Abstract
Myelin in the mammalian nervous system has a high concentration of galactolipids (galactosylceramide and sulfatide) with 2-hydroxy fatty acids. We recently reported that fatty acid 2-hydroxylase, encoded by the FA2H gene, is the major fatty acid 2-hydroxylase in the mouse brain. In this report, we show that FA2H also plays a major role in the formation of 2-hydroxy galactolipids in the peripheral nervous system. FA2H mRNA and fatty acid 2-hydroxylase activity in the neonatal rat sciatic nerve rapidly increased during developmental myelination. The contents of 2-hydroxy fatty acids were approximately 5% of total galactolipid fatty acids at 4 days-of-age, and increased to 60% in galactosylceramide and to 35% in sulfatides at 60 days-of-age. The chain length of galactolipid fatty acids also significantly increased during myelination. FA2H expression in cultured rat Schwann cells was highly elevated in response to dibutyryl cyclic AMP, which stimulates Schwann cell differentiation and upregulates myelin genes, such as CGT and protein zero. These observations indicate that FA2H is a myelination-associated gene. FA2H-directed RNA interference by short-hairpin RNA expression resulted in a reduction of cellular 2-hydroxy fatty acids and 2-hydroxy galactosylceramide in D6P2T Schwannoma cells, providing direct evidence that FA2H-dependent fatty acid 2-hydroxylation is required for the formation of 2-hydroxy galactolipids in peripheral nerve myelin. Interestingly, FA2H-directed RNA interference enhanced migration of D6P2T cells, suggesting that, in addition to their structural role in myelin, 2-hydroxy lipids may greatly influence the migratory properties of Schwann cells.
Supplementary key words: Fatty acid 2-hydroxylase, fatty acid alpha-hydroxylase, hydroxy fatty acids, myelin, galactolipids, galactosylceramide, sulfatide
INTRODUCTION
In higher vertebrates, nerve conduction is greatly facilitated by myelin, a lipid-rich membrane that wraps around the axon. Myelin is formed by oligodendrocytes in the central nervous system (CNS) and by Schwann cells in the peripheral nervous system (PNS). Myelin lipid compositions are very unique: approximately 25–30% of all lipids consist of galactosylceramides (GalCer)2 and sulfatides (3-sulfate ester of GalCer) in both CNS and PNS (1). As much as 60% of the amide-linked fatty acids in myelin galactolipids are hydroxylated at the C2 position (2-hydroxy fatty acids) (2,3). No other mammalian tissues contain such high concentrations of 2-hydroxy fatty acids, suggesting an essential role for the 2-hydroxyl modification in maintaining myelin function.
Biophysical studies of 2-hydroxy galactolipids in model membranes have shown that the 2-hydroxyl group facilitates tight lipid packing via hydrogen bonds at the surface of the membrane (4–6). The 2-hydroxyl group also enhanced carbohydrate-carbohydrate interactions between galactolipids on apposing membranes, presumably by altering the conformation of the carbohydrate head groups (7). These studies suggest that 2-hydroxy galactolipids play critical roles in lipid-lipid and lipid-protein interactions within myelin.
GalCer is synthesized from ceramide and UDP-galactose by the enzyme UDP-galactose:ceramide galactosyltransferase (CGT) (8). CGT-knockout mice, which cannot synthesize galactolipids, are able to form myelin, but develop neurological abnormalities and have a short lifespan (9,10). Detailed analyses of these mice revealed crucial roles for GalCer and sulfatide in myelination and axo-glial organization (11–13). Myelin of CGT-knockout mice is devoid of GalCer and sulfatide, and instead contains glucosylceramides (GlcCer), which are not found in normal myelin (9,10). Interestingly, the GlcCer in myelin of CGT-knockout mice contained 2-hydroxy fatty acids, suggesting a specific need for monoglycosylceramides with both non-hydroxy and 2-hydroxy fatty acids for proper myelin function and stability.
Recently, Fewou et al. reported that CGT-transgenic mice had unstable and uncompacted myelin and developed progressive hindlimb paralysis and demyelination (14). While total myelin galactolipids in these mice were not significantly altered, the 2-hydroxy GalCer to non-hydroxy GalCer ratio was reduced. It was therefore concluded that the underlying cause of the unstable myelin was reduced 2-hydroxy GalCer.
