synthesis no longer occurs in differentiated epithelial cells, making them inherently incapable of supporting HPV replication. Consequently, papillomaviruses need to uncouple the processes of cellular differentiation and proliferation. The strategies that some HPVs have developed to achieve this contribute to their transforming activities. Infected cells leaving the basal layer remain competent to support DNA synthesis largely due to the actions of the E7 protein (Cheng et al., 1995). There appear to be HPV type-specific differences with regard to the specific roles that E7 proteins play in the viral life cycle, as HPV11 and 31 E7 are where shown to be required for episomal maintenance of the viral genome (Oh et al., 2004b; Thomas et al., 1999), whereas HPV16 and 18 E7 are necessary for the productive stage of the viral life cycle (Flores et al., 2000; McLaughlin-Drubin et al., 2005). While many of the biological activities of E7 that are necessary for the viral life cycle remain to be elucidated, it has been shown that the ability of HPV16 E7 to allow suprabasal cells to support DNA synthesis is linked to E7's ability to bind pRB family members but not with its ability to induce their degradation (Collins et al., 2005). On the other hand, the capacity of HPV16 E7 to perturb differentiation correlates with both E7's binding to and degradation of pRB family members, suggesting that different key stages of the productive stage of the HPV16 life cycle rely on different functions of E7 (Collins et al., 2005).
Activation of E2F-dependent transcription through degradation of the retinoblastoma tumor suppressor protein, pRB
HPV E7 proteins associate with pRB and the related pocket proteins, p107 and p130, via the LXCXE motif in CR2 (Dyson et al., 1992; Munger et al., 1989b). The best-studied function of the pocket proteins is the ability to regulate G1/S entry and progression by modulating the transcriptional activities of E2F transcription factors (reviewed in Frolov et al., 2004). E2F transcription factors are heterodimers that contain an E2F (E2F1-8) and a DP (DP-1, DP-2) subunit and are critical regulators of G1 exit and S-phase progression. In addition, a number of other cellular processes, including cellular differentiation apoptosis, and genomic instability, are controlled by E2Fs (reviewed in Dyson, 1998). The G1 specific pRB/E2F complex acts a transcriptional repressor. In normal cells, disruption of the pRB/E2F repressor complex is triggered by cdk4/6 and cdk2 mediated pRB phosphorylation in late G1; the dissociated E2F acts as a transcriptional activator of genes necessary for S-phase entry and progression. Like SV40 T Ag, high-risk HPV E7 preferentially associates with G1-specific, E2F-bound pRB (Dyson et al., 1992). This causes disruption of pRB/E2F repressor complexes and, thus, uncontrolled G1 exit and S-phase entry. In addition to the LXCXE motif in CR2, carboxyl terminal E7 sequences are necessary for disruption of pRB/E2F complexes (Huang et al., 1993; Wu et al., 1993).
However, unlike SV40 T Ag and Ad E1A, which inactivate pRB/E2F complexes by stoichiometric pRB association, high-risk HPV E7 proteins destabilize pRB through proteasomal degradation (Boyer et al., 1996; Jones et al., 1997b) via a mechanism that involves association with and reprogramming of the cullin 2 ubiquitin ligase complex by HPV16 E7 (Huh et al., 2007). The induction of the aberrant pRB degradation by high-risk HPV E7 proteins and the resulting activation of E2F-mediated transcription represent important mechanisms by which these viruses achieve and maintain S-phase competence in differentiated epithelial cells. In addition to the core pRB binding site in CR2, sequences in the CR1 homology domain are necessary for pRB degradation and mutational studies suggest that the ability of high-risk HPV E7 proteins to destabilize pRB family members is necessary for cellular transformation (Jones et al., 1997b). Low-risk HPV E7 proteins bind pRB with lower efficiency (approximately 10-fold lower) than the high-risk HPV E7 proteins (Gage et al., 1990; Munger et al., 1989b). This difference in pRB binding efficiency maps to a single amino acid (Asp 21 in HPV16 E7 versus Gly 22 in HPV6 E7) and substitution experiments indicated that this single amino acid residue was the primary determinant for pRB-binding affinity and transformation capacity of the mucosal HPV E7 proteins (Heck et al., 1992; Sang et al., 1992). Interestingly, for cutaneous HPV E7 proteins, the presence of an aspartate residue and efficient pRB binding is not associated with a high-risk clinical classification in vivo and does not predict transforming potential in vitro (Ciccolini et al., 1994).
HPV16 E7 can directly bind E2F1 and enhance E2F1-mediated transcription (Hwang et al., 2002). E2F1 plays a role in mediating the transcriptional control of the E2F6 gene, which is upregulated at the G1/S-phase transition to exert an opposing effect on the activities of E2F-responsive promoters, thereby directing appropriate cell cycle exit and differentiation (Lyons et al., 2006). Interestingly, HPV E7 associates with E2F6 and abrogates its ability to function as a transcriptional repressor (McLaughlin-Drubin et al., 2008), suggesting that the functional deregulation of E2F6 by HPV E7 is needed to counterbalance the up-regulation of E2F6 as a consequence of the activation of E2F1 by E7, thus ensuring that the cells remain in an S-phase-competent state that is necessary for the viral life cycle. Moreover, given E2F6′s possible role in maintaining quiescence (Ogawa et al., 2002), it is conceivable that the deregulation of E2F6 by HPV E7 aids in allowing HPVs to bypass negative growth signals, thus allowing cells to exit from G0.
Other mechanisms of G1/S cell checkpoint dysregulation
In addition to targeting pRB for proteasomal degradation, high-risk HPV E7 proteins also contribute to cell cycle dysregulation through several additional mechanisms.
Cyclin dependent kinases (cdks) are the motors that drive the cell division cycle. Multiple mechanisms, most importantly association with positive regulatory subunits, cyclins, and negative regulators, cyclin-dependent kinase inhibitors (CKIs), regulate their activity. Expression of cyclins E and A, the regulatory subunits of cdk2, which drive S-phase entry and progression, is under E2F control and they are both expressed at higher levels in E7 expressing cells (Zerfass et al., 1995).
HPV16 E7 has been shown to interact with and abrogate the growth-inhibitory activities of the CKIs p21CIP1 (Funk et al., 1997; Jones et al., 1997a) and p27KIP1 (Zerfass-Thome et al., 1996), which are induced by anti-proliferative signals, including growth factor withdrawal (Firpo et al., 1994), activation of p53 (el-Deiry et al., 1993), and loss of cellular adhesion (Assoian, 1997; Fang et al., 1996). Interestingly, p21CIP1 has been implicated in coupling cell cycle arrest and differentiation in keratinocytes (Alani et al., 1998; Di Cunto et al., 1998; Missero et al., 1996). The steady state levels of p21CIP1 increase during differentiation, subsequently inhibiting cdk2 activity and inducing a G1 growth arrest; interestingly, p21CIP1 levels are increased further through a non-transcriptional mechanism in the presence of HPV E7. Although HPV16 E7 expression increases p21CIP1 levels through protein stabilization (Jian et al., 1998; Jones et al., 1999; Noya et al., 2001), cdk2 remains active in HPV E7-expressing cells (Funk et al., 1997; Jones et al., 1997a; Ruesch et al., 1997). The ability of HPV E7 to abrogate CKIs, together with its ability to disrupt pRB/E2F complexes, which results in increased cyclin E and cyclin A levels, retains differentiating keratinocytes in a DNA synthesis competent state. HPV16 E7 can also directly associate with cdk2/cyclin A and cyclin E complexes (He et al., 2003; Nguyen et al., 2008b; Tommasino et al., 1993) resulting in increased cdk2 activity as measured on a pRB-derived substrate (He et al., 2003).
HPV16 E7 and epigenetic reprogramming
In addition to a DP1/2 containing repressive complex, E2F6 is also a component of polycomb group (PcG) transcription factor complexes. Mammalian PcG complexes act as transcriptional repressors, some of which contain histone methyltransferases (Attwooll et al., 2005; Ogawa et al., 2002; Trimarchi et al., 2001). Given that in addition to DP1 and DP2 we also identified the polycomb group proteins Ring1, Bmi1, Mel18, hpc2, and L3MBTL2 as HPV16 E7 associated proteins by tandem affinity purification (McLaughlin-Drubin et al., 2008), we presume that HPV16 E7 associates with both E2F6/DP and E2F/PcG complexes. This is supported by the finding that some E2F6 specific cellular genes are upregulated, and that E2F6 containing polycomb bodies are detected at lower levels in E7 expressing cells (McLaughlin-Drubin et al., 2008).
