Abstract
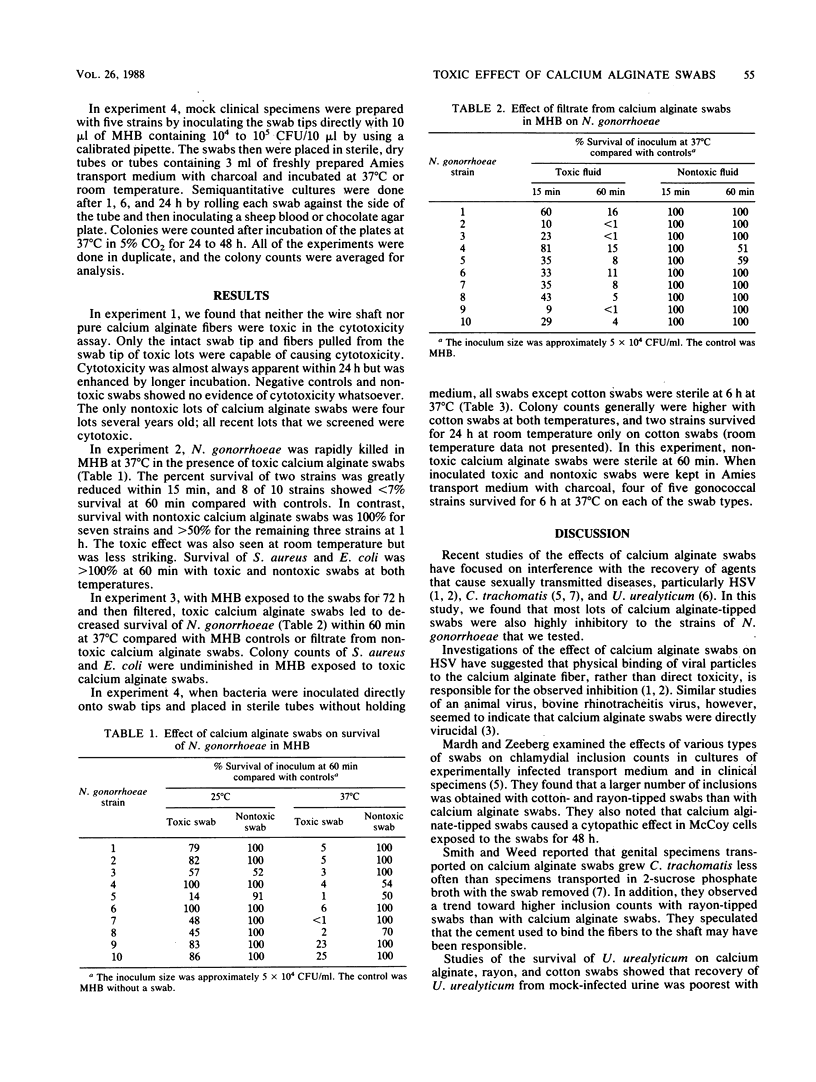
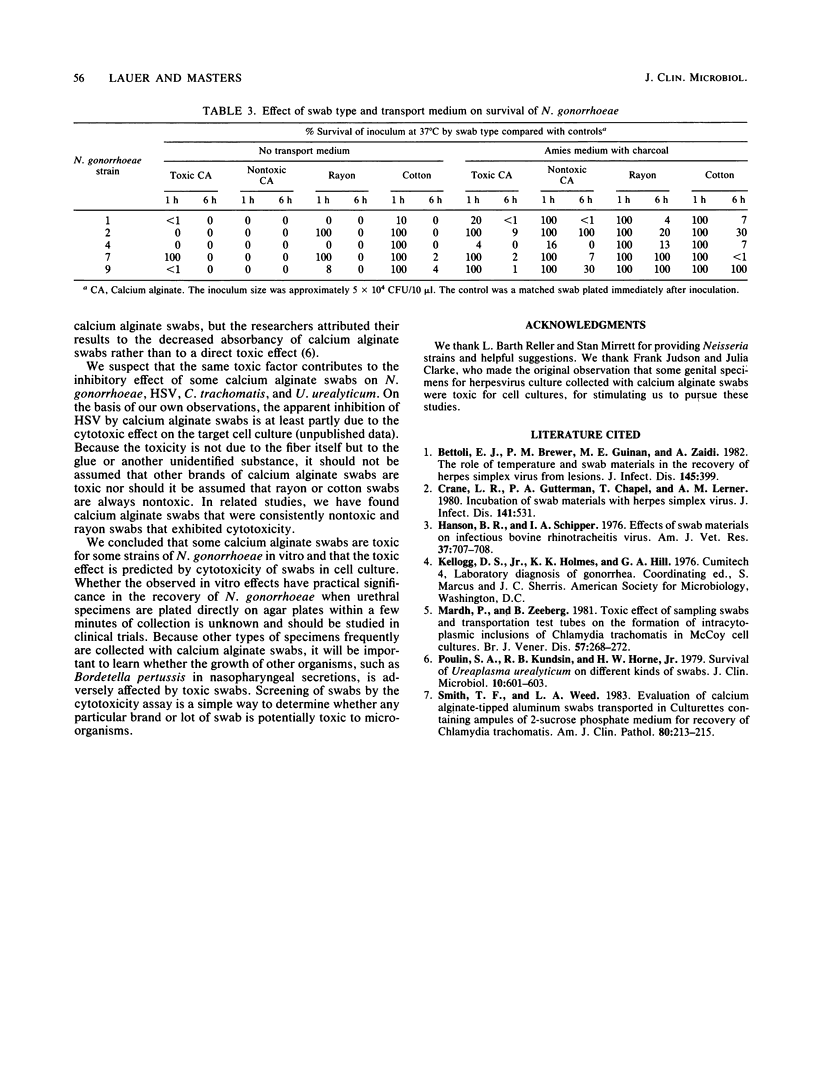
Calcium alginate (CA)-tipped swabs have been reported to interfere with the recovery of herpes simplex virus, Chlamydia trachomatis, and Ureaplasma urealyticum and may cause cytotoxicity in cell culture. To determine whether CA swabs also inhibit the growth of Neisseria gonorrhoeae, we carried out a series of experiments using either CA swabs that were toxic or nontoxic in a cell culture cytotoxicity assay or nontoxic rayon or cotton swabs. Leaving a toxic CA swab in 3 ml of Mueller-Hinton broth inoculated with 10(4) CFU/ml caused rapid killing within 6 h at 37 degrees C; colony counts of five strains were less than 1% of those of Mueller-Hinton broth controls. When the tips of toxic CA swabs were inoculated directly and kept at 37 degrees C without holding medium, the swabs were sterile at 6 h. If the same swabs were placed in Amies medium with charcoal, organisms could still be recovered at 6 h. Toxicity was less at room temperature than at 37 degrees C. Inhibition of growth of N. gonorrhoeae was not seen with rayon or cotton swabs. The toxic component was neither the CA fiber nor the aluminum wire but probably the glue used to attach the fibers. We concluded that some lots of CA swabs kill N. gonorrhoeae in vitro. Survival of N. gonorrhoeae is improved with nontoxic swabs, particularly cotton swabs, and Amies medium with charcoal regardless of swab type.