Abstract
BRCA2 is closely related to the pathogenesis of breast cancer. In the present study, we found that estrogen can activate BRCA2 transcription, which is estrogen receptor (ER) α-dependent. During estrogen treatment, ERα interacted with CREB-binding protein/p300, p68/p72, and MyoD and formed an activating transcriptional complex that could bind to many Sp1 sites on the BRCA2 promoter and activate its transcription by inducing histone acetylations. MyoD is a new component of ERα complex. ERβ or p53 attenuated ERα-mediated transcriptional activation by preventing the recruitment of ERα transcriptional complex and histone acetylations on the BRCA2 promoter. ERβ interacted with ERα and CREB-binding protein/p300 and formed a weak activating transcriptional complex that competed for binding to Sp1 sites with ERα transcriptional complex and slightly attenuated BRCA2 transcription. Different from ERβ, p53 interacted with HDAC1 and CtBP1 and formed an inhibiting transcriptional complex that could compete for binding to Sp1 sites with ERα transcriptional complex and inhibit BRCA2 transcription more significantly.
Breast cancer is the second leading cause of death in American women, accounting for more than 50,000 deaths each year. The breast cancer and ovarian susceptibility genes 1 and 2 (BRCA1 and BRCA2)3 were identified based on their genetic linkage to familial early onset of breast and ovarian cancer syndromes (1–3). Mutations in the BRCA1 and BRCA2 are characterized by predisposition to familial breast and ovarian cancer. However, reduced levels of wild-type BRCA1 and BRCA2 expression have been detected in a large percentage of sporadic breast tumors in the absence of BRCA1 and BRCA2 mutations (4–6), suggesting that defects in transcriptional regulation of the BRCA1 and BRCA2 genes contribute to sporadic breast and ovarian tumorigenesis (7, 8). Detection of this transcriptional regulation in cancer cells may provide a molecular mechanistic basis for sporadic breast and ovarian tumor formation.
In addition to attenuation of BRCA1 and BRCA2 expression by mutation or promoter hypermethylation, BRCA1 and BRCA2 expression are also controlled by transcriptional factors (9). There are only a few studies about the promoter transcription of BRCA2. Nuclear factor-κB can activate the BRCA2 promoter activity (10); p53 represses the BRCA2 promoter activity and down-regulates BRCA2 mRNA and protein levels in response to DNA damage (11). So far, there is no report on how estrogen receptor (ER) α activates BRCA2 transcription during E2 treatment and how ERβ or p53 attenuate the BRCA2 transcription by competing with ERα transcriptional complex for binding to Sp1 sites on the BRCA2 promoter region upstream of the transcription start site. In this study, we investigated changes in these complexes on the BRCA2 promoter region and their effects on BRCA2 transcription.
EXPERIMENTAL PROCEDURES
Cell Lines, Culture, Plasmids, and Transfection—Human breast cancer cell lines MDA-MB-231 and MCF-7 were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. Cells were checked routinely and found to be free of contamination by Mycoplasma or fungi. All the cell lines were discarded after 3 months, and new lines were obtained from frozen stocks.
BRCA2 promoter/luciferase construct pGL3-BRCA2 (–1470 to +129) was kindly provided by Dr. Penelope Miron of the Department of Cancer Biology, Dana-Farber Cancer Institute, Harvard University. CBP and p300 expression vectors were kindly provided by Dr. Changjiang Xu of Shanghai Innovative Research Center of Traditional Chinese Medicine, Shanghai, China. ERα and ERβ expression vectors were kindly provided by Dr. Yifeng Hou in our hospital. pcDNA3.0-CtBP1 plasmid was a gift from Dr. Yang Shi of Harvard Medical School. p53 expression vector (pcDNA3.1-p53-Flag) was purchased from Shanghai GeneChem Co. Ltd. (Zhangjiang, Shanghai, China). MyoD expression vector (pCMV-MyoD) was purchased from Origene Co. (Rockville, MD).
Briefly for transient transfection, cells were seeded in 6-well plates at a density of 4 × 105 cells/well. The following day, cells were transfected with the indicated expression vector for 8 h. Following transfection, cells were maintained in RPMI 1640 medium plus 5% charcoal-stripped fetal bovine serum (FBS) and allowed to recover for 16 h. Cells were then treated in RPMI 1640 medium containing either control (ethanol vehicle) or 10 nm 17β-estradiol (E2) (Sigma) for the times indicated.
Reverse Transcription-PCR—Total RNA was extracted from cells with TRIzol reagent (Invitrogen) and quantified by UV absorbance spectroscopy. The reverse transcription reaction was performed using the Superscript First-Strand Synthesis System (Invitrogen) in a final volume of 20 μl containing 5 μg of total RNA, 200 ng of random hexamers, 1× reverse transcription buffer, 2.5 mm MgCl2, 1 mm deoxynucleotide triphosphate mixture, 10 mm dithiothreitol, RNaseOUT recombinant ribonuclease inhibitor, 50 units of superscript reverse transcriptase, and diethyl pyrocarbonate-treated water. After incubation at 42 °C for 50 min, the reverse transcription reaction was terminated by heating at 85 °C for 5 min. The newly synthesized cDNA was amplified by PCR. The reaction mixture contained 2 μl of cDNA template, 1.5 mm MgCl2, 2.5 units of Taq polymerase, and 0.5 μm BRCA2 primer (5′-TGATCCAAAGGGTCCCAAAGTTTC-3′ and 5′-TTCACAGCTTTTTGCAGAGCCTCACA-3′); glyceraldehyde-3-phosphate dehydrogenase primer (5′-GCCAAAAGGGTCATCATCTC-3′ and 5′-GTAGAGGCAGGGATGATGTTC-3′) was used as an internal control. Amplification cycles were as follows: 94 °C for 3 min, then 33 cycles at 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1.5 min followed by 72 °C for 10 min. Aliquots of PCR product were electrophoresed on 1.5% agarose gels, and PCR fragments were visualized by ethidium bromide staining.
Western Blot Analysis—Cells were washed twice with phosphate-buffered saline (PBS) containing 1 mm phenylmethylsulfonyl fluoride and lysed in mammalian protein extraction buffer (Pierce). The lysates were transferred to Eppendorf tubes and clarified by centrifugation at 12,000 × g for 40 min at 4 °C. Identical amounts (50 μg of protein) of cell lysates were resolved by SDS-PAGE. The proteins were transferred to nitrocellulose. The membranes were incubated in blocking solution consisting of 5% powered milk in PBST (PBS plus 0.1% Tween 20) at room temperature for 1 h and then immunoblotted with BRCA2 (Millipore Corp., Billerica, MA), ERβ, p53, MyoD (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or tubulin (Sigma-Aldrich) antibodies, respectively. Detection by enzyme-linked chemiluminescence was performed according to the manufacturer's protocol (ECL, Amersham Biosciences).