In addition, E7 interacts with class I histone deacetylases (HDACs) (Brehm et al., 1999; Longworth et al., 2004), which function as transcriptional co repressors by inducing chromatin remodeling by reversing acetyl modifications of lysine residues on histones. The association between E7 and HDACs results in increased levels of E2F2-mediated transcription in differentiating cells (Longworth et al., 2005), possibly influencing S-phase progression. Moreover, E7 can also associate, directly or indirectly, with histone acetyl transferases (HATs) including p300, pCAF, and SRC1 (Avvakumov et al., 2003; Baldwin et al., 2006; Bernat et al., 2003; Huang et al., 2002) and has been shown to abrogate SRC1 associated HAT activity (Baldwin et al., 2006).
Subversion of p53 functions by HPV E7
The levels and half-life of p53 are increased in E7 expressing cells, indicating that E7 may perturb p53 degradation (Demers et al., 1994; Jones et al., 1997a; Jones et al., 1997b). Although the mechanism of p53 stabilization in E7 expressing cells is unclear, it is known that it is independent of p14ARF, an inhibitor of mdm2-mediated p53 degradation (Bates et al., 1998; Zhang et al., 1998). Nevertheless, normal mdm2 mediated p53 turnover is defective in high-risk HPV E6/E7 expressing cervical carcinoma cells lines (Hengstermann et al., 2001), and the p53-specific ubiquitin ligase mdm2 is not as efficiently bound to p53 in E7 expressing cells as compared to normal cells (Seavey et al., 1999). This is intriguing, as mdm2 has been implicated in the degradation of pRB (Uchida et al., 2005), and there is a correlation between E7 mediated pRB degradation and p53 stabilization (Jones et al., 1997b). Despite the high levels of p53 in E7 expressing cells, the transcriptional activity of p53 is not activated (Jones et al., 1999), in fact, E7 has been shown to inhibit p53 transcriptional activity in reporter assays (Eichten et al., 2002).
Moreover, HPV16 E7 interferes with p53-mediated G1 growth arrest signaling in response to DNA damage (Demers et al., 1994; Hickman et al., 1994; Slebos et al., 1994; Vousden et al., 1993). The mechanistic details of this are unclear but may include aberrant expression of E2F transcriptional targets (Hickman et al., 1994; Katich et al., 2001), inactivation of the p53-responsive CKI p21CIP1 (Funk et al., 1997; Jones et al., 1997a), and destabilization of pRB (Jones et al., 1997b).
HPV E7 and cell death signaling
Primary human fibroblasts expressing HPV16 E7 are predisposed to apoptosis, especially under conditions of growth factor deprivation or when the cells reach confluence. This has been observed with other viral and cellular oncogenes (Evan et al., 1992; White et al., 1991) and is sometimes referred to as the “trophic sentinel response”. The trophic sentinel response represents a cell intrinsic tumor suppressive pathway that is triggered when the oncogenic aberrant proliferative signal is in conflict with a growth inhibitory signal generated by a lack of mitogenic stimulation. Generation of this signal in E7 expressing fibroblasts requires integrity of the p53 tumor suppressor pathway, but does not involve increased expression of p53-responsive genes (Eichten et al., 2004). While it is accompanied by caspase activation, the HPV16 E7 induced trophic sentinel response still occurs when caspases are inhibited and hence represents a form of cell death that is distinct from apoptosis. Recent experiments with normal human keratinocytes have revealed that HPV16 E7 expression may cause metabolic stress that causes activation of an autophagy-like process (Zhou et al., 2008).
Anoikis, a form of apoptosis, is triggered when cells attempt to enter S-phase despite a lack of matrix attachment (Frisch et al., 2001). The resistance to anoikis is a hallmark of cellular transformation and is characterized by the ability of transformed cells to form foci and display anchorage independent growth. High- and low-risk, as well as BPV1 E7, associate with the 600 kD retinoblastoma protein-associated factor, p600 (DeMasi et al., 2005; Huh et al., 2005). Although the biological functions of p600 have not been fully elucidated in mammalian cells, reports on p600 homologs in Drosophila and Arabidopsis suggest that p600 may play a role in chromosome segregation (Sekelsky et al., 1999), synaptic transmission at the neuromuscular junction (Richards et al., 1996), calcium influx (Xu et al., 1998), and/or polar auxin transport (Gil et al., 2001). A recent study identified p600 as a novel microtubule-associated protein (MAP) that is developmentally regulated in neurons (Shim et al., 2008). The p600 protein contains a RING finger/UBR domain and functions as a ubiquitin ligase in the N-end rule pathway (Tasaki et al., 2005) that is involved in the ubiquitination of proteins through an interaction with their N-terminus (reviewed in Mogk et al., 2007). Experiments involving RNAi knockdown of p600 in mammalian cells have implicated p600 in anoikis (DeMasi et al., 2007; Huh et al., 2005). Therefore, the interaction between E7 and p600 may deregulate anoikis and protect detached cells from apoptosis, thereby contributing to viral transformation (DeMasi et al., 2007; Huh et al., 2005), Consistent with this idea, HPV16 E7 associates with p600 through the CR1 domain, which, is necessary for the transformation capability of HPV16 E7 (Edmonds et al., 1989; Gulliver et al., 1997; Phelps et al., 1992), as well as BPV1 E7 (DeMasi et al., 2005).
Modulation of cytostatic cytokine signaling by HPV E7
Transforming growth factor β (TGF-β) is potent inhibitor of epithelial cell growth, and acquisition of TGF-β resistance is a hallmark of epithelial tumors (reviewed in Polyak, 1996). Indeed, cervical carcinoma cell lines are TGF-β resistant (De Geest et al., 1994), and ectopic HPV16 E7 expression abrogates TGF-β mediated growth inhibition (Pietenpol et al., 1990). Both p21CIP1 and p27KIP1 have been implicated in TGF-β mediated growth inhibition (Datto et al., 1995; Elbendary et al., 1994; Polyak et al., 1994); and HPV16 E7's ability to inactivate these CKIs may contribute to its ability to abrogate TGF-β mediated growth inhibition. TGF-β also induces a cdk4/cdk6 specific CKI, p15INKB (Hannon et al., 1994) and p15INKB-induced growth suppression may require functional pRB, which is targeted fro degradation by HPV16 E7.
However, acquisition of TGF-β resistance in HPV positive cell line is a multi-step process. TGF-β treatment leads to decreased expression of the HPV E6 and E7 oncoproteins; consequently, freshly derived HPV-containing epithelial cell lines remain sensitive to TGF-β (Creek et al., 1995; Woodworth et al., 1990). This effect is mediated by a mechanism that involves the ski oncogene and NFI sites in the viral upstream regulatory region (URR) and acquisition of TGF-β resistance involves this complex (Baldwin et al., 2004).
HPV positive cervical carcinoma cell lines also acquire resistance to tumor necrosis factor α (TNF) (Villa et al., 1992), an important immune response mediator that is produced by cytotoxic T cells in response to a viral infection. Normal keratinocytes undergo G1 growth arrest and cellular differentiation in response to TNF (Basile et al., 2001) through NF-kB-mediated induction of p21CIP1 (Basile et al., 2003). TNF-related cytokines such as Fas ligand and TNF-related apoptosis inducing ligand (TRAIL) do not affect keratinocyte proliferation (Basile et al., 2001). In contrast, HPV16 E7-expressing keratinocytes continue to proliferate in the presence of TNF (Vieira et al., 1996; Villa et al., 1992).
TNF triggers apoptosis when co-administered with the protein synthesis inhibitor cycloheximide, and HPV E7 expressing keratinocytes exhibit increased apoptosis upon TNF/cycloheximide treatment (Basile et al., 2001; Stoppler et al., 1998).
HPV E7 also compromises interferon (IFN) signaling through association with and inhibition of IFN-α induced nuclear translocation of p48, the DNA binding component of ISGF-3 (Barnard et al., 1999; Barnard et al., 2000). Interferon expression is activated by interferon regulatory factors (IRFs) and HPV16 E7 can associate with IRF-1 and impair its transcriptional activity (Park et al., 2000; Perea et al., 2000). Moreover, IFN-γ has been shown to inhibit HPV16 E7 expression, and IFN-γ-induced suppressor of cytokine signaling-1 (SOCS-1)/JAB can associate with and induce ubiquitin-mediated degradation of E7 (Kamio et al., 2004).