The 2-hydroxyl group on the N-acyl chain of myelin galactolipids is incorporated during de novo biosynthesis of GalCer. In the biosynthesis pathway for 2-hydroxy galactolipids, FA2H is the major enzyme that catalyzes fatty acid 2-hydroxylation in the CNS (15). FA2H activity increases in the CNS during developmental myelination to provide precursors for the synthesis of myelin 2-hydroxy galactolipids. Biochemical studies of fatty acid 2-hydroxylase have been conducted almost exclusively in the CNS, and little is known about this enzyme in the PNS. The current study is aimed at establishing that FA2H is also responsible for the formation of 2-hydroxy galactolipids in PNS myelin. Furthermore, a novel function of 2-hydroxy lipids in cell migration was discovered during the study using RNA interference.
EXPERIMENTAL PROCEDURES
Materials
Fetal bovine serum was purchased from Atlanta Biologicals (Norcross, GA). Bovine sulfatides, cerebrosides (kerasin, phrenosin), glucocerebrosides, and odd chain fatty acids (C15–C21) were purchased from Matreya (Pleasant Gap, PA). Deuterated tetracosanoic acid [3,3,5,5-D4] was purchased from Larodan Fine Chemicals (Malmö, Sweden). Purified human NADPH:cytochrome P450 reductase and NADPH-regenerating system solutions were purchased from BD Biosciences (Bedford, MA). Recombinant heregulin-β1177–244 was a generous gift from Genentech, Inc. (South San Francisco, CA). Forskolin and dibutyryl cAMP were purchased from Sigma (St. Louis, MO).
Animals
Fisher and Sprague-Dawley rats were maintained in animal care facilities of the Medical University of South Carolina and University of Miami Miller School of Medicine, respectively, with water and food ad libitum. Rats were treated in accordance with the Institutional Animal Care and Use Committee approved procedures at these institutions.
Cell Cultures
Rat Schwann cells were obtained from sciatic nerves of 3-month-old Fisher rats by the method of Morrissey (16) with modifications. Nerve segments were explanted in DMEM (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated FBS (Hyclone) and depleted of fibroblasts by sequential transplantation (2–3 times) to new plastic dishes. After two weeks, tissue explants were dissociated with 0.25% dispase (Roche) and 0.05% collagenase (Worthington) and the resulting cell suspension was plated on dishes coated with poly-L-lysine substrate (200 μg/ml). Cells were grown in medium containing DMEM-10% FBS supplemented with a mixture of mitogens [2 μM forskolin, 20 μg/ml bovine pituitary extract (Biomedical Tech., Stoughton, MA), and 2.5 nM recombinant heregulin-β1177–244 (Genentech)]. After 1 week in culture, the cells were trypsinized and remaining fibroblasts were removed by a 30-min incubation with anti-Thy 1.1 antibodies (conditioned medium from mouse hybridoma cells, ATCC) followed by an addition of rabbit complement (ICN). Primary Schwann cells from passage 1 to 4 (2–8 population doublings) were cultured on poly-L-lysine-coated dishes in medium containing mitogens. These cells were >98% Schwann cells based on immunostaining with anti-S100 (Dako), a Schwann cell-specific marker. To induce differentiation, Schwann cells were plated in 10 cm poly-L-lysine-laminin-coated dishes (2×106 cells/dish) in DMEM containing 1% FBS and stimulated for 3 days with 1 mM dibutyryl cAMP (Sigma). Under this condition, myelin-associated genes are induced, and Schwann cells acquire a differentiated phenotype (17).
Rat Schwannoma-derived D6P2T cells (18) were purchased from ATCC and grown in DMEM containing 10% FBS. Cells were harvested at approximately 60–70% confluency.