ChIP Assay and ChIP-ReChIP—ChIP assays were carried out according to the manufacturer's protocol (Active Motif, Carlsbad, CA). Briefly cells in 150-mm tissue culture dishes were fixed with 1% formaldehyde and incubated for 10 min at 37 °C. The cells were then washed twice with ice-cold PBS, harvested, and resuspended in ice-cold TNT lysis buffer (20 mm Tris-HCl, pH 7.4, 200 mm NaCl, 1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 1% aprotinin). The lysates were sonicated to shear the DNA to fragments of 200–600 bp and subjected to immunoprecipitation with the following antibodies, respectively, CBP (Chemicon, Rosemont, IL), Sp1, ERα, ERβ, p53, MyoD (Santa Cruz Biotechnology, Inc.), acetylated histone H3 (Abcam Inc., Cambridge, MA), histone deacetylase (HDAC) 1, p300, CtBP1, acetylated histone H2A, acetylated histone H2B, acetylated histone H4 (Millipore Corp.), or IgG (Santa Cruz Biotechnology, Inc.) as negative control. 3 μg of antibody was used for each immunoprecipitation. The antibody·protein complexes were collected by Protein G beads and washed three times with ChIP wash buffer (5% SDS, 1 mm EDTA, 0.5% bovine serum albumin, 40 mm NaHPO4, pH 7.2). The immune complexes were eluted with 1% SDS and 1 m NaHCO3, and the cross-links were reversed by incubation at 65 °C for 4 h in the presence of 200 mm NaCl and RNase A. The samples were then treated with proteinase K for 2 h, and DNA was purified by minicolumn, ethanol-precipitated, and resuspended in 100 ml of H2O. The primers corresponding to the BRCA2 promoter region –191 and +30 upstream of the transcription start site (sense, 5′-AGGGTCAGCGAGAAGA-3′; and antisense, 5′-CTGCCGCCTAGTTTCA-3′) (221 bp) were used for PCR to detect the presence of the BRCA2 promoter DNA. As negative controls, we tested for the recruitment of ERα, CBP, p300, and MyoD at exon 7 of the BRCA2 gene using the primers (sense, 5′-AGCATTCTGCCTCATACAGG-3′; and antisense, 5′-TCAACCTCATCTGCTCTTTCTT-3′) (284 bp).
In brief, for ChIP-reChIP assay after sonication, chromatin was incubated overnight with 5 μg of ERα, ERβ, or p53 antibody, respectively, or IgG as negative control. After several washings, the beads were incubated with 50 μl of buffer containing 0.5% SDS and 0.1 m NaHCO3 for 10 min at 65 °C. The supernatant was collected after spinning; diluted with 1 mm EDTA, 150 mm NaCl, 50 mm HEPES, pH 7.5, 0.1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate; and incubated with 3 μg of the CBP, p300, MyoD, Sp1, HDAC1, or CtBP1 antibody, respectively, overnight. After washing, protein·DNA complexes were eluted from beads and treated with proteinase K overnight. DNA was purified with a minicolumn, and the DNA binding to the BRCA2 endogenous promoter was quantified by PCR using the primers described above.
siRNA and Transfection—MyoD siRNA and non-targeting siRNA were purchased from Santa Cruz Biotechnology, Inc. Cells in exponential phase of growth were plated in 6-well plates at 5 × 105 cells/well, grown for 24 h, and then transfected with MyoD-targeted siRNA or non-targeting siRNA at a final concentration of 100 nm using Oligofectamine and Opti-MEM I reduced serum medium (Invitrogen) according to the manufacturer's protocol. Silencing was examined 48 h after transfection.
Luciferase Reporter Gene Assay—In these experiments, MDA-MB-231 or MCF-7 cells were seeded in 6-well plates at a density of 1–2 × 105 cells/well and cultured for 24 h. Cells were then transfected with the BRCA2 promoter/luciferase construct (0.5 μg/well) or co-transfected with 0.5 μg of pcDNA3.0, ERα, CBP, p300, MyoD, ERβ, p53, or CtBP1 expression vector, respectively, together with 20 ng of control Renilla luciferase reporter construct pRL-TK (Promega, Madison, WI). The total amount of DNA per well was adjusted to 1.5 μg by the addition of sonicated salmon sperm DNA. Luciferase assays were performed as recommended by the vendor (Promega) and normalized relative to protein concentration as determined by the bicinchoninic acid protein assay (Pierce). The promoter activity was then expressed as luminescence units, which was the ratio of luminescence counts of cell lysate and the absorbance at 595 nm for the same amount of cell lysate stained with bicinchoninic acid protein assay reagent.
Mutagenesis—BRCA2 promoter/luciferase construct (–1470 to +129) was used as template. Plasmid DNA was methylated with DNA methylase at 37 °C for 1 h. The plasmid was amplified in a mutagenesis reaction with two overlapping primers, one of which contained the target mutation. The product was linear, double-stranded DNA containing the mutation. The mutagenesis mixture was transformed into wild-type Escherichia coli. The host cell circularized the linear mutated DNA, and McrBC endonuclease in the host cell digested the methylated template DNA, leaving only unmethylated, mutated product. For individual mutations, the sequences of Sp1-binding sites were mutated as follows: CGCGGG (sp1A site, –9 to –4) was converted to GAGAAA, GGGTGG (sp1B site, –55 to –50) was converted to AAAGAG, and CCCACCC (sp1C site, –75 to –69) was converted to CCCATTC.
In Vivo Co-immunoprecipitation (co-IP) Assays—Approximately 1 × 107 exponentially growing MCF-7 cells or the ERα-, ERβ-, MyoD-, p53-, or CtBP1-transfected MCF-7 cells were harvested 24 h later, washed in cold PBS, and resuspended in 1 ml of lysis buffer (1% Nonidet P-40, 150 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml pepstatin A, 50 mm Tris-HCl, pH 8.0). The cell lysate (2 mg of protein) was incubated on ice for 20 min and clarified by centrifugation. ERα, ERβ, MyoD, p53, or CtBP1 antibodies were added to achieve a 5 μg/ml final concentration, respectively, and incubation was continued for 1 h at 4 °C. To precipitate the immune complexes, 50 μl of Protein G-Sepharose 4 Fast Flow beads (Amersham Biosciences) was added to the lysate with incubation for 1 h at 4 °C. The immune complexes were pelleted by centrifugation; washed extensively with lysis buffer and a final wash with 50 mm Tris-HCl, pH 8.0; and resuspended in SDS sample buffer for analysis by Western blot. To detect proteins in the immune complexes, the following antibodies were used respectively in Western blot: CBP (Chemicon), Sp1, ERα, ERβ, p53, p68, MyoD, CtBP1, CtBP2 (Santa Cruz Biotechnology, Inc.), HDAC1, p300, CtBP1 (Millipore Corp.), and p72 (Novus Biologicals, Inc., Littleton, CO).
Statistical Analysis—Data shown represent means ± S.D. Statistical analyses for detection of significant differences between the control and experimental groups were carried out using Student's t test.
RESULTS
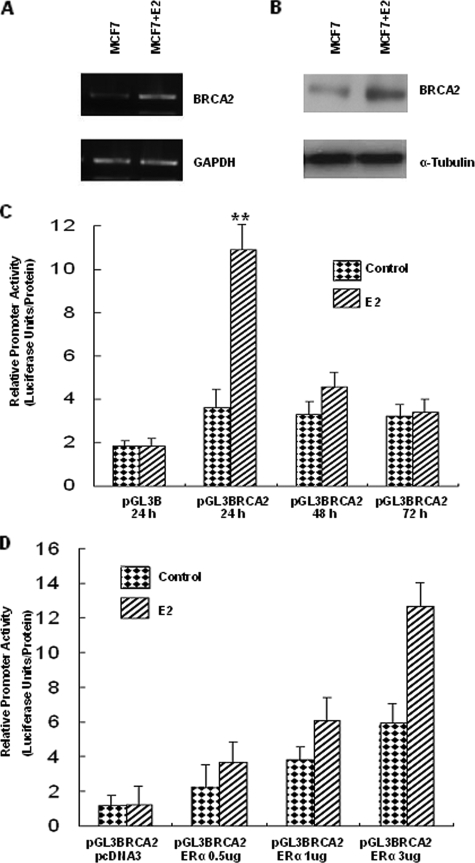
Estrogen Activates BRCA2 Promoter Activity—To test whether E2 could induce BRCA2 expression, MCF-7 cells were precultured for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS (Hyclone Laboratories, Logan, UT), and then MCF-7 cells were cultured for 24 h in RPMI 1640 medium plus 10 nm E2. Fig. 1A shows that, as compared with control MCF-7 cells, the level of the endogenous BRCA2 mRNA in the cells with E2 treatment at 24 h increased as determined by reverse transcription-PCR. Fig. 1B shows that, as compared with control MCF-7 cells, the level of the endogenous BRCA2 protein in the cells with E2 treatment at 24 h increased as determined by Western blot.