HPV16 E7 may also interfere with insulin-like growth factor (IGF) signaling, which regulates cell survival. HPV16 E7 has been reported to associate with insulin like growth factor binding protein-3 (IGFBP-3) (Mannhardt et al., 2000) and accelerate its proteasome-mediated degradation (Santer et al., 2007). HPV16 E7 expressing cells express increased IGFBP-2 and IGFBP-5 levels through an NF-kB dependent mechanism, specifically under conditions of growth factor depletion (Eichten et al., 2004).
Effects of HPV E7 on cellular metabolism
The metabolism of cancer cells is based on energy generation through glycolytic processes rather than mitochondrial respiration (reviewed in Aisenberg, 1961; Warburg, 1936). HPV16 E7 can associate with and alter the activity of the metabolic enzyme pyruvate kinase (PK) (Zwerschke et al., 1999). Presumably as a consequence of this association, HPV16 E7 transformed cells contain high levels of a relatively inactive, dimeric form of PK and such cells derive their metabolic energy mostly from glycolytic processes rather than from oxidative phosphorylation (Mazurek et al., 2001).
HPV16 E7 has also been found to associate with and allosterically activate α-glucosidase (Zwerschke et al., 2000). This enzyme regulates glycogen catabolism and similar to many other tumor types, HPV-associated cervical cancers contain relatively low glycogen levels (Pedersen, 1975).
Transformed cells often have a higher pH and HPV E7 expression in NIH 3T3 cells causes intracellular alkalinization potentially due to increased activity of the Na+/H+ exchanger protein. Inhibition of this process decreased cell proliferation and inhibited anchorage independent growth of HPV16 E7 transformed NIH 3T3 cells (Reshkin et al., 2000).
HPV E7 and chromosomal instability
High-risk HPV oncogene expression in primary human keratinocytes causes cellular immortalization, and these cells exhibit many of the hallmarks of premalignant lesions. However, these cells are not fully transformed, as they do not form tumors when injected into nude mice. Malignant progression of these cells occurs after prolonged passaging in culture or when additional oncogenes, such as ras or fos, are expressed (DiPaolo et al., 1989; Durst et al., 1989; Hurlin et al., 1991; Pei et al., 1993). Similarly, the development of cervical cancer in HPV16 E6 and E7 transgenic mice is dependent on long-term estrogen exposure (Arbeit et al., 1996). This is comparable to the extended period of time between initial HPV infection and the development of invasive cervical carcinoma (reviewed in Schiffman et al., 2007).
Thus, while the expression of the HPV oncogenes is necessary and sufficient for the initiation of cervical carcinogenesis, additional host genome mutations are needed for malignant progression. Indeed, cervical cancer cells have accumulated a wide range of numerical and structural chromosomal abnormalities (Mitelman et al., 2007) some of which likely contribute to malignant progression. Gains of chromosome 3q have been reported to correlate with progression of severe dysplasia to invasive carcinoma (Heselmeyer et al., 1997; Heselmeyer et al., 1996) and transfer of chromosome 11 suppresses the malignant phenotype of the HPV18-positive HeLa cervical carcinoma cell line (Rosl et al., 1991; Stanbridge et al., 1982). Development of aneuploidy is specifically associated with high-risk, but not low-risk, HPV infection in vivo (Rihet et al., 1996) and is detected in premalignant lesions (Bibbo et al., 1989; Steinbeck, 1997) prior to the integration of HPV genomes into host chromosomes (Bulten et al., 1998; Southern et al., 1997).
A variety of cytogenetic abnormalities have also been detected in HPV immortalized keratinocytes (Cottage et al., 2001; Smith et al., 1989) suggesting that HPV oncogene expression may somehow facilitate genomic destabilization. This concept was experimentally proven when it was shown that expression of high-risk HPV E6 and/or E7 in primary human cells increases genomic instability (Hashida et al., 1991; White et al., 1994).
Aneuploidy in HPV E6 and/or E7 expressing cells arises as a consequence of gains and/or losses of entire chromosomes during mitosis that can arise as a consequence of multiple types of mitotic abnormalities including lagging chromosomal material, anaphase bridges, and multipolar mitoses (Duensing et al., 2002).
Abnormal, multipolar mitoses are histopathological hallmarks of high-risk HPV-associated premalignant lesions and cancers (Winkler et al., 1984) and are induced by supernumerary centrosomes (Duensing et al., 2000). The synthesis of centrosomes is intimately linked to the cell division cycle. After cell division each daughter cell contains a single centrosomes, which consist of two centrioles. Upon S-phase entry the two centrioles separate and each centriole then serve as a template for the synthesis of one daughter centriole. After completion of S-phase, cells contain two centrosomes that form the poles of the bipolar mitotic spindle, thus ensuring equal and symmetric chromosome segregation during cell division (reviewed in Bettencourt-Dias et al., 2007).
High-risk, but not low-risk, HPV E6 and E7 proteins cooperate to generate mitotic defects and aneuploidy through induction of supernumerary centrosomes and multipolar mitoses in primary human epithelial cells (Duensing et al., 2000). Supernumerary centrosomes and associated multipolar mitoses have been detected in cells that express episomal HPV16 at low copy number (Duensing et al., 2001b) and their incidence increases in cells with integrated HPV (Pett et al., 2004; Skyldberg et al., 2001) presumably due to higher E6/E7 expression. In addition, supernumerary centrosomes have been documented in HPV16 E6 and/or E7 expressing transgenic mice that develop cervical (Balsitis et al., 2003; Riley et al., 2003) or skin lesions (Schaeffer et al., 2004). HPV16 E7 induces supernumerary centrosomes by uncoupling centriole synthesis from the cell division cycle causing aberrant centrioles synthesis (Duensing et al., 2001a; Guarguaglini et al., 2005) through concurrent formation of multiple daughter centrioles from a single maternal template (Duensing et al., 2007). Unlike normal centrosome duplication, the ability of HPV16 E7 to induce aberrant centriole synthesis is dependent on cdk2 activity. The inhibition of cdk2 interferes with E7 mediated induction of supernumerary centrosomes and decreases the incidence of centrosome abnormalities and aneuploidy in E7 expressing cells (Duensing et al., 2006; Duensing et al., 2004). As discussed in a previous section, HPV16 E7 expression causes aberrant cdk2 activation through multiple pathways. Since HPV16 E7 expression augments the incidence of supernumerary centrosomes in mouse embryo fibroblasts that lack pRB, p107 and p130, however, the ability of HPV16 E7 to induce supernumerary centrosomes is at least in part independent of the ability to target pRB family members (Duensing et al., 2003). A possible pRB/p107/p130 independent mechanism for the emergence of supernumerary centrosomes in HPV16 E7 expressing cells involves the association of E7 with the centrosomal regulator γ-tubulin through sequences that overlap the pRB core-binding site and interfere with γ-tubulin recruitment to centrosomes. The association of HPV16 E7 with γ-tubulin was pRB/p107/p130 independent and binding correlated with the ability of HPV16 E7 to induce supernumerary centrosome abnormalities in pRB/p107/p130 deficient cells. Moreover, HPV16 E7 expression significantly inhibited γ-tubulin recruitment to the centrosome (Nguyen et al., 2007).
Supernumerary centrosomes do not necessarily cause multipolar mitoses due to a cellular defense mechanism, centrosome coalescence, which causes formation of a single mitotic spindle pole by multiple centrosomes. Such cells undergo pseudo-bipolar mitoses, although such mitotic events may lead to chromosome segregation errors. A recent study with high-risk HPV associated anal carcinomas has revealed that the vast majority of cells with supernumerary centrosomes appear to undergo pseudo-bipolar mitosis (Duensing et al., 2008). Nonetheless, tripolar mitoses are hallmarks of high-risk HPV associated lesions and cancers (Crum et al., 1984; Skyldberg et al., 2001) and a significant fraction of HPV16 E6/E7 expressing keratinocytes undergo multipolar mitoses (Duensing et al., 2000). In some cell types, most notably U2OS osteosarcoma cells, E7 expression is sufficient for the induction of multipolar mitoses, suggesting that HPV E7 contributes to subversion of centrosome coalescence.