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettoli E. J., Brewer P. M., Oxtoby M. J., Zaidi A. A., Guinan M. E. The role of temperature and swab materials in the recovery of herpes simplex virus from lesions. J Infect Dis. 1982 Mar;145(3):399–399. doi: 10.1093/infdis/145.3.399. [DOI] [PubMed] [Google Scholar]
- Crane L. R., Gutterman P. A., Chapel T., Lerner A. M. Incubation of swab materials with herpes simplex virus. J Infect Dis. 1980 Apr;141(4):531–531. doi: 10.1093/infdis/141.4.531. [DOI] [PubMed] [Google Scholar]
- Hanson B. R., Schipper I. A. Effects of swab materials on infectious bovine rhinotracheitis virus. Am J Vet Res. 1976 Jun;37(6):707–708. [PubMed] [Google Scholar]
- Mårdh P. A., Zeeberg B. Toxic effect of sampling swabs and transportation test tubes on the formation of intracytoplasmic inclusions of Chlamydia trachomatis in McCoy cell cultures. Br J Vener Dis. 1981 Aug;57(4):268–272. doi: 10.1136/sti.57.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin S. A., Kundsin R. B., Horne H. W., Jr Survival of Ureaplasma urealyticum on different kinds of swabs. J Clin Microbiol. 1979 Oct;10(4):601–603. doi: 10.1128/jcm.10.4.601-603.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Weed L. A. Evaluation of calcium alginate-tipped aluminum swabs transported in Culturettes containing ampules of 2-sucrose phosphate medium for recovery of Chlamydia trachomatis. Am J Clin Pathol. 1983 Aug;80(2):213–215. doi: 10.1093/ajcp/80.2.213. [DOI] [PubMed] [Google Scholar]