FIGURE 1.
E2 induces BRCA2 expression in MCF-7 cells. A and B, E2 induces BRCA2 mRNA and protein levels in MCF-7 cells. MCF-7 cells were precultured for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS, and then MCF-7 cells were cultured for 24 h in RPMI 1640 medium plus 10 nm E2. A, mRNA expression levels of BRCA2. B, protein expression levels of BRCA2. C, E2 induces BRCA2 promoter activity in transiently transfected MCF-7 cells. MCF-7 cells were precultured for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS, and then BRCA2 promoter construct (pGL3-BRCA2) was transiently transfected into MCF-7 cells, and cells were cultured for 24, 48, or 72 h in RPMI 1640 medium or RPMI 1640 medium plus 10 nm E2. Luciferase activity in the cells treated with E2 was compared with that in the control cells. **, p < 0.01. D, E2 induces BRCA2 promoter activity with the help of ERα in transiently transfected MDA-MB-231 cells. MDA-MB-231 cancer cells were co-transfected with pGL3-BRCA2 with ERα expression vector or an empty vector (pcDNA3). Transfected cells were cultured for 24 h in RPMI 1640 medium or RPMI 1640 medium plus 10 nm E2. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In transient transfection assays with ERα-positive MCF-7 breast cancer cells, we found that E2 stimulated BRCA2 promoter activity at the 24-h time point significantly (Fig. 1C). In transient transfection assays with ERα-negative MDA-MB-231 breast cancer cells, we found that E2 could not stimulate BRCA2 promoter activity at the 24-h time point. However, BRCA2 transcription became responsive to E2 following co-transfection with various amounts of ERα expression vector (Fig. 1D).
Estrogen Stimulates Histone Acetylations and the Recruitment of ERα, CBP/p300, MyoD, and Sp1 on the BRCA2 Promoter Region—In this study, we investigated whether or not E2 stimulated the recruitment of some transcriptional factors to the BRCA2 promoter. ChIP experiments showed that E2 stimulated the recruitment of ERα to the BRCA2 promoter. This was accompanied by the recruitment of CBP/p300, MyoD, and Sp1 to the BRCA2 promoter (Fig. 2A). Control experiments indicated that the co-incubation of cross-linked chromatin with preimmune IgG did not generate a corresponding BRCA2 amplification product. Neither did E2 stimulate the recruitment of ERα, CBP/p300, or MyoD to the coding region of exon 7 in the BRCA2 gene (Fig. 2B).
FIGURE 2.
ERα, CBP/p300, and MyoD increase BRCA2 promoter activity in MCF-7 cells during E2 treatment. A, binding of ERα, CBP/p300, MyoD, and Sp1 to the BRCA2 promoter in MCF-7 cells with E2 treatment. MCF-7 cells were precultured for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS, and then MCF-7 cells were cultured for 24 h in RPMI 1640 medium plus 10 nm E2. Nucleic extracts were prepared from MCF-7 cells or MCF-7 cells treated with 10 nm E2. ChIP assays were performed using antibody against ERα, CBP, p300, MyoD, and Sp1, respectively, as described under “Experimental Procedures.” The primers corresponding to the BRCA2 promoter region –191 and +30 upstream of the transcriptional start site were used for PCR to detect the presence of the BRCA2 promoter DNA. Con, control. B, ERα, CBP/p300, and MyoD cannot bind to exon 7 in the BRCA2 gene in MCF-7 cells with E2 treatment. Con, control. ChIP assays were performed using antibody against ERα, CBP, p300, and MyoD, respectively, as described above. The primers correspond to the BRCA2 exon 7 region. C, detection of ERα complex on the BRCA2 promoter. A ChIP-reChIP assay was performed in MCF-7 cells with E2 treatment. Chromatin was incubated with ERα antibody and then immunoprecipitated sequentially with CBP, p300, MyoD, or Sp1 antibody. The BRCA2 promoter DNA bound to ERα/CBP, ERα/p300, ERα/MyoD, or ERα/Sp1 was amplified by PCR. Con, control. D, histone modifications on the BRCA2 promoter. Nucleic extracts were prepared from MCF-7 cells or MCF-7 cells treated with E2. ChIP assays were performed using antibody against acetyl-H2A, acetyl-H2B, acetyl-H3, and acetyl-H4, respectively, as described under “Experimental Procedures.” Con, control. E, activation of the BRCA2 promoter by ERα, CBP, p300, and MyoD. MCF-7 cells were plated in 6-well tissue culture plates and then co-transfected with 0.5 μg of pGL3-BRCA2 with 0.5 μg of ERα, CBP, p300, or MyoD expression vector or pcDNA3 control vector. Renilla luciferase reporter construct pRL-TK (20 ng) was used as an internal control for transfection efficiency. Forty hours after transfection, luciferase activity was measured with equivalent amounts of protein extracts. Luciferase activity of the BRCA2 promoter was normalized to the activity of a co-transfected Renilla luciferase expression vector and protein content. Luciferase activity in the cells transfected with ERα, CBP, p300, or MyoD expression vector was compared with that in the cells transfected with pcDNA3 control vector. **, p < 0.01. F, effect of siRNA on MyoD expression in MCF-7 cells. MCF-7 cells were transfected with control siRNA or MyoD siRNA for 48 h, and then Western blot was performed. G, reduction of MyoD expression by siRNA attenuates the E2-induced BRCA2 promoter activity in MCF-7 cells. BRCA2 promoter construct (pGL3-BRCA2) was transiently co-transfected with MyoD siRNA or non-targeting siRNA (Control siRNA) into MCF-7 cells, cells were cultured for 24 h and then exposed to 10 nm E2 for 24 h, and luciferase activity was detected. Luciferase activity in the cells treated with MyoD siRNA was compared with that in the cells treated with non-targeting siRNA. *, p < 0.05.
To demonstrate that ERα was recruited to the BRCA2 promoter through interaction with CBP, p300, MyoD, and Sp1, we performed ChIP-reChIP assays. Cross-linked and fragmented chromatin was prepared from MCF-7 cells and sequentially subjected to the first step ChIP with ERα antibody and the second step immunoprecipitation with CBP, p300, MyoD, and Sp1 antibody, respectively. As shown in Fig. 2C, the two-step ChIP-reChIP successfully precipitated the BRCA2 promoter, indicating that ERα, CBP/p300, MyoD, and Sp1 formed a protein complex on the BRCA2 promoter.