A study from Bill Saunder's group has shown that the nuclear and mitotic apparatus protein-1 (NuMA) and the microtubule motor protein dynein play significant roles in regulating this process. Oral cancer cell lines with NuMA overexpression show delocalization of dynein from the mitotic spindles, which in turn was correlated with abrogation of centrosome coalescence (Quintyne et al., 2005). HPV16 E7 expression causes dynein delocalization from mitotic spindles (Nguyen et al., 2008a). This activity of E7 maps to a carboxyl terminal domain that is distinct from the LXCXE sequences that are necessary for the induction of supernumerary centrosomes. Interestingly, however, in the context of a normal cell, HPV16 E7 mediated dynein delocalization is not sufficient to induce induction of multipolar mitoses (Nguyen et al., 2008a). It will be interesting to determine whether the ability of HPV16 E7 to induce dynein delocalization is mediated through NuMA and whether it contributes to induction of multipolar mitoses in the context of HPV16 E6 expression which has been shown to greatly increase the incidence of multipolar mitoses in E7 expressing keratinocytes (Duensing et al., 2000).
HPV16 E7 expression also causes other types of mitotic abnormalities including lagging chromosomal material and anaphase bridges that may represent chromosomal fusions caused by double stand DNA breaks (Duensing et al., 2002). The presence of DNA repair foci seen in HPV16 E7 expressing cells indicates that E7 may induce double strand DNA breaks or interfere with break repair. This may facilitate viral genome integration. Consequently, E7 may be a driving force for integration of high-risk HPV genomes into host cellular chromosomes (Kessis et al., 1996), an event that frequently accompanies malignant progression of high-risk HPV associated lesions. Induction of genomic instability and HPV genome integration may also arise as a direct consequence of HPV genome replication, through “onion skin” DNA replication, where multiple replication initiation events occur on a single viral genome template so that replication is occurring at different stages simultaneously (Kadaja et al., 2007; Mannik et al., 2002). This may result in double stranded HPV DNA fragments breaking off of the circular genome and being integrated into the host genome via the endogenous DNA double strand break (DSB) repair machinery (Kadaja et al., 2007). If the upstream regulatory region is integrated into the host DNA, it may be the site of continued “onion skin” replication as long as the viral E1 and E2 replication proteins are expressed (Kadaja et al., 2007). This abnormal generation of multiple double stranded DNA fragments can also initiate DNA DSB repair, which may result in several possible outcomes: excision of HPV DNA, excision of host DNA, rearrangement of host or HPV DNA, repeated integration of HPV DNA, or repeated fragments of host DNA.
Fanconi anemia (FA) patients have an increased incidence of squamous cell carcinomas at sites that are infected by HPVs (reviewed in zur Hausen, 2002) and oral cancers arising in FA patients are HPV positive at a significantly higher rate than in the general population (Kutler et al., 2003). The FA pathway is activated by DNA crosslinking agents and stalled replication forks (reviewed in Kennedy et al., 2005), and it was recently demonstrated that the FA pathway is activated by HPV16 E7 expression and that the capacity of HPV16 E7 to induce DNA repair foci is enhanced in FA patient derived cell lines (Spardy et al., 2007).
Concluding Remarks
At the time this review was written it has been more that 20 years since the discovery of transforming activity of the HPV16 E7 protein. Thus one might question (and some grant reviewers do, indeed), whether or not continued studies on HPV oncoproteins can still yield conceptually novel insights. Does the fact that one of the authors of this article (KM) has been studying biological activities and cellular targets of the HPV E7 proteins for more than 20 years represent relentless scientific curiosity or is simply a reflection of intellectual lethargy? While readers of this article might disagree on the answer of the latter question, these authors would strongly argue that HPV16 E7 will continue to provide a powerful tool to discover novel cellular signaling pathways that importantly contribute to tumorigenesis. HPV associated cancers are unique amongst human solid tumors since they are uniformly caused by the same carcinogens, expression of high-risk HPV E6 and E7 oncoproteins. The findings that HPV16 E7 expression in a transgenic mouse model is sufficient for cervical cancer development (Riley et al., 2003) and that its continued expression remains necessary for the maintenance of the transformed state of cervical cancer derived cells (DeFilippis et al., 2003) clearly validate the premise that the HPV E7 oncoprotein importantly contributes to induction and progression of HPV associated cancers. Clearly, genome-wide RNA expression analyses as well as novel proteomic methodologies made it possible to identify novel and unexpected cellular pathways that HPV16 E7 targets. The finding that HPV16 E7 expression induces certain aspects of angiogenesis serves as a powerful illustration of this point (Chen et al., 2007; Toussaint-Smith et al., 2004).
With the availability of genome wide libraries of siRNA and cDNA expression clones, it is now possible to perform loss- and gain-of-function experiments in mammalian cells that for all practical purposes approach genetic screens in genetically tractable model organisms. Such studies, in combination with sophisticated analytical tools that are developed through the emerging field of network biology, will yield novel and unexpected insights into the molecular signaling networks that are targeted by HPV E7. We have recently performed a small-scale, proof-of-principle shRNA screen to investigate whether HPV16 E7 expression alters the kinase requirements of the RKO colon cancer cell line. These experiments revealed that E7 expression in these cells abrogates the requirement of RKO cells for CDK6, ERBB3, FYN, AAK1, and TSSK2. The reduced requirement for CDK6 in E7 expressing RKO cells is not surprising given that HPV16 E7 targets its major phosphorylation substrate, the retinoblastoma tumor suppressor pRB, for degradation. On the other hand, the biochemical mechanisms by which E7 expression abrogates the requirement for the four other kinases in RKO cells are unknown (Baldwin et al., 2008). Given that kinase signaling pathways are remarkably distinct in different cell types (Grueneberg et al., 2008), similar studies that are currently performed in a more appropriate “wild-type” cellular background promise to reveal exciting new avenues of research.
Acknowledgments
This article is dedicated to all Münger Lab members, past and present, whose hard and dedicated work has provided many critical insights into the biochemistry and biology of human papillomavirus oncoproteins. KM also acknowledges the spiritual support of M. An Hattens and Chung Hwa, and MEM-D acknowledges the support of P. R. Cocet. Supported by NIH grants R01CA066980 and R01CA081135 (KM) and American Cancer Society Postdoctoral Fellowship PF-07-072-01-MBC (MEM-D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisenberg AC. Studies on normal and neoplastic mitochondria. I. Respiration. Cancer Res. 1961;21:295–303. [PubMed] [Google Scholar]
- Alani RM, Hasskarl J, Munger K. Alterations in cyclin-dependent kinase 2 function during differentiation of primary human keratinocytes. Mol Carcinog. 1998;23(4):226–33. doi: 10.1002/(sici)1098-2744(199812)23:4<226::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Angeline M, Merle E, Moroianu J. The E7 oncoprotein of high-risk human papillomavirus type 16 enters the nucleus via a nonclassical Ran-dependent pathway. Virology. 2003;317(1):13–23. doi: 10.1016/j.virol.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93(7):2930–5. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DJ, Roman A. The Anomalous Electrophoretic Behavior of the Human Papillomavirus Type-16 E7-Protein Is Due to the High Content of Acidic Amino Acid Residues. Biochemical and Biophysical Research Communications. 1993;192(3):1380–1387. doi: 10.1006/bbrc.1993.1569. [DOI] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136(1):1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwooll C, Oddi S, Cartwright P, Prosperini E, Agger K, Steensgaard P, Wagener C, Sardet C, Moroni MC, Helin K. A novel repressive E2F6 complex containing the polycomb group protein, EPC1, that interacts with EZH2 in a proliferation-specific manner. J Biol Chem. 2005;280(2):1199–208. doi: 10.1074/jbc.M412509200. [DOI] [PubMed] [Google Scholar]
- Avvakumov N, Torchia J, Mymryk JS. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene. 2003;22(25):3833–41. doi: 10.1038/sj.onc.1206562. [DOI] [PubMed] [Google Scholar]