Isolation of galactolipids from rat sciatic nerve
Rats at postnatal days (P) 4, 7, 10, 15, 20, 30, and 60 were euthanized according to guidelines established by the institutional IACUC committee. Sciatic nerves were exposed through a longitudinal incision in the thigh and excised immediately. Whole nerves were homogenized in PBS using a PT1200E Polytron with a 7-mm generator. GalCer and sulfatides (2-hydroxy and non-hydroxy) were isolated as described by Coetzee et al. (10) with minor modifications. Lipids were extracted with 19 volumes of chloroform/methanol (2:1, v/v) (19). The extracts were washed with 0.2 vol of 0.9% NaCl, and the lower phase was collected and dried under an N2 stream. Dry lipid extracts were subjected to mild alkaline hydrolysis (0.5 N KOH for 10 min at 50 °C) to remove glycerolipids. TLC plates (Silica gel 60, Merck) were saturated with solvent vapor [chloroform/methanol/water (70:30:4, v/v)] for 45 min prior to development. Lipid spots were visualized under UV after spraying with primuline solution (0.005% primuline in acetone/water, 80:20, v/v) and identified using the corresponding standards. GalCer and sulfatide were separately removed from the plates, and internal standards (C15, C17, C19, and C21 fatty acids) were added prior to alkaline hydrolysis (4 N KOH, overnight at 80 °C). Lipids were acidified by an addition of glacial acetic acid, and free fatty acids were extracted three times with 3 ml diethyl ether. Combined ether extracts were brought to dryness under N2.
Fatty acid determination by gas chromatography/mass spectrometry (GC/MS)
Fatty acid methyl esters were prepared by adding 1 ml of methanolic HCl to each sample and incubated at 65 °C for 45 min and brought to dryness under N2. To prepare trimethylsilyl derivatives of hydroxyl groups, 100 μl of Tri-Sil Reagent (Pierce Biotechnology, Rockford, IL) was added and incubated for 30 min at room temperature. Derivatized samples were applied to a GC-2010 gas chromatograph (Shimadzu Scientific, Columbia, MD) with the injector in splitless mode. The injection port and transfer line were maintained at 250 °C. Analytes were fractionated on a Restek RTX-5 column (5% diphenyl and 95% dimethyl polysiloxane; 0.25 mm inner diameter, 0.25 μm D.F., 30 m). The initial oven temperature was 110 °C, and increased to 300 °C at 10 °C/min. Mass spectra data were obtained on a Shimadzu GC/MS-QP2010 mass spectrometer following electron impact ionization. Peaks of the target analytes and internal standards were processed using the GC-MS Lab Solutions software (Shimadzu Scientific). Calibration curves were constructed by plotting peak area ratios of the target analytes to their respective internal standards against concentration.
Fatty acid 2-hydroxylase assay
FA2H activity was determined in rat sciatic nerves and in D6P2T cells using the method described previously by Alderson et al. (20). Briefly, crude sciatic nerve or D6P2T homogenates (50 μg of protein) were added to an assay mixture containing 2.7 mM Tris-HCl, pH 7.6, 1.20 mM NADP+, 3.3 mM glucose 6-phosphate, 3.3 mM MgCl2, 0.2 unit of glucose 6-phosphate dehydrogenase, and 1 μg of human NADPH:cytochrome P-450 reductase, in a total volume of 1.4 ml. The substrate, 1 μg (2.7 nmol) of [3,3,5,5-D4] C24 fatty acid (stock solution: 10 μg/ml in 1.5 mM α-cyclodextrin), was added at time zero. After gentle mixing by swirling, the assay mixture was incubated with shaking (100 rpm) at 37 °C for 180 min. At the end of the incubation, 1 pmol of C23 fatty acid was added to each sample as an internal standard, and samples were acidified by an addition of glacial acetic acid (20 μl). Fatty acids were extracted three times with diethyl ether (2 ml), and combined diethyl ether extracts were brought to dryness under N2. Fatty acids were derivatized and quantified as described in §Fatty acid determination by gas chromatography/mass spectrometry (GC/MS).