In the above experiments, we detected that CBP/p300 and ERα formed a complex and bound to the BRCA2 promoter. Because CBP and p300 are histone acetyltransferases, next we investigated whether treatment of E2 might affect histone acetylation on the BRCA2 promoter. ChIPs with antibodies against acetyl-H2A, acetyl-H2B, acetyl-H3, and acetyl-H4 were performed between E2-treated MCF-7 cells and untreated control MCF-7 cells. In cells treated by E2, increases of acetyl-H2A, acetyl-H2B, acetyl-H3, and acetyl-H4 were found (Fig. 2D). These results indicated that CBP and p300 bound to the BRCA2 promoter and that CBP/p300 might induce specific changes of histone acetylation levels on the BRCA2 promoter during E2 treatment.
To identify the roles of ERα, p300, CBP, and MyoD in regulating BRCA2 promoter transcription, we co-transfected the BRCA2 promoter/luciferase construct with ERα, CBP, p300, or MyoD expression vector, respectively. Fig. 2E shows that the luciferase activity was enhanced significantly by ERα, p300, CBP, and MyoD, further indicating that ERα, p300, CBP, and MyoD were involved in the activation of the BRCA2 promoter during E2 treatment.
To further analyze the role of MyoD in BRCA2 transcription, we knocked down the expression of MyoD with siRNA and determined whether inhibition of MyoD expression would influence BRCA2 transcription. Fig. 2F shows that the expression of MyoD was significantly inhibited by MyoD siRNA. Fig. 2G shows that inhibition of MyoD expression by siRNA attenuated the E2-induced activation of BRCA2 promoter activity in MCF-7 cells.
Overexpression of ERβ or p53 Attenuates BRCA2 Expression and the Recruitment of ERα, CBP/p300, MyoD, and Histone Acetylations on the BRCA2 Promoter Region Induced by E2—To test the effect of ERβ or p53 on BRCA2 expression, we precultured MCF-7 cells for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS (Hyclone Laboratories), and then transfected ERβ, p53, or pcDNA3 vector, respectively, into the MCF-7 cells. Cells were cultured for 6 h, and then the medium was replaced with RPMI 1640 medium plus 10 nm E2 for 24 h. Western blot analysis demonstrated that overexpression of ERβ or p53 in MCF-7 cells reduced the expression level of BRCA2 induced by E2 compared with MCF-7 cells transfected with the empty plasmid pcDNA3. p53 reduced BRCA2 expression more significantly than did ERβ (Fig. 3A).
FIGURE 3.
Overexpression of ERβ or p53 attenuates BRCA2 expression and the recruitment of ERα, CBP/p300, MyoD, and histone acetylations on the BRCA2 promoter region induced by E2. A, overexpression of ERβ or p53 in MCF-7 cells attenuates BRCA2 expression induced by E2. MCF-7 cells were precultured for 4 days in phenol red-free RPMI 1640 medium containing 5% charcoal dextran-stripped FBS, then ERβ or p53 expression vector was transiently transfected into MCF-7 cells, and cells were cultured for 6 h. Then the medium was replaced with RPMI 1640 medium plus 10 nm E2 for 24 h, and Western blot analysis was performed. B, ERα increases BRCA2 promoter activity induced by E2, and ERβ or p53 attenuates BRCA2 promoter activity induced by E2. MCF-7 cells were precultured as in A. Then BRCA2 promoter construct (pGL3-BRCA2) was transiently co-transfected with ERα, ERβ, or p53 expression vector or pcDNA3 control vector, respectively, into MCF-7 cells, and cells were cultured for 24 h in RPMI 1640 medium or RPMI 1640 medium plus 10 nm E2. Luciferase activity in the cells transfected with ERα, ERβ, or p53 expression vector was compared with that in the cells transfected with pcDNA3 control vector. *, p < 0.05; **, p < 0.01. C, ERβ or p53 attenuates the recruitment of ERα, p300, CBP, and MyoD to the BRCA2 promoter region stimulated by E2. MCF-7 cells were precultured as in A, and then ERβ or p53 expression vector was transiently transfected with into MCF-7 cells. Cells were cultured for 24 h in RPMI 1640 medium or RPMI 1640 medium plus 10 nm E2. ChIP assays were performed using antibody against ERα, p300, CBP, and MyoD as described under “Experimental Procedures.” Con, pcDNA3. D, ERβ or p53 attenuates histone acetylations on the BRCA2 promoter region stimulated by E2. MCF-7 cells were treated as in C. ChIP assays were performed using antibody against acetylated histones H2A, H2B, H3, and H4 as described under “Experimental Procedures.” Con, pcDNA3. E, ERα or ERβ increases BRCA2 promoter activity, and p53 represses BRCA2 promoter activity. MCF-7 cells were precultured as in A, and then BRCA2 promoter construct (pGL3-BRCA2) was transiently co-transfected with ERα, ERβ, or p53 expression vector or pcDNA3 control vector, respectively, into MCF-7 cells. Cells were cultured for 24 h. Luciferase activity in the cells transfected with ERα, ERβ, or p53 expression vector was compared with that in the cells transfected with pcDNA3 control vector. **, p < 0.01.
In transient transfection experiments with MCF-7 cells, we found that E2-induced BRCA2 promoter activity was attenuated following co-transfection with ERβ or wild-type p53 expression vector. p53 reduced BRCA2 promoter activity more significantly than did ERβ (Fig. 3B).
As seen in Fig. 3C, ChIP experiments showed that both ERβ and p53 could attenuate the recruitment of ERα, CBP/p300, and MyoD to the BRCA2 promoter region stimulated by E2. Different from ERβ, p53 repressed the recruitment of ERα, CBP/p300, and MyoD to the BRCA2 promoter region more significantly.
ChIP experiments also showed that both ERβ and p53 attenuated histone acetylations of H2A, H2B, H3, and H4 on the BRCA2 promoter region stimulated by E2. Different from ERβ, p53 repressed histone acetylations more significantly (Fig. 3D). In transient transfection experiments with MCF-7 cells, we found that both ERα and ERβ increased BRCA2 promoter activity, but ERβ only increased BRCA2 promoter activity slightly compared with ERα, whereas p53 repressed BRCA2 promoter activity significantly (Fig. 3E).
Detection of ERβ or p53 Complex on the BRCA2 Promoter—To determine the potential ERβ transcriptional complex, exponentially growing MCF-7 cells were transfected with ERβ expression vector. ChIP assays demonstrated that ERβ could bind directly to the BRCA2 promoter (Fig. 4A). ChIP-reChIP assays demonstrated that ERβ could interact with ERα and form a heterodimer on the BRCA2 promoter; at the same time, ERβ could interact with CBP/p300 and form a protein complex on the BRCA2 promoter, but ERβ could not interact with MyoD on the BRCA2 promoter (Fig. 4B).
FIGURE 4.