- Baldwin A, Huh KW, Munger K. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J Virol. 2006;80(13):6669–77. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Li W, Grace M, Pearlberg J, Harlow E, Münger K, Grueneberg DA. Kinase Requirements in Human Cells: II Genetic Interaction Screens Identify Alterations in Kinase Requirements Following HPV16 E7 Expression in Cancer Cells. Proc Natl Acad Sci U S A. 2008;105 doi: 10.1073/pnas.0806195105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Pirisi L, Creek KE. NFI-Ski interactions mediate transforming growth factor beta modulation of human papillomavirus type 16 early gene expression. J Virol. 2004;78(8):3953–64. doi: 10.1128/JVI.78.8.3953-3964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the Effects of the Human Papillomavirus Type 16 E7 Oncogene on Mouse Epithelium by Somatic Rb Deletion and Detection of pRb-Independent Effects of E7 In Vivo. Mol Cell Biol. 2003;23(24):9094–103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MS, Edmonds C, Fisher C, Schiller JT, Lowy DR, Vousden KH. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. Embo J. 1990;9(1):153–60. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MS, Lowy DR, Schiller JT. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989;63(3):1404–7. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MS, Vass WC, Lowy DR, Schiller JT. In vitro biological activities of the E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991;65(1):292–8. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259(2):305–13. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- Barnard P, Payne E, McMillan NA. The human papillomavirus E7 protein is able to inhibit the antiviral and anti-growth functions of interferon-alpha. Virology. 2000;277(2):411–9. doi: 10.1006/viro.2000.0584. [DOI] [PubMed] [Google Scholar]
- Basile JR, Eichten A, Zacny V, Munger K. NF-kappaB-mediated induction of p21(Cip1/Waf1) by tumor necrosis factor alpha induces growth arrest and cytoprotection in normal human keratinocytes. Mol Cancer Res. 2003;1(4):262–70. [PubMed] [Google Scholar]
- Basile JR, Zacny V, Munger K. The cytokines tumor necrosis factor-alpha (TNF-alpha) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J Biol Chem. 2001;276(25):22522–8. doi: 10.1074/jbc.M010505200. [DOI] [PubMed] [Google Scholar]
- Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395(6698):124–5. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Jones KH, Grossman SR, Laimins LA. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989;63(3):1247–55. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat A, Avvakumov N, Mymryk JS, Banks L. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene. 2003;22(39):7871–81. doi: 10.1038/sj.onc.1206896. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8(6):451–63. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Bibbo M, Dytch HE, Alenghat E, Bartels PH, Wied GL. DNA ploidy profiles as prognostic indicators in CIN lesions. Am J Clin Pathol. 1989;92(3):261–5. doi: 10.1093/ajcp/92.3.261. [DOI] [PubMed] [Google Scholar]
- Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56(20):4620–4. [PubMed] [Google Scholar]
- Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. Embo J. 1999;18(9):2449–58. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulten J, Poddighe PJ, Robben JCM, Gemmink JH, de Wilde PCM, Hanselaar AGJM. Interphase cytogenetic analysis of cervical intraepithelial neoplasia. Am J Pathol. 1998;152:495–503. [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li F, Mead L, White H, Walker J, Ingram DA, Roman A. Human papillomavirus causes an angiogenic switch in keratinocytes which is sufficient to alter endothelial cell behavior. Virology. 2007;367(1):168–74. doi: 10.1016/j.virol.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9(19):2335–49. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Di Pasquale G, Carlotti F, Crawford L, Tommasino M. Functional studies of E7 proteins from different HPV types. Oncogene. 1994;9(9):2633–8. [PubMed] [Google Scholar]
- Clemens KE, Brent R, Gyuris J, Munger K. Dimerization of the human papillomavirus E7 oncoprotein in vivo. Virology. 1995;214(1):289–93. doi: 10.1006/viro.1995.9926. [DOI] [PubMed] [Google Scholar]
- Cole ST, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Collins AS, Nakahara T, Do A, Lambert PF. Interactions with pocket proteins contribute to the role of human papillomavirus type 16 E7 in the papillomavirus life cycle. J Virol. 2005;79(23):14769–80. doi: 10.1128/JVI.79.23.14769-14780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottage A, Dowen S, Roberts I, Pett M, Coleman N, Stanley M. Early genetic events in HPV immortalised keratinocytes. Genes Chromosomes Cancer. 2001;30(1):72–9. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1060>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Creek KE, Geslani G, Batova A, Pirisi L. Progressive loss of sensitivity to growth control by retinoic acid and transforming growth factor-beta at late stages of human papillomavirus type 16-initiated transformation of human keratinocytes. Adv Exp Med Biol. 1995;375:117–35. doi: 10.1007/978-1-4899-0949-7_11. [DOI] [PubMed] [Google Scholar]
- Crum CP, Ikenberg H, Richart RM, Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310(14):880–3. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin- dependent kinase inhibitor p21 through a p53-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest K, Bergman CA, Turyk ME, Frank BS, Wilbanks GD. Differential response of cervical intraepithelial and cervical carcinoma cell lines to transforming growth factor-beta 1. Gynecol Oncol. 1994;55(3 Pt 1):376–85. doi: 10.1006/gyno.1994.1310. [DOI] [PubMed] [Google Scholar]
- DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77(2):1551–63. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMasi J, Chao MC, Kumar AS, Howley PM. Bovine papillomavirus E7 oncoprotein inhibits anoikis. J Virol. 2007;81(17):9419–25. doi: 10.1128/JVI.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMasi J, Huh KW, Nakatani Y, Munger K, Howley PM. Bovine papillomavirus E7 transformation function correlates with cellular p600 protein binding. Proc Natl Acad Sci U S A. 2005;102(32):11486–91. doi: 10.1073/pnas.0505322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers GW, Foster SA, Halbert CL, Galloway DA. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc Natl Acad Sci U S A. 1994;91(10):4382–6. doi: 10.1073/pnas.91.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, Dotto GP. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280(5366):1069–72. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- DiPaolo JA, Woodworth CD, Popescu NC, Notario V, Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989;4(4):395–9. [PubMed] [Google Scholar]
- Duensing A, Chin A, Wang L, Kuan SF, Duensing S. Analysis of centrosome overduplication in correlation to cell division errors in high-risk human papillomavirus (HPV)-associated anal neoplasms. Virology. 2008;372(1):157–64. doi: 10.1016/j.virol.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene. 2007;26(43):6280–8. doi: 10.1038/sj.onc.1210456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25(20):2943–9. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Duensing A, Crum CP, Munger K. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 2001a;61(6):2356–60. [PubMed] [Google Scholar]
- Duensing S, Duensing A, Flores ER, Do A, Lambert PF, Munger K. Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J Virol. 2001b;75(16):7712–6. doi: 10.1128/JVI.75.16.7712-7716.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Duensing A, Lee DC, Edwards KM, Piboonniyom SO, Manuel E, Skaltsounis L, Meijer L, Munger K. Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene. 2004;23(50):8206–15. doi: 10.1038/sj.onc.1208012. [DOI] [PubMed] [Google Scholar]
- Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97(18):10002–7. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62(23):7075–82. [PubMed] [Google Scholar]
- Duensing S, Munger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol. 2003;77(22):12331–5. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst M, Gallahan D, Jay G, Rhim JS. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989;173(2):767–71. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12(15):2245–62. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66(12):6893–902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C, Vousden KH. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989;63(6):2650–6. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichten A, Rud DS, Grace M, Piboonniyom SO, Zacny V, Munger K. Molecular pathways executing the “trophic sentinel” response in HPV-16 E7-expressing normal human diploid fibroblasts upon growth factor deprivation. Virology. 2004;319(1):81–93. doi: 10.1016/j.virol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Eichten A, Westfall M, Pietenpol JA, Münger K. Stabilization and functional impairment of the tumor suppressor p53 by the human papillomavirus type 16 E7 oncoprotein. Virology. 2002;295:74–95. doi: 10.1006/viro.2002.1375. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Elbendary A, Berchuck A, Davis P, Havrilesky L, Bast RC, Jr, Iglehart JD, Marks JR. Transforming growth factor beta 1 can induce CIP1/WAF1 expression independent of the p53 pathway in ovarian cancer cells. Cell Growth Differ. 1994;5:1301–1307. [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69(1):119–28. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271(5248):499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Figge J, Webster T, Smith TF, Paucha E. Prediction of similar transforming regions in simian virus 40 large T, adenovirus E1A, and myc oncoproteins. J Virol. 1988;62(5):1814–8. doi: 10.1128/jvi.62.5.1814-1818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14(7):4889–901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firzlaff JM, Galloway DA, Eisenman RN, Luscher B. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 1989;1(1):44–53. [PubMed] [Google Scholar]
- Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74(14):6622–31. doi: 10.1128/jvi.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117(Pt 11):2173–81. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11(16):2090–100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage JR, Meyers C, Wettstein FO. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990;64(2):723–30. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 2001;15(15):1985–97. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield I, Nickerson J, Penman S, Stanley M. Human papillomavirus 16 E7 protein is associated with the nuclear matrix. Proc Natl Acad Sci U S A. 1991;88(24):11217–21. doi: 10.1073/pnas.88.24.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg DA, Degot S, Pearlberg J, Li W, Davies JE, Baldwin A, Endege W, Doench J, Sawyer J, Hu Y, Boyce F, Xian J, Münger K, Harlow E. Kinase Requirements in Human Cells: I Comparing Kinase Requirements Across Various Cell Types. Proc Natl Acad Sci U S A. 2008;105 doi: 10.1073/pnas.0808019105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell. 2005;16(3):1095–107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver GA, Herber RL, Liem A, Lambert PF. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71(8):5905–14. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activty in human epithelial cells. Journal of Virology. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ, Beach D. p15(INK4B) is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hashida T, Yasumoto S. Induction of chromosome abnormalities in mouse and human epidermal keratinocytes by the human papillomavirus type 16 E7 oncogene. J Gen Virol. 1991;72(Pt 7):1569–77. doi: 10.1099/0022-1317-72-7-1569. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. Embo J. 1989;8(12):3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Staples D, Smith C, Fisher C. Direct activation of cyclin-dependent kinase 2 by human papillomavirus E7. J Virol. 2003;77(19):10566–74. doi: 10.1128/JVI.77.19.10566-10574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DV, Yee CL, Howley PM, Munger K. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc Natl Acad Sci U S A. 1992;89(10):4442–6. doi: 10.1073/pnas.89.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt AM, Funk JO, Galloway DA. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. J Virol. 2002;76(20):10559–68. doi: 10.1128/JVI.76.20.10559-10568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt AM, Galloway DA. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 e7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J Virol. 2001;75(15):6737–47. doi: 10.1128/JVI.75.15.6737-6747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci U S A. 2001;98(3):1218–23. doi: 10.1073/pnas.031470698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heselmeyer K, Macville M, Schrock E, Blegen H, Hellstrom AC, Shah K, Auer G, Ried T. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosomes Cancer. 1997;19:233–240. [PubMed] [Google Scholar]
- Heselmeyer K, Schrock E, du Manoir S, Blegen H, Shah K, Steinbeck R, Auer G, Ried T. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc Natl Acad Sci U S A. 1996;93(1):479–84. doi: 10.1073/pnas.93.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman ES, Picksley SM, Vousden KH. Cells expressing HPV16 E7 continue cell cycle progression following DNA damage induced p53 activation. Oncogene. 1994;9(8):2177–81. [PubMed] [Google Scholar]
- Huang PS, Patrick DR, Edwards G, Goodhart PJ, Huber HE, Miles L, Garsky VM, Oliff A, Heimbrook DC. Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol Cell Biol. 1993;13(2):953–60. doi: 10.1128/mcb.13.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002;76(17):8710–21. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JB, Bedell MA, McCance DJ, Laiminis LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64(2):519–26. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81(18):9737–47. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102(32):11492–7. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Kaur P, Smith PP, Perez-Reyes N, Blanton RA, McDougall JK. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc Natl Acad Sci U S A. 1991;88(2):570–4. doi: 10.1073/pnas.88.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SG, Lee D, Kim J, Seo T, Choe J. Human papillomavirus type 16 E7 binds to E2F1 and activates E2F1-driven transcription in a retinoblastoma protein-independent manner. J Biol Chem. 2002;277(4):2923–30. doi: 10.1074/jbc.M109113200. [DOI] [PubMed] [Google Scholar]
- Jewers RJ, Hildebrandt P, Ludlow JW, Kell B, McCance DJ. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J Virol. 1992;66(3):1329–35. doi: 10.1128/jvi.66.3.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Y, Schmidt-Grimminger DC, Chien WM, Wu X, Broker TR, Chow LT. Post-transcriptional induction of p21cip1 protein by human papillomavirus E7 inhibits unscheduled DNA synthesis reactivated in differentiated keratinocytes. Oncogene. 1998;17(16):2027–38. doi: 10.1038/sj.onc.1202142. [DOI] [PubMed] [Google Scholar]
- Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997a;11(16):2101–11. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997b;239(1):97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Suh-Burgmann E, Grace M, Munger K. Expression of the HPV E7 oncoprotein mimics but does not evoke a p53-dependent cellular DNA damage response pathway. Virology. 1999;258(2):406–14. doi: 10.1006/viro.1999.9733. [DOI] [PubMed] [Google Scholar]
- Kadaja M, Sumerina A, Verst T, Ojarand M, Ustav E, Ustav M. Genomic instability of the host cell induced by the human papillomavirus replication machinery. Embo J. 2007;26(8):2180–91. doi: 10.1038/sj.emboj.7601665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio M, Yoshida T, Ogata H, Douchi T, Nagata Y, Inoue M, Hasegawa M, Yonemitsu Y, Yoshimura A. SOCS1 [corrected] inhibits HPV-E7-mediated transformation by inducing degradation of E7 protein. Oncogene. 2004;23(17):3107–15. doi: 10.1038/sj.onc.1207453. [DOI] [PubMed] [Google Scholar]
- Kanda T, Furuno A, Yoshiike K. Human papillomavirus type 16 open reading frame E7 encodes a transforming gene for rat 3Y1 cells. J Virol. 1988;62(2):610–3. doi: 10.1128/jvi.62.2.610-613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katich SC, Zerfass-Thome K, Hoffmann I. Regulation of the Cdc25A gene by the human papillomavirus Type 16 E7 oncogene. Oncogene. 2001;20(5):543–50. doi: 10.1038/sj.onc.1204130. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19(24):2925–40. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene. 1996;13(2):427–431. [PubMed] [Google Scholar]
- Knapp AA, McManus PM, Bockstall K, Moroianu J. Identification of the Nuclear Localization and Export Signals of High Risk HPV16 E7 Oncoprotein. Virology. 2008 doi: 10.1016/j.virol.2008.09.037. accepted pending revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, Huvos AG, Giampietro P, Levran O, Pujara K, Diotti R, Carlson D, Huryn LA, Auerbach AD, Singh B. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95(22):1718–21. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- Lee C, Laimins LA. The differentiation-dependent life cycle of human papillomaviruses in keratinocytes. In: Garcea RL, DiMaio D, editors. The Papillomaviruses. Springer; New York: 2007. pp. 45–68. [Google Scholar]
- Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281(1):578–86. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J Virol. 2004;78(7):3533–41. doi: 10.1128/JVI.78.7.3533-3541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS, Wilson R, Laimins LA. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. Embo J. 2005;24(10):1821–30. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TE, Salih M, Tuana BS. Activating E2Fs mediate transcriptional regulation of human E2F6 repressor. Am J Physiol Cell Physiol. 2006;290(1):C189–99. doi: 10.1152/ajpcell.00630.2004. [DOI] [PubMed] [Google Scholar]
- Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol. 2000;20(17):6483–95. doi: 10.1128/mcb.20.17.6483-6495.2000. In Process Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik A, Runkorg K, Jaanson N, Ustav M, Ustav E. Induction of the bovine papillomavirus origin “onion skin”-type DNA replication at high E1 protein concentrations in vivo. J Virol. 2002;76(11):5835–45. doi: 10.1128/JVI.76.11.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi P, Banks L. Differential phosphorylation of the HPV-16 E7 oncoprotein during the cell cycle. Virology. 2000;276(2):388–94. doi: 10.1006/viro.2000.0514. [DOI] [PubMed] [Google Scholar]