Quantitative RT-PCR (qPCR)
Whole sciatic nerves of P4, 7, 10, 15, 20, 30, and 60 rats were homogenized in PBS using a PT1200E Polytron with a 7-mm generator. Total RNA was isolated using the QIAGEN RNeasy Lipid Tissue kit. Total RNA from cultured Schwann cells was isolated using the QIAGEN RNeasy kit. RNA was quantified using a NanoDrop ND-1000 Spectrophotometer (Nanodrop Technologies, Inc.). cDNA was generated using the Bio-Rad iScript cDNA Synthesis kit. Real-time quantitative PCR was performed on a BioRAD MyiQ single-color real-time PCR detection system. The primers used for each gene were as follows: for rat FA2H, rFA2H-F1 (cca tta cta cct gca ctt tgg) and rFA2H-R1 (tct gga atg agg gtg tgg a); for rat CGT, rCGT-F1 (gtt cat ggg tcc agc ttg tg) and rCGT-R1 (ctg gcc ggc ttt gtt agg); for rat P0, rP0-F1 (ctg cac tgc tcc ttc tgg t) and rP0-R1 (cct tgg cat agt gga aga ttg); for rat 18S rRNA, r18S-F1 (ggc ccg aag cgt tta ctt) and r18S-R1 (cgg ccg tcc ctc tta atc). PCR reactions were performed in a 96-well plate with a reaction mixture containing 15 μl iQ SYBR Green Supermix (Bio-Rad), cDNA template, and 200 nM each of forward and reverse primers in a total volume of 30 μL. All reactions were performed in triplicate. The thermal cycling conditions were set at 95 °C for 3 min, followed by 40 cycles of 2-step amplification (10 sec at 95 °C and 45 sec at 57 °C). Data were analyzed with MyiQ software. The abundance of FA2H, CGT, and P0 mRNA were normalized against 18S rRNA contents, using the ΔΔCt method (21).
Construction of short-hairpin RNA (shRNA) expression plasmids
Four siRNA against rat FA2H were designed using the Ambion siRNA Target Finder, and screened for the ability to reduce cellular 2-hydroxy fatty acids. The most effective sequence (aagagattattcacttgtggt) was selected for the construction of an shRNA expression plasmid. The control shRNA expression plasmid was constructed based on the sequence of Control siRNA #5 from Ambion (Ambion, Austin, TX). Each insert contained a BamHI linker, sense strand, loop sequence (ttcaagaga), antisense strand, RNA Pol III terminator, and a HindIII linker. Double-stranded DNA inserts were prepared by annealing 63-base complementary synthetic oligonucleotides and cloned into BamHI and HindIII sites of pSilencer 5.1-U6 Retro (Ambion). The correct inserts were confirmed by sequencing.
Transfection of D6P2T cells
D6P2T cells were transfected with double stranded siRNA (2 μg) or shRNA expression plasmids (2.5 μg) using the Nucleofector kit T (amaxa, Gaithersburg, MD). Transfections were performed according to the manufacturer’s instructions (Program T-20). Briefly, cells were harvested at 60–70% confluency by trypsin-EDTA treatment. Approximately 2×106 cells were transfected for each treatment condition, and immediately plated in 75-cm2 flasks to propagate for 48 hr. Transfection efficiency was monitored for each experiment using pmaxGFP and visualization with fluorescence microscopy. Typical experiments averaged approximately 80–90% transfection efficiency with high viability.
[14C] Acetate labeling in D6P2T cells
D6P2T cells were transfected with 2.5 μg of control shRNA or FA2H-shRNA expression plasmids using the Nucleofector kit T (amaxa). After 48 hr, puromycin (1 μg/ml) was added to the culture media, and resistant cells were selected for two weeks. Stably transfected cells were grown to approximately 50% confluency and harvested for fatty acid analysis or metabolically labeled with [14C] acetate (2 μCi/ml) for 48 hr. Labeled cells were harvested by trypsin-EDTA treatment and washed with PBS. Cellular lipids were extracted with 19 volumes of chloroform/methanol (2:1, v/v) (19). The extracts were washed with 0.2 vol of 0.9% NaCl, and the lower phase was collected and dried under an N2 stream. Dried lipid extracts were subjected to mild alkaline hydrolysis (0.5 N KOH for 10 min at 50 °C) to remove glycerolipids. A small aliquot was removed from each sample for determination of total lipid [14C] counts to confirm equal [14C] incorporation. TLC plates (Silica gel 60) were immersed in 1% sodium tetraborate (in methanol) for 15 sec and air-dried overnight, followed by heating at 110 °C for 30 min prior to sample application. Non-hydroxy and 2-hydroxy GalCer standards were added to each sample as carriers, and equal [14C] counts were spotted on borate-impregnated TLC plates (Supplemental Fig. S1). TLC plates were saturated with solvent vapor [chloroform/methanol/water (70:30:4, v/v)] for 45 min prior to development. Lipid spots were visualized under UV after spraying with primuline solution (0.005% primuline in acetone/water, 80:20 v/v) and identified using corresponding standards. Non-hydroxy and 2-hydroxy GalCer spots were removed from the plates separately, and [14C] incorporation was measured by liquid scintillation counting.