Detection of ERβ or p53 complex on the BRCA2 promoter. A, ERβ binding to the BRCA2 promoter in MCF-7 cells transfected with ERβ expression vector. MCF-7 cells were precultured as in Fig. 3A, then ERβ expression vector was transiently transfected into MCF-7 cells, and cells were cultured for 24 h. ChIP assays were performed using antibody against ERβ as described under “Experimental Procedures.” Con, pcDNA3. B, detection of ERβ complex on the BRCA2 promoter. A ChIP-reChIP assay was performed in MCF-7 cells transfected with ERβ vector. Chromatin was incubated with ERβ antibody and then immunoprecipitated sequentially with ERα, CBP, p300, and MyoD antibodies. The BRCA2 promoter DNA bound to ERα/ERβ, ERβ/CBP, ERβ/p300, or ERβ/MyoD was amplified by PCR. Con, pcDNA3. C, binding of p53, HDAC1, and CtBP1 to the BRCA2 promoter in MCF-7 cells transfected with p53 vector. MCF-7 cells were precultured as in Fig. 3A, then p53 expression vector was transiently transfected into MCF-7 cells, and cells were cultured for 24 h. ChIP assays were performed using antibody against p53, HDAC1, and CtBP1 as described under “Experimental Procedures.” Con, pcDNA3. D, detection of p53 complex on the BRCA2 promoter. A ChIP-reChIP assay was performed in MCF-7 cells transfected with p53 vector. Chromatin was incubated with p53 antibody and then immunoprecipitated sequentially with HDAC1 and CtBP1 antibodies. The BRCA2 promoter DNA bound to p53/HDAC1 and p53/CtBP1 was amplified by PCR. Con, pcDNA3. E, p53 and CtBP1 synergistically represses BRCA2 promoter activity. MCF-7 cells were precultured as in Fig. 3A, then BRCA2 promoter construct (pGL3-BRCA2) was transiently co-transfected with p53 or CtBP1 expression vector or pcDNA3 control vector into MCF-7 cells, and cells were cultured for 24 h. Luciferase activity in the cells transfected with p53 or CtBP1 expression vector was compared with that in the cells transfected with pcDNA3 control vector. *, p < 0.05; **, p < 0.01.
To determine the potential p53 transcriptional complex, exponentially growing MCF-7 cells were transfected with p53 expression vector. ChIP assays demonstrated that p53 could bind directly to the BRCA2 promoter; this was accompanied by recruitment of HDAC1 and CtBP1 to the BRCA2 promoter (Fig. 4C). ChIP-reChIP assays demonstrated that p53 could interact with HDAC1 and CtBP1 and form a protein complex on the BRCA2 promoter (Fig. 4D). These results indicated that p53 might recruit HDAC1 and CtBP1 and form an inhibiting transcriptional complex on the BRCA2 promoter.
To test whether HDAC1 or CtBP1 was involved in the p53-induced repression of BRCA2 promoter activity, we chose CtBP1 and tested its role in regulating BRCA2 promoter activity. We transfected p53 and CtBP1 expression vectors into MCF-7 cells and found that both p53 and CtBP1 could inhibit BRCA2 promoter activity. p53 and CtBP1 could synergistically repress BRCA2 promoter activity more significantly (Fig. 4E).
Detection of ERα, ERβ, MyoD, p53, and CtBP1 Transcriptional Complex in Vivo—To confirm the potential ERα transcriptional complex, MCF-7 cells were transfected with ERα expression vector. ERα was immunoprecipitated with anti-ERα antibody, and the immunoprecipitated proteins were separated by electrophoresis and analyzed by Western blot. As shown in Fig. 5A, CBP, p300, and MyoD were co-precipitated by anti-ERα antibody in extracts of cells transfected with ERα, indicating that ERα interacted with CBP/p300 and MyoD. The results also showed that Sp1 was co-precipitated by anti-ERα antibody, indicating that ERα complex might bind to the Sp1 site. But ERβ was not co-precipitated by anti-ERα antibody.
FIGURE 5.
Detection of ERα, ERβ, MyoD, p53, or CtBP1 transcriptional complex, respectively, by co-IP assays. The process was described under “Experimental Procedures.” A, cell lysates extracted from ERα-overexpressing MCF-7 cells were subjected to immunoprecipitation with IgG or ERα antibody followed by immunoblotting with ERα, CBP, p300, MyoD, Sp1, or ERβ antibody, respectively. B, cell lysates extracted from ERβ-overexpressing MCF-7 cells were subjected to immunoprecipitation with IgG or ERβ antibody followed by immunoblotting with ERα, CBP, p300, MyoD, Sp1, or ERβ antibody, respectively. C, cell lysates extracted from MyoD-overexpressing MCF-7 cells were subjected to immunoprecipitation with IgG or MyoD antibody followed by immunoblotting with MyoD, p68, p72, ERα, or ERβ antibody, respectively. D, cell lysates extracted from p53-overexpressing MCF-7 cells were subjected to immunoprecipitation with IgG or p53 antibody followed by immunoblotting with HDAC1, CtBP1, Sp1, or p53 antibody, respectively. E, cell lysates extracted from CtBP1-overexpressing MCF-7 cells were subjected to immunoprecipitation with IgG or CtBP1 antibody followed by immunoblotting with HDAC1, p53, CtBP1, and CtBP2 (Santa Cruz Biotechnology, Inc.) antibodies, respectively.
To confirm the potential ERβ transcriptional complex, MCF-7 cells were transfected with ERβ expression vector. ERβ was immunoprecipitated with anti-ERβ antibody. As shown in Fig. 5B, CBP/p300 was co-precipitated by anti-ERβ antibody in extracts of cells transfected with ERβ, indicating that ERβ interacted with CBP/p300 in vivo. MyoD was not co-precipitated by anti-ERβ antibody, indicating that ERβ could not interact with MyoD. ERα was co-precipitated by anti-ERβ antibody, indicating that ERα and ERβ could form a heterodimer. The results also showed that Sp1 was co-precipitated by anti-ERβ antibody, indicating that ERα/ERβ heterodimer might bind to the Sp1 site.
To determine the potential MyoD transcriptional complex, MCF-7 cells were transfected with MyoD expression vector. MyoD was immunoprecipitated with anti-MyoD antibody. As shown in Fig. 5C, ERα, p68, and p72 were co-precipitated by anti-MyoD antibody in extracts of cells transfected with MyoD, indicating that MyoD interacted with ERα, p68, and p72. ERβ was not co-precipitated by anti-MyoD antibody, indicating that ERβ could not interact with MyoD in vivo.
To confirm the potential p53 transcriptional complex, MCF-7 cells were transfected with p53 expression vector. p53 was immunoprecipitated with anti-p53 antibody. As shown in Fig. 5D, HDAC1 and CtBP1 were co-precipitated by anti-p53 antibody, indicating that p53 interacted with HDAC1 and CtBP1. The results also showed that Sp1 was co-precipitated by anti-p53 antibody, indicating that p53 complex might bind to the Sp1 site.
To determine the potential CtBP1 transcriptional complex, MCF-7 cells were transfected with CtBP1 expression vector. CtBP1 was immunoprecipitated with anti-CtBP1 antibody. As shown in Fig. 5E, HDAC1, p53, and CtBP2 were co-precipitated by anti-CtBP1 antibody, indicating that CtBP1 interacted with HDAC1, p53, and CtBP2.
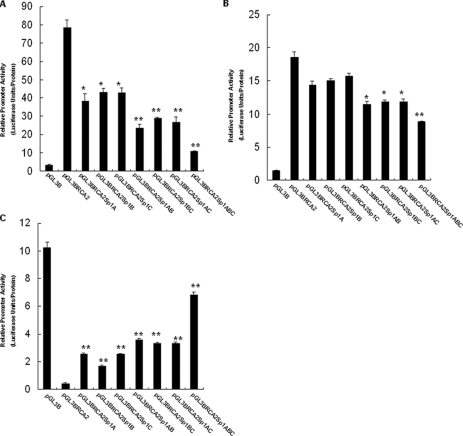
Many Sp1-binding Sites on the BRCA2 Promoter Region Appear to Be Important for ERα-, ERβ-induced Transcription Activation or p53-induced Transcription Inhibition—In all, we found eight Sp1 sites from –462 to +1 bp on the BRCA2 promoter as shown in Table 1. To determine the potential roles of these Sp1 elements in regulation of BRCA2 gene transcription, we individually and combinatorially mutated the three Sp1 sites (Sp1A, Sp1B, and Sp1C) close to the transcriptional start site on the BRCA2 promoter and examined ERα-, ERβ-, or p53-induced BRCA2 promoter activity in MCF-7 cells.