- Matlashewski G, Schneider J, Banks L, Jones N, Murray A, Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. Embo J. 1987;6(6):1741–6. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem J. 2001;356(Pt 1):247–56. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci U S A. 1988;85(19):7169–73. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre MC, Frattini MG, Grossman SR, Laimins LA. Human papillomavirus type 18 E7 protein requires intact Cys-X-X-Cys motifs for zinc binding, dimerization, and transformation but not for Rb binding. Journal of Virology. 1993;67:3142–3150. doi: 10.1128/jvi.67.6.3142-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Bromberg-White JL, Meyers C. The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology. 2005;338(1):61–8. doi: 10.1016/j.virol.2005.04.036. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Huh KW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J Virol. 2008;82(17):8695–705. doi: 10.1128/JVI.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10(23):3065–75. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F. Mitelman database of chromosome aberrations in cancer (2007) http://cgap.nci.nih.gov/Chromosomes/Mitelman 2007
- Mogk A, Schmidt R, Bukau B. The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007;17(4):165–72. doi: 10.1016/j.tcb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989a;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. Embo J. 1989b;8(13):4099–105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K, Yee CL, Phelps WC, Pietenpol JA, Moses HL, Howley PM. Biochemical and biological differences between E7 oncoproteins of the high- and low risk human papillomavirus types are determined by amino-terminal sequences. Journal of Virology. 1991;65:3943–3948. doi: 10.1128/jvi.65.7.3943-3948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol. 2007;81(24):13533–43. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, McLaughlin-Drubin ME, Münger K. Delocalization of the Microtubule Motor Dynein from Mitotic Spindles by the Human Papillomavirus E7 Oncoprotein is Not Sufficient for Induction of Multipolar Mitoses. Cancer Res. 2008a;68(22) doi: 10.1158/0008-5472.CAN-08-1303. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Munger K. Direct association of the HPV16 E7 oncoprotein with cyclin A/CDK2 and cyclin E/CDK2 complexes. Virology. 2008b doi: 10.1016/j.virol.2008.07.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noya F, Chien WM, Broker TR, Chow LT. p21cip1 Degradation in differentiated keratinocytes is abrogated by costabilization with cyclin E induced by human papillomavirus E7. J Virol. 2001;75(13):6121–34. doi: 10.1128/JVI.75.13.6121-6134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296(5570):1132–6. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Kalinina A, Wang J, Nakayama K, Nakayama KI, Bagchi S. The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and Cullin 1- and Skp2-containing E3 ligase. J Virol. 2004a;78(10):5338–46. doi: 10.1128/JVI.78.10.5338-5346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ST, Longworth MS, Laimins LA. Roles of the E6 and E7 proteins in the life cycle of low-risk human papillomavirus type 11. J Virol. 2004b;78(5):2620–6. doi: 10.1128/JVI.78.5.2620-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlenschlager O, Seiboth T, Zengerling H, Briese L, Marchanka A, Ramachandran R, Baum M, Korbas M, Meyer-Klaucke W, Durst M, Gorlach M. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene. 2006 doi: 10.1038/sj.onc.1209584. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275(10):6764–9. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- Patrick DR, Oliff A, Heimbrook DC. Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. J Biol Chem. 1994;269(9):6842–50. [PubMed] [Google Scholar]
- Pedersen SN. Enzymatic studies of glycogen metabolism in nonmalignant and malignant biopsies from the human uterine cervix. Acta Obstet Gynecol Scand. 1975;54(5):443–8. doi: 10.3109/00016347509157107. [DOI] [PubMed] [Google Scholar]
- Pei XF, Meck JM, Greenhalgh D, Schlegel R. Cotransfection of HPV-18 and v-fos DNA induces tumorigenicity of primary human keratinocytes. Virology. 1993;196(2):855–60. doi: 10.1006/viro.1993.1546. [DOI] [PubMed] [Google Scholar]
- Perea SE, Massimi P, Banks L. Human papillomavirus type 16 E7 impairs the activation of the interferon regulatory factor-1. Int J Mol Med. 2000;5(6):661–6. doi: 10.3892/ijmm.5.6.661. [DOI] [PubMed] [Google Scholar]
- Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, Coleman N. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64(4):1359–68. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- Phelps WC, Münger K, Yee CL, Barnes JA, Howley PM. Structure-function analysis of the human papillomavirus E7 oncoprotein. Journal of Virology. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps WC, Yee CL, Munger K, Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53(4):539–47. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Pietenpol JA, Stein RW, Moran E, Yaciuk P, Schlegel R, Lyons RM, Pittelkow MR, Munger K, Howley PM, Moses HL. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990;61(5):777–85. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Polyak K. Negative regulation of cell growth by TGF beta. Biochim Biophys Acta. 1996;1242(3):185–99. doi: 10.1016/0304-419x(95)00009-5. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27(kip1), a cyclin-cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes & Development. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307(5706):127–9. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- Reinstein E, Scheffner M, Oren M, Ciechanover A, Schwartz A. Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene. 2000;19(51):5944–50. doi: 10.1038/sj.onc.1203989. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB Journal. 2000;14:2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- Ressler S, Scheiden R, Dreier K, Laich A, Muller-Holzner E, Pircher H, Morandell D, Stein I, Viertler HP, Santer FR, Widschwendter A, Even J, Jansen-Durr P, Capesius C, Zwerschke W. High-risk human papillomavirus E7 oncoprotein detection in cervical squamous cell carcinoma. Clin Cancer Res. 2007;13(23):7067–72. doi: 10.1158/1078-0432.CCR-07-1222. [DOI] [PubMed] [Google Scholar]
- Richards S, Hillman T, Stern M. Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics. 1996;142(4):1215–23. doi: 10.1093/genetics/142.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihet S, Lorenzato M, Clavel C. Oncogenic human papillomaviruses and ploidy in cervical lesions. J Clin Pathol. 1996;49(11):892–6. doi: 10.1136/jcp.49.11.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63(16):4862–71. [PubMed] [Google Scholar]
- Rosl F, Achtstatter T, Bauknecht T, Hutter KJ, Futterman G, zur Hausen H. Extinction of the HPV18 upstream regulatory region in cervical carcinoma cells after fusion with non-tumorigenic human keratinocytes under non-selective conditions. EMBO J. 1991;10(6):1337–45. doi: 10.1002/j.1460-2075.1991.tb07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesch MN, Laimins LA. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J Virol. 1997;71(7):5570–8. doi: 10.1128/jvi.71.7.5570-5578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang BC, Barbosa MS. Single amino acid substitutions in “low-risk” human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the “high-risk” HPV E7 oncoproteins. Proc Natl Acad Sci U S A. 1992;89(17):8063–7. doi: 10.1073/pnas.89.17.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer FR, Moser B, Spoden GA, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein inhibits apoptosis mediated by nuclear insulin-like growth factor-binding protein-3 by enhancing its ubiquitin/proteasome-dependent degradation. Carcinogenesis. 2007;28(12):2511–20. doi: 10.1093/carcin/bgm199. [DOI] [PubMed] [Google Scholar]
- Sato H, Watanabe S, Furuno A, Yoshiike K. Human papillomavirus type 16 E7 protein expressed in Escherichia coli and monkey COS-1 cells: immunofluorescence detection of the nuclear E7 protein. Virology. 1989;170(1):311–5. doi: 10.1016/0042-6822(89)90386-3. [DOI] [PubMed] [Google Scholar]
- Schaeffer AJ, Nguyen M, Liem A, Lee D, Montagna C, Lambert PF, Ried T, Difilippantonio MJ. E6 and E7 oncoproteins induce distinct patterns of chromosomal aneuploidy in skin tumors from transgenic mice. Cancer Res. 2004;64(2):538–46. doi: 10.1158/0008-5472.can-03-0124. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. Embo J. 1988;7(10):3181–7. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seavey SE, Holubar M, Saucedo LJ, Perry ME. The E7 oncoprotein of human papillomavirus type 16 stabilizes p53 through a mechanism independent of p19(ARF) J Virol. 1999;73(9):7590–8. doi: 10.1128/jvi.73.9.7590-7598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky JJ, McKim KS, Messina L, French RL, Hurley WD, Arbel T, Chin GM, Deneen B, Force SJ, Hari KL, Jang JK, Laurencon AC, Madden LD, Matthies HJ, Milliken DB, Page SL, Ring AD, Wayson SM, Zimmerman CC, Hawley RS. Identification of novel Drosophila meiotic genes recovered in a P-element screen. Genetics. 1999;152(2):529–42. doi: 10.1093/genetics/152.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SY, Wang J, Asada N, Neumayer G, Tran HC, Ishiguro K, Sanada K, Nakatani Y, Nguyen MD. Protein 600 is a microtubule/endoplasmic reticulum-associated protein in CNS neurons. J Neurosci. 2008;28(14):3604–14. doi: 10.1523/JNEUROSCI.5278-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyldberg B, Fujioka K, Hellstrom AC, Sylven L, Moberger B, Auer G. Human papillomavirus infection, centrosome aberration, and genetic stability in cervical lesions. Mod Pathol. 2001;14(4):279–84. doi: 10.1038/modpathol.3880303. [DOI] [PubMed] [Google Scholar]