Migration assay
D6P2T cells were transfected with control (Ambion Control siRNA #5) or FA2H siRNA (target sequence: aagagattattcacttgtggt). Cells were harvested 72 hr after transfection, and migration was measured by a method based on the Boyden chamber principle. Equal number (50,000) of viable cells were placed on the top surface of 24-well cell culture inserts (polyethylene terephthalate membrane, 8-μm pore, Falcon No. 353097) with 300 μl of serum-free DMEM. Each insert was placed in a well containing 500 μl of DMEM with 10% FBS and placed in a CO2 incubator at 37 °C for 4 hr. Cells remaining on the top surface of the inserts were removed with a cotton swab, and migratory cells on the bottom surface were stained and counted using light microscopy. Migration assays were also performed with D6P2T cells stably transfected with control shRNA or FA2H-shRNA expression plasmids.
RESULTS
FA2H expression is upregulated in sciatic nerve during myelination
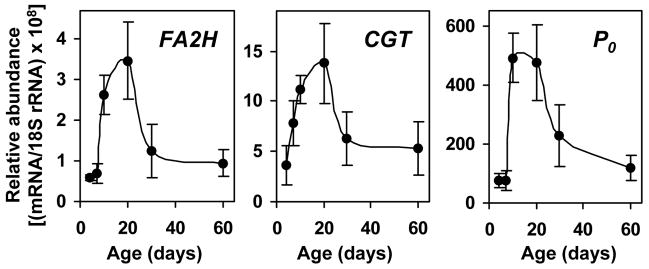
We have previously demonstrated that FA2H is expressed in oligodendrocytes in the CNS (15). We further demonstrated that FA2H gene expression was up-regulated approximately 400-fold during postnatal myelination in mouse brain in parallel to other myelin-associated genes. To show FA2H is also involved in PNS myelination, we first examined the expression of FA2H during postnatal myelination of the PNS by quantitative RT-PCR (qPCR). mRNA for other well-characterized PNS myelin genes, CGT and P0, were also determined. CGT encodes UDP-galactose:ceramide galactosyltransferase that catalyzes the synthesis of GalCer. FA2H was presumed to function in the same biosynthetic pathway as CGT, and therefore the two genes were expected to be coordinately expressed during myelination. Protein zero (P0) is the major structural protein of PNS myelin, and serves as a good marker for myelination. As shown in Fig. 1, FA2H was expressed in sciatic nerve at all ages. FA2H mRNA increased significantly after P7, and reached the highest at P20 (approximately 6-fold of the levels at P4), which coincides with the period of active myelination in rat sciatic nerve. During this same period, CGT and P0 increased 3.7-fold and 6.3-fold, respectively. By P60, the mRNA levels of the three genes were decreased to 25–38% of the levels measured at P20. The temporal changes in FA2H expression closely paralleled the two well-established myelin genes, suggesting a role in PNS myelination.
Fig. 1.
FA2H expression is upregulated during developmental myelination of rat sciatic nerve. Total RNA was isolated from rat sciatic nerves and FA2H, CGT, and P0 mRNA levels were determined by quantitative RT-PCR. Data are normalized to 18S rRNA levels. The mean and SD are shown (n=5 for P7, P15, P20, P30, and P60; n=4 for P30). The data at P4 represents a pool of sciatic nerves from 4 rats in triplicate measurements.