TABLE 1.
Sp1 sites on the BRCA2 promoter
|
No.
|
BRCA2
|
|
|---|---|---|
| Region | Sp1 | |
| bp | ||
| 1 | -4 to -9 | CGCGGG |
| 2 | -50 to -55 | GGGTGG |
| 3 | -69 to -75 | CCCACCC |
| 4 | -80 to -85 | CCCGCA |
| 5 | -205 to -210 | GGGTGG |
| 6 | -220 to -228 | GGGCGGCCC |
| 7 | -287 to -292 | GGGCGC |
| 8 | -441 to -446 | CCCGCC |
As shown in Fig. 6, A and B, relative to the control wild-type BRCA2 promoter construct, when co-transfected with ERα or ERβ, mutation of any one of the three Sp1 sites resulted in low levels of decreased BRCA2 promoter activity. Mutations of any two Sp1 sites simultaneously caused a further decrease of BRCA2 promoter activity, and the combined mutations of all three Sp1 sites resulted in maximal levels of decreased BRCA2 promoter activity. These results suggested that all three Sp1 sites contributed in a concerted mechanism to the ERα- or ERβ-induced transcription of BRCA2 gene and that ERα or ERβ activated BRCA2 transcription by binding to a number of Sp1 sites on the BRCA2 promoter.
FIGURE 6.
Many Sp1-binding sites on the BRCA2 promoter region appear to be important for ERα-, ERβ-induced transcription activation or p53-induced transcription inhibition. A, effects of mutations of Sp1-binding sites on ERα-induced BRCA2 promoter activity in MCF-7 cells. Single or combined mutations of Sp1A, Sp1B, and Sp1C sites were separately made in pGL3-BRCA2 as described under “Experimental Procedures.” Cells were co-transfected in duplicate with either the wild-type pGL3-BRCA2 plasmid or one of the mutant pGL3-BRCA2 constructs with ERα expression vector and then incubated for 40 h. Luciferase assays were performed as described under “Experimental Procedures.” Luciferase activity in the cells transfected with the mutant pGL3-BRCA2 constructs was compared with that in the cells transfected with the wild-type pGL3-BRCA2 plasmid. *, p < 0.05; **, p < 0.01. B, effects of mutations of Sp1-binding sites on ERβ-induced BRCA2 promoter activity in MCF-7 cells. Cells were co-transfected in duplicate with either the wild-type pGL3-BRCA2 plasmid or one of the mutant pGL3-BRCA2 constructs with ERβ expression vector and then incubated for 40 h. Luciferase assays were performed as described under “Experimental Procedures.” Luciferase activity in the cells transfected with the mutant pGL3-BRCA2 constructs was compared with that in the cells transfected with the wild-type pGL3-BRCA2 plasmid. *, p < 0.05; ** p < 0.01. C, effects of mutations of Sp1-binding sites on p53-induced BRCA2 promoter activity in MCF-7 cells. Cells were co-transfected in duplicate with either the wild-type pGL3-BRCA2 plasmid or one of the mutant pGL3-BRCA2 constructs with p53 expression vector and then incubated for 40 h. Luciferase assays were performed as described under “Experimental Procedures.” Luciferase activity in the cells transfected with the mutant pGL3-BRCA2 constructs was compared with that in the cells transfected with the wild-type pGL3-BRCA2 plasmid. *, p < 0.05; **, p < 0.01.
As shown in Fig. 6C, relative to the control wild-type BRCA2 promoter construct, when co-transfected with p53, mutation of any one of the three Sp1 sites resulted in low levels of increased BRCA2 promoter activity. Mutations of any two Sp1 sites simultaneously caused a further increase of BRCA2 promoter activity, and the combined mutations of all three Sp1 sites resulted in maximal levels of increased BRCA2 promoter activity. These results suggested that all three Sp1 sites contributed in a concerted mechanism to the p53-reduced transcription of BRCA2 gene and that p53 inhibited BRCA2 transcription by binding to a number of Sp1 sites on the BRCA2 promoter.
DISCUSSION
Estrogens influence the pathological processes of hormone-dependent diseases, such as breast, endometrial, and ovarian cancers as well as osteoporosis (12). The biological actions of estrogens are mediated by binding to one of two specific ERs, ERα or ERβ, which belong to the nuclear receptor superfamily, a family of ligand-regulated transcription factors (13).
It is reported that E2 can elevate BRCA2 mRNA levels in BT-483 and MCF-7 breast cancer cell lines (14). So we detected the potential transcriptional complex influencing the BRCA2 promoter activity during E2 treatment. In this study, we found that E2 could elevate BRCA2 mRNA and protein levels in human MCF-7 breast cancer cells. We also found that E2 induced BRCA2 promoter activity in ERα-positive MCF-7 breast cancer cells but E2 could not induce BRCA2 promoter activity in ERα-negative MDA-MB-231 breast cancer cells. However, BRCA2 transcription became responsive to E2 following co-transfection with various amounts of ERα expression vector in MDA-MB-231 cells. These results indicated that ERα was required and essential in the E2-dependent regulation of BRCA2 transcription.
ChIP and ChIP-reChIP assays demonstrated that ERα could interact with p300, CBP, and MyoD and form an activating transcriptional complex on the BRCA2 promoter during E2 treatment; this was confirmed by co-IP assays in vivo. ERα, p300, CBP, and MyoD activated BRCA2 promoter activity, further indicating that ERα, p300, CBP, and MyoD were synergistically involved in the activation of the BRCA2 promoter during E2 treatment.
In this study, we found that MyoD is a new component of ERα complex. Overexpression of MyoD increased BRCA2 promoter activity, and inhibition of MyoD expression by siRNA attenuated the E2-induced activation of BRCA2 promoter, indicating that MyoD played an important role in activating BRCA2 transcription. ChIP-reChIP assays demonstrated that ERα could interact with MyoD on the BRCA2 promoter, but ERβ could not interact with MyoD on the BRCA2 promoter; co-IP assays confirmed that MyoD could interact with ERα but not ERβ in vivo. Co-IP assays also demonstrated that MyoD could interact with p68/p72 and ERα. It is reported that p68/p72 directly bind the SRC-1/TIF2 family proteins and ERα but not ERβ (15, 16), and MyoD interacts with p72/p68 (17). Based on these reports and our experiments, we concluded that MyoD is a new component of ERα complex and involved in E2-induced BRCA2 transcription. p68/p72 could interact with MyoD and form a part of the ERα transcriptional complex.