- Slebos RJ, Lee MH, Plunkett BS, Kessis TD, Williams BO, Jacks T, Hedrick L, Kastan MB, Cho KR. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994;91(12):5320–4. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PP, Bryant EM, Kaur P, McDougall JK. Cytogenetic analysis of eight human papillomavirus immortalized human keratinocyte cell lines. Int J Cancer. 1989;44(6):1124–31. doi: 10.1002/ijc.2910440631. [DOI] [PubMed] [Google Scholar]
- Smith-McCune K, Kalman D, Robbins C, Shivakumar S, Yuschenkoff L, Bishop JM. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc Natl Acad Sci U S A. 1999;96(12):6999–7004. doi: 10.1073/pnas.96.12.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986;83(13):4680–4. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D, Wettstein FO. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987;61:1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern SA, Evans MF, Herrington CS. Basal cell tetrasomy in low-grade cervical squamous intraepithelial lesions infected with high-risk human papillomaviruses. Cancer Res. 1997;57(19):4210–3. [PubMed] [Google Scholar]
- Spardy N, Duensing A, Charles D, Haines N, Nakahara T, Lambert PF, Duensing S. The human papillomavirus type 16 E7 oncoprotein activates the Fanconi Anemia (FA) pathway and causes accelerated chromosomal instability in FA cells. J Virol. 2007 doi: 10.1128/JVI.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E, Dez CJ, Dorsen CJ, Nishioni RY, Pechl DM, Weissman BE, Wilkinson JE. Human cell hybrids: Analysis of transformation and tumorigenicity. Science. 1982;215:252–256. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Steinbeck RG. Proliferation and DNA aneuploidy in mild dysplasia imply early steps of cervical carcinogenesis. Acta Oncol. 1997;36:3–12. doi: 10.3109/02841869709100723. [DOI] [PubMed] [Google Scholar]
- Stoppler H, Stoppler MC, Johnson E, Simbulan-Rosenthal CM, Smulson ME, Iyer S, Rosenthal DS, Schlegel R. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene. 1998;17(10):1207–14. doi: 10.1038/sj.onc.1202053. [DOI] [PubMed] [Google Scholar]
- Storey A, Almond N, Osborn K, Crawford L. Mutations of the human papillomavirus type 16 E7 gene that affect transformation, transactivation and phosphorylation by the E7 protein. J Gen Virol. 1990;71(Pt 4):965–70. doi: 10.1099/0022-1317-71-4-965. [DOI] [PubMed] [Google Scholar]
- Stubenrauch F, Laimins LA. Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol. 1999;9(6):379–86. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Noda T, Yajima H, Hatanaka M, Ito Y. Identification of a transforming gene of human papillomavirus type 16. J Virol. 1989;63(3):1465–9. doi: 10.1128/jvi.63.3.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Mulder LC, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT. A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol. 2005;25(16):7120–36. doi: 10.1128/MCB.25.16.7120-7136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JT, Hubert WG, Ruesch MN, Laimins LA. Human papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc Natl Acad Sci U S A. 1999;96(15):8449–54. doi: 10.1073/pnas.96.15.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasino M, Adamczewski JP, Carlotti F, Barth CF, Manetti R, Contorni M, Cavalieri F, Hunt T, Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8(1):195–202. [PubMed] [Google Scholar]
- Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23(17):2988–95. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Fairchild B, Wen J, Lees JA. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc Natl Acad Sci U S A. 2001;98(4):1519–24. doi: 10.1073/pnas.041597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida C, Miwa S, Kitagawa K, Hattori T, Isobe T, Otani S, Oda T, Sugimura H, Kamijo T, Ookawa K, Yasuda H, Kitagawa M. Enhanced Mdm2 activity inhibits pRB function via ubiquitin-dependent degradation. Embo J. 2005;24(1):160–9. doi: 10.1038/sj.emboj.7600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira KBL, Goldstein DJ, Villa LL. Tumor necrosis factor alpha interferes with the cell cycle of normal and papillomavirus-immortalized human keratinocytes. Cancer Research. 1996;56:2452–2457. [PubMed] [Google Scholar]
- Villa LL, Vieira KB, Pei XF, Schlegel R. Differential effect of tumor necrosis factor on proliferation of primary human keratinocytes and cell lines containing human papillomavirus types 16 and 18. Mol Carcinog. 1992;6(1):5–9. doi: 10.1002/mc.2940060103. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Doniger J, DiPaolo JA, Lowy DR. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3(2):167–75. [PubMed] [Google Scholar]
- Vousden KH, Jat PS. Functional similarity between HPV16E7, SV40 large T and adenovirus E1a proteins. Oncogene. 1989;4(2):153–8. [PubMed] [Google Scholar]
- Vousden KH, Vojtesek B, Fisher C, Lane D. HPV-16 E7 or adenovirus E1A can overcome the growth arrest of cells immortalized with a temperature-sensitive p53. Oncogene. 1993;8(6):1697–702. [PubMed] [Google Scholar]
- Warburg O. Ueber den Stoffwechsel der Tumoren. Springer; Berlin: 1936. [Google Scholar]
- Watanabe S, Kanda T, Sato H, Furuno A, Yoshiike K. Mutational analysis of human papillomavirus type 16 E7 functions. J Virol. 1990;64(1):207–14. doi: 10.1128/jvi.64.1.207-214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Yoshiike K. Transformation of rat 3Y1 cells by human papillomavirus type-18 DNA. Int J Cancer. 1988;41(6):896–900. doi: 10.1002/ijc.2910410622. [DOI] [PubMed] [Google Scholar]
- White AE, Livanos EM, Tlsty TD. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 1994;8(6):666–77. doi: 10.1101/gad.8.6.666. [DOI] [PubMed] [Google Scholar]
- White E, Cipriani R, Sabbatini P, Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991;65(6):2968–78. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler B, Crum CP, Fujii T, Ferenczy A, Boon M, Braun L, Lancaster WD, Richart RM. Koilocytotic lesions of the cervix. The relationship of mitotic abnormalities to the presence of papillomavirus antigens and nuclear DNA content. Cancer. 1984;53(5):1081–7. doi: 10.1002/1097-0142(19840301)53:5<1081::aid-cncr2820530511>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Bowden PE, Doniger J, Pirisi L, Barnes W, Lancaster WD, DiPaolo JA. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 1988;48(16):4620–8. [PubMed] [Google Scholar]
- Woodworth CD, Doniger J, DiPaolo JA. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989;63(1):159–64. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth CD, Waggoner S, Barnes W, Stoler MH, DiPaolo JA. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Research. 1990;50:3709–3715. [PubMed] [Google Scholar]
- Wu EW, Clemens KE, Heck DV, Munger K. The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. J Virol. 1993;67(4):2402–7. doi: 10.1128/jvi.67.4.2402-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Wes PD, Chen H, Li HS, Yu M, Morgan S, Liu Y, Montell C. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J Biol Chem. 1998;273(47):31297–307. doi: 10.1074/jbc.273.47.31297. [DOI] [PubMed] [Google Scholar]
- Yasumoto S, Burkhardt AL, Doniger J, DiPaolo JA. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986;57(2):572–7. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutsudo M, Okamoto Y, Hakura A. Functional dissociation of transforming genes of human papillomavirus type 16. Virology. 1988;166(2):594–7. doi: 10.1016/0042-6822(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Zatsepina O, Braspenning J, Robberson D, Hajibagheri MA, Blight KJ, Ely S, Hibma M, Spitkovsky D, Trendelenburg M, Crawford L, Tommasino M. The human papillomavirus type 16 E7 protein is associated with the nucleolus in mammalian and yeast cells. Oncogene. 1997;14(10):1137–45. doi: 10.1038/sj.onc.1200946. [DOI] [PubMed] [Google Scholar]
- Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansen-Durr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995;69(10):6389–99. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13(11):2323–30. [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92(6):725–34. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Münger K. Expression of the Human Papillomavirus type 16 E7 oncoprotein induces an autophagy-related process and sensitizes normal human keratinocytes to cell death in response to growth factor deprivation. 2008 doi: 10.1016/j.virol.2008.12.003. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mannhardt B, Massimi P, Nauenburg S, Pim D, Nickel W, Banks L, Reuser AJ, Jansen-Durr P. Allosteric activation of acid alpha-glucosidase by the human papillomavirus E7 protein. J Biol Chem. 2000;275(13):9534–41. doi: 10.1074/jbc.275.13.9534. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96(4):1291–6. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]