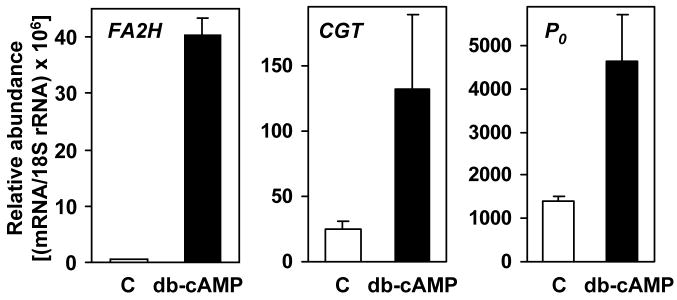
FA2H expression was also determined in primary cultures of Schwann cells isolated from adult rat sciatic nerve. Adult-derived Schwann cells expanded in vitro display features of undifferentiated cells (16). However, in the absence of axons the cells can be induced to re-acquire a differentiated phenotype (i.e. high myelin gene expression) by exposure to agents increasing the intracellular levels of cAMP (17). As shown in Fig. 2, FA2H was expressed at very low levels in quiescent, non-differentiated Schwann cells compared to the relatively high basal levels of CGT and P0 expression. Upon dibutyryl cAMP-stimulated differentiation, FA2H mRNA was increased 86-fold, whereas CGT and P0 expression was elevated 3–4 fold. These results indicate that FA2H is highly inducible in Schwann cells in response to signals for differentiation, as other myelin-associated genes.
Fig. 2.
FA2H expression in primary Schwann cells is highly upregulated upon differentiation. Schwann cells were isolated from adult rat sciatic nerve. Total RNA was isolated from proliferating cells, control (C), or cells treated with 1 mM dibutyryl cAMP (db-cAMP). FA2H, CGT, and P0 mRNA levels were determined by quantitative RT-PCR. Data are normalized to 18S rRNA levels.
FA2H activity in sciatic nerve increases during myelination
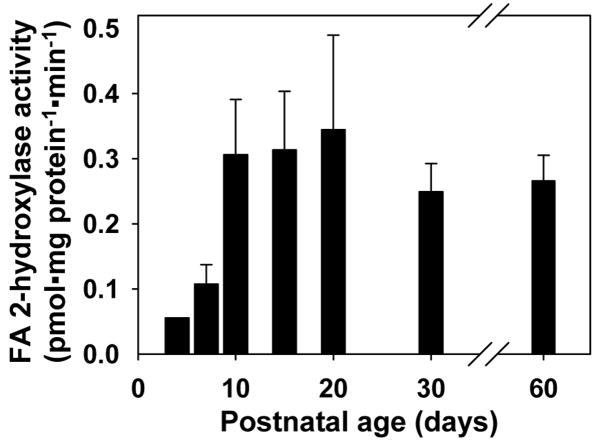
The high expression of FA2H in myelinating sciatic nerve and differentiated Schwann cells is indicative of FA2H being involved in the biosynthesis of myelin 2-hydroxy galactolipids. To show FA2H expression correlates with the FA2H enzymatic activity, fatty acid 2-hydroxylase activity was determined in sciatic nerve during postnatal myelination, utilizing the method developed in our laboratory (20). This assay specifically measures the conversion of the exogenous substrate, deuterated C24 free fatty acid, to deuterated C24 2-hydroxy fatty acid. Consistent with the increase in FA2H mRNA, fatty acid 2-hydroxylase activity increased progressively to P20, reaching approximately 700% of activity levels measured at P4 (Fig. 3). These data provide evidence supporting a role of FA2H in the synthesis of 2-hydroxy galactolipids in the PNS.
Fig. 3.
Fatty acid 2-hydroxylase activity in postnatal rat sciatic nerve. Crude nerve tissue homogenates (50 μg protein) were used for fatty acid 2-hydroxylase assays. The mean and SD are shown (n=5 for P15, P20, P30, and P60; n=3 for P7; n=2 for P10). The data at P4 represents a pool of sciatic nerve from 4 animals.