The best studied histone modification involved in transcriptional activation is acetylation. Levels of acetylated histones have been correlated with the transcription status of many genes. Transcriptionally active chromatin regions of the genome are often associated with hyperacetylated histones, whereas transcriptionally silent regions are associated with hypoacetylated histones. CBP and p300 are histone acetyltransferases and key regulators in the assembly and mobilization of the basal transcription machinery. CBP and p300 are identified as transcriptional co-activators, and they catalyze acetylation of all four core histones (18, 19), which is believed to aid in chromatin remodeling and promote target gene transcription (20). Conversely competition for CBP/p300 binding has been suggested to mediate some examples of signal-induced transcriptional repression (21). Our experiments showed that histones H2A, H2B, H3, and H4 were acetylated in the BRCA2 promoter induced by E2 because CBP and p300 were recruited by ERα to the BRCA2 promoter, and CBP/p300 might be responsible for specific changes of histone acetylation levels on the BRCA2 promoter during E2 treatment.
In the following experiments, we found that overexpression of ERβ or p53 attenuated BRCA2 expression induced by E2 through Western blot and promoter activity assay. During these processes, ChIP experiments demonstrated that ERβ, p53, HDAC1, or CtBP1 could bind to the BRCA2 promoter; at the same time, ERα, CBP, p300, and MyoD were released from the promoters, and histones were deacetylated. These results indicated that ERβ or p53 attenuated BRCA2 transcription by inhibition of the recruitment of activating transcriptional factors and histone acetylations.
ERβ displayed a weak transcriptional potency in this context compared with ERα, and ERβ could neutralize the BRCA2 transcriptional activation induced by ERα during E2 treatment. This probably occurred as a consequence of the formation of a heterodimeric complex between ERα and ERβ on the BRCA2 promoter. Our co-IP and ChIP-reChIP assays demonstrated that ERβ interacted with ERα, CBP/p300, and Sp1, indicating that ERβ and ERα formed a heterodimer, which interacted with CBP/p300 and formed a weak activating transcriptional complex to bind to Sp1 sites on the BRCA2 promoter. ERα-induced gene activation requires the combination of its two activation parts (AF-1 and AF-2) for synergistic transcriptional activation, but the individual regions exhibit independent activity in a cell type- and promoter-dependent manner (22). Unlike ERα, ERβ contains a weaker N-terminal AF-1, which may possess repressive activity (23).
HDAC and histone acetyltransferase are enzymes that influence transcription by selectively deacetylating or acetylating the core histone proteins. Chromatin acetylation correlates with transcriptional activity, whereas chromatin deacetylation correlates with gene silencing. CtBP1 can interact with HDAC1 and HDAC2 and form a transcriptional complex that suppresses gene transcription (24). Our results demonstrated that p53 and CtBP1 could synergistically repress BRCA2 promoter activity. Our co-IP and ChIP-reChIP assays demonstrated that p53 interacted with HDAC1, CtBP1/CtBP2, and Sp1, indicating that p53 could recruit HDAC1 and CtBP1/CtBP2 and form an inhibiting transcriptional complex on Sp1 sites of the BRCA2 promoter.
Taken together, ERα, CBP/p300, p68/p72, and MyoD could form an activating transcriptional complex; ERα/ERβ and CBP/p300 could form a weak activating transcriptional complex; and p53, HDAC1, and CtBP1/CtBP2 could form an inhibiting transcriptional complex. All these complexes could compete for binding to Sp1 sites on the BRCA2 promoter. When ERα complex was replaced by ERα·ERβ complex, histone acetylations were attenuated, and gene transcription was reduced slightly. When ERα complex was replaced by p53 complex, histones were deacetylated, and gene transcription was repressed more significantly.
Our experiments showed that ERα, ERβ, and p53 complex could bind to Sp1, indicating that Sp1 sites might contribute to the formation of transcription complexes at this promoter region. In all, we found that there are eight Sp1 sites within 500 bp in front of the transcriptional start site of BRCA2 promoter. To test the important roles of these Sp1 sites, we used BRCA2 promoter/luciferase constructs containing Sp1 mutations and found that all three Sp1 sites contributed in a concerted mechanism to the BRCA2 transcription. Mutation of any of these Sp1 sites close to the start site reduced ERα- and ERβ-activated transcriptional activity, suggesting that these Sp1 sites were essential in the formation of ERα and ERβ transcriptional complex. On the contrary, mutation of these Sp1 sites close to the start site abrogated the p53-induced transcription inhibition, suggesting that these Sp1 sites were also essential in the formation of p53 transcriptional complex. All these results indicated that the synergistic actions of these Sp1 sites were responsible for maximal control of BRCA2 gene transcription.
Taken together, as shown in Fig. 7, our findings support a model in which an ERα·Sp1 complex modulates BRCA2 transcription under conditions of estrogen stimulation. Conversely the formation of this transcription complex is abrogated in cells overexpressing ERβ or p53. So the expression status of the various proteins in these complexes is important for BRCA2 transcription. Approximately two-thirds of all breast cancers are ERα-positive. Patients with tumors that express ERα have a longer disease-free interval and overall survival than patients with tumors that lack ERα expression; this indicates that ERα may be a key regulator of breast cancer susceptibility (25). However, the molecular basis for the association between ERα expression, hormonal responsiveness, and breast cancer prognosis remains unclear (26), and the other genes involved in breast cancer susceptibility need to be found. ERβ is another estrogen receptor in sporadic breast tumors. Although ERβ expression showed wide variations, its range was smaller than that of ERα, suggesting that ERβ is more tightly controlled than ERα (27). Approximately 40% of sporadic breast tumors contain p53 mutations, and the functionality of the ATM-p53-mediated DNA damage response is compromised (28, 29). So far, it is still unclear that the expression level of ERβ or p53 will influence sporadic breast cancers, and there is no report on the expression status of CBP, p300, or MyoD in sporadic breast cancers. The relationship between these proteins and breast cancer deserves to be further studied.
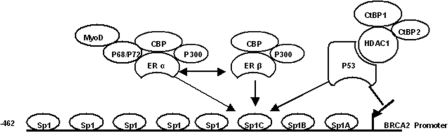
FIGURE 7.
Model depicting binding of ERα, ERβ, or p53 transcriptional complex to the Sp1 sites on the BRCA2 promoter. ERα interacts with CBP/p300, p68/p72, and MyoD and forms an activating transcriptional complex that can bind to many Sp1 sites on the BRCA2 promoter and activate its transcription. ERβ or p53 attenuates ERα-mediated transcriptional activation by preventing the recruitment of ERα transcriptional complex on the BRCA2 promoter; ERβ interacts with ERα and CBP/p300 and forms a weak activating transcriptional complex that competes for binding to Sp1 sites with ERα transcriptional complex. Different from ERβ, p53 interacts with HDAC1 and CtBP1 and forms an inhibiting transcriptional complex that can compete for binding to Sp1 sites with ERα transcriptional complex and inhibit BRCA2 transcription more significantly.
We conclude that ERα interacts with CBP/p300, p68/p72, and MyoD and forms an activating transcriptional complex that can bind to many Sp1 sites on the BRCA2 promoter and activate its transcription by inducing histone acetylations. MyoD is a new component of ERα complex. ERβ and p53 attenuate ERα-mediated transcriptional activation by preventing the recruitment of ERα transcriptional complex and histone acetylations on the BRCA2 promoter. ERβ interacts with ERα and CBP/p300 and forms a weak activating transcriptional complex that competes for binding to Sp1 sites with ERα transcriptional complex and slightly attenuates BRCA2 transcription. Different from ERβ, p53 interacts with HDAC1 and CtBP1/CtBP2 and forms an inhibiting transcriptional complex that can compete for binding to Sp1 sites with ERα transcriptional complex and inhibit BRCA2 transcription more significantly. The interplay between the positive regulation and the negative regulation by ERα, ERβ, and p53, respectively, on BRCA2 expression may be part of an integral signaling pathway that determines and explains breast cancer susceptibility. Detection of expression status of the various proteins in these complexes may predict the onset of sporadic breast cancer.
This work was supported in part by 973 (Grant 2006CB0D0901), the National Natural Science Foundation of China (Grants 30371580 and 30572109), the Shanghai Science and Technology Committee (Grants 03J14019, 06DJ14004, and 06DZ19504); the Cancer Hospital/Cancer Institute of Fudan University (Grant YJ200604), and the Shanghai Pujiang Program. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: BRCA, breast cancer and ovarian susceptibility gene; E2, 17β-estradiol; ER, estrogen receptor; HDAC, histone deacetylase; PBS, phosphate-buffered saline; co-IP, co-immunoprecipitation; FBS, fetal bovine serum; CBP, cAMP-response element-binding protein (CREB)-binding protein; siRNA, small interfering RNA; ChIP, chromatin immunoprecipitation.
References
- 1.Futreal, P. A., Liu, Q., Shattuck-Eidens, D., Cochran, C., Harshman, K., Tavtigian, S., Bennett, L. M., Haugen-Strano, A., Swensen, J., Miki, Y., Eddington, K., McClure, M., Frye, C., Weaver-Feldhaus, J., Ding, W., Gholami, Z., Sderkvist, P., Terry, L., Jhanwar, S., Berchuck, A., Iglehart, J. D., Marks, J., Ballinger, D. G., Barrett, J. C., Skolnick, M. H., Kamb, A., and Wiseman, R. (1994) Science 266 120–122 [DOI] [PubMed] [Google Scholar]
- 2.Gayther, S. A., Russell, P., Harrington, P., Antoniou, A. C., Easton, D. F., and Ponder, B. A. (1999) Am. J. Hum. Genet. 65 1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkitaraman, A. R. (2002) Cell 108 171–182 [DOI] [PubMed] [Google Scholar]
- 4.Wilson, C. A., Ramos, L., Villasenor, M. R., Anders, K. H., Press, M. F., Clarke, K., Karlan, B., Chen, J. J., Scully, R., and Livingston, D. (1999) Nat. Genet. 21 236–240 [DOI] [PubMed] [Google Scholar]
- 5.Thompson, M. E., Jensen, R. A., Obermiller, P. S., Page, D. L., and Holt, J. T. (1995) Nat. Genet. 9 444–450 [DOI] [PubMed] [Google Scholar]
- 6.Sourvinos, G., and Spandidos, D. A. (1998) Biochem. Biophys. Res. Commun. 245 75–80 [DOI] [PubMed] [Google Scholar]
- 7.Turner, N., Tutt, A., and Ashworth, A. (2004) Nat. Rev. Cancer 4 814–819 [DOI] [PubMed] [Google Scholar]
- 8.Thakur, S., and Croce, C. M. (1999) J. Biol. Chem. 274 8837–8843 [DOI] [PubMed] [Google Scholar]
- 9.Mueller, C. R., and Roskelley, C. D. (2003) Breast Cancer Res. 5 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, K. J., Jiang, S. W., Thangaraju, M., Wu, G. J., and Couch, F. J. (2000) J. Biol. Chem. 275 35548–35556 [DOI] [PubMed] [Google Scholar]
- 11.Wu, K. J., Jiang, S. W., and Couch, F. J. (2003) J. Biol. Chem. 278 15652–15660 [DOI] [PubMed] [Google Scholar]
- 12.Couse, J. F., and Korach, K. S. (1999) Endocr. Rev. 20 358–417 [DOI] [PubMed] [Google Scholar]
- 13.Pettersson, K., and Gustafsson, J. A. (2001) Annu. Rev. Physiol. 63 165–192 [DOI] [PubMed] [Google Scholar]
- 14.Spillman, M. A., and Bowcock, A. M. (1996) Oncogene 13 1639–1645 [PubMed] [Google Scholar]
- 15.Watanabe, M., Yanagisawa, J., Kitagawa, H., Takeyama, K., Ogawa, S., Arao, Y., Suzawa, M., Kobayashi, Y., Yano, T., Yoshikawa, H., Masuhiro, Y., and Kato, S. (2001) EMBO J. 20 1341–1352 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Kato, S., Masuhiro, Y., Watanabe, M., Kobayashi, Y., Takeyama, K. I., Endoh, H., and Yanagisawa, J. (2000) Genes Cells 5 593–601 [DOI] [PubMed] [Google Scholar]
- 17.Caretti, G., Schiltz, R. L., Dilworth, F. J., Di Padova, M., Zhao, P., Ogryzko, V., Fuller-Pace, F. V., Hoffman, E. P., Tapscott, S. J., and Sartorelli, V. (2006) Dev. Cell 11 547–560 [DOI] [PubMed] [Google Scholar]
- 18.Bannister, A. J., and Kouzarides, T. (1996) Nature 384 641–643 [DOI] [PubMed] [Google Scholar]
- 19.Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H., and Nakatani, Y. (1996) Cell 87 953–959 [DOI] [PubMed] [Google Scholar]
- 20.Blobel, G. A. (2002) J. Leukoc. Biol. 71 545–556 [PubMed] [Google Scholar]
- 21.Vo, N., and Goodman, R. H. (2001) J. Biol. Chem. 276 13505–13508 [DOI] [PubMed] [Google Scholar]
- 22.Nilsson, S., Makela, S., Treuter, E., Tujague, M., Thomsen, J., Andersson, G., Enmark, E., Pettersson, K., Warner, M., and Gustafsson, J. A. (2001) Physiol. Rev. 81 1535–1565 [DOI] [PubMed] [Google Scholar]
- 23.Ogawa, S., Inoue, S., Watanabe, T., Hiroi, H., Orimo, A., Hosoi, T., Ouchi, Y., and Muramatsu, M. (1998) Biochem. Biophys. Res. Commun. 243 122–126 [DOI] [PubMed] [Google Scholar]
- 24.Shi, Y., Sawada, J., Sui, G., Affar, E. B., Whetstine, J. R., Lan, F., Ogawa, H., Luke, M. P., Nakatani, Y., and Shi, Y. (2003) Nature 422 735–738 [DOI] [PubMed] [Google Scholar]
- 25.Shek, L. L., and Dodolphin, W. (1989) Eur. J. Cancer Clin. Oncol. 25 243–250 [DOI] [PubMed] [Google Scholar]
- 26.Abba, M. C., Hu, Y., Sun, H., Drake, J. A., Gaddis, S., Baggerly, K., Sahin, A., and Aldaz, C. M. (2005) BMC Genomics 6 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieche, I., Parfait, B., Laurendeau, I., Girault, I., Vidaud, M., and Lidereau, R. (2001) Oncogene 20 8109–8115 [DOI] [PubMed] [Google Scholar]
- 28.Coles, C., Condie, A., Chetty, U., Steel, C. M., Evans, H. J., and Prosser, J. (1992) Cancer Res. 52 5291–5298 [PubMed] [Google Scholar]
- 29.Angéle, S., Treilleux, I., Taniére, P., Martel-Planche, G., Vuillaume, M., Bailly, C., Brémond, A., Montesano, R., and Hall, J. (2000) Clin. Cancer Res. 6 3536–3544 [PubMed] [Google Scholar]