2-Hydroxy galactolipids in sciatic nerve increase during developmental myelination
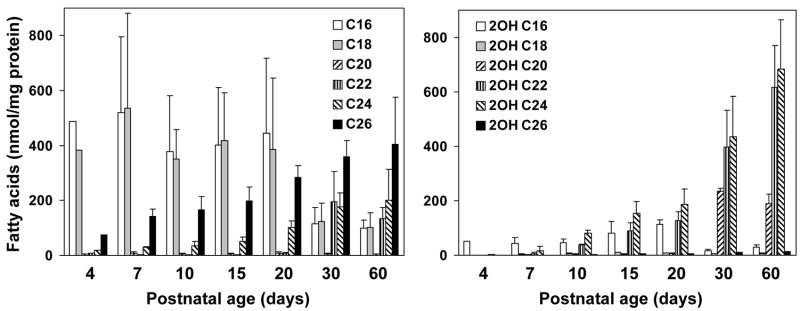
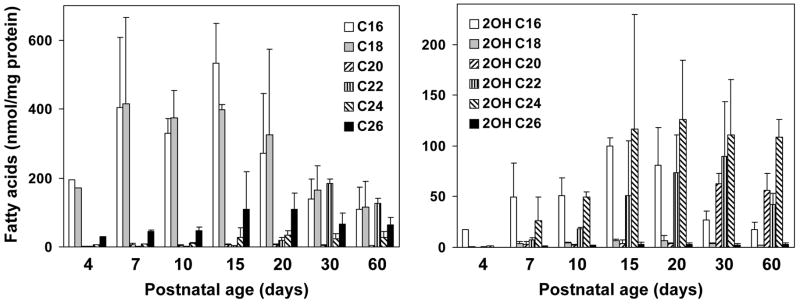
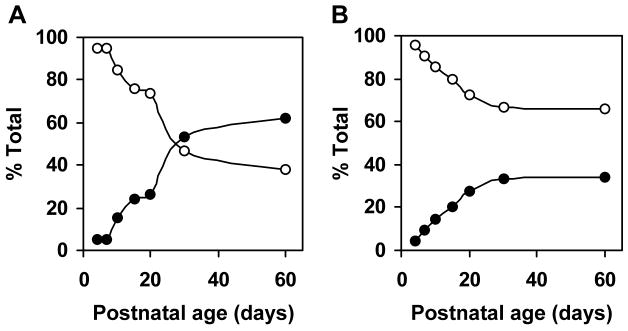
Fatty acid compositions of myelin galactolipids have been extensively studied in the mammalian CNS. Relative contents of very-long-chain fatty acids (>C20) and 2-hydroxy fatty acids in brain galactolipids increase during developmental myelination. Although the high galactolipid content in the mammalian PNS are known, developmental changes in fatty acid compositions of galactolipids are not fully defined. If FA2H was responsible for the synthesis of 2-hydroxy galactolipids in the PNS, 2-hydroxy fatty acid contents in galactolipids would be expected to increase in parallel with the sharp increase in FA2H expression and activity during myelination of the sciatic nerve. We therefore quantified the non-hydroxy and 2-hydroxy fatty acids associated with GalCer and sulfatides in sciatic nerve during postnatal myelination (Fig. 4 and 5). The changes in relative non-hydroxy and 2-hydroxy fatty acid contents are shown in Fig. 6. There were two distinct features in the changes of GalCer-associated fatty acids from P4 to P60 (Fig. 4). First, 2-hydroxy fatty acids were minor components in GalCer in neonates and markedly increased as animals matured, whereas total non-hydroxy GalCer contents (per protein) remained relatively unchanged. 2-Hydroxy fatty acids continued to increase after the peak myelination period, and 2-hydroxy C22 and C24 fatty acids were the predominant species at P30 and P60 (Fig. 4, right). Second, fatty acid chain length (both non-hydroxy and 2-hydroxy) shifted from long-chain (C16–C20) in neonates to predominantly very-long-chain (C22–C26) in adults (Fig. 4, left). While fatty acids in GalCer were almost exclusively C16 and C18 at P4, very-long-chain fatty acids (C22–C26) continually increased and were major species at P30 and P60. Interestingly, there was little 2-hydroxy C26 fatty acid even though non-hydroxy C26 was present at all ages. Unsaturated fatty acids (C18:1 and C24:1, both hydroxy and non-hydroxy) were below detectable levels. The shift from non-hydroxy to 2-hydroxy fatty acids is evident when the relative fatty acid contents are calculated as percent total GalCer-associated fatty acids (Fig. 6A). The proportion of 2-hydroxy fatty acids increased from 5% at P4 to 62% at P60.
Fig. 4.
Fatty acid compositions of rat sciatic nerve GalCer. Total lipids were extracted from sciatic nerve. GalCer were purified by TLC and fatty acid compositions determined by GC/MS. Average and SD of 3–4 animals are shown, except at day 4, which represents a pool of sciatic nerves from 4 animals. In all samples C18:1 and C24:1 fatty acids were not detectable.
Fig. 5.
Fatty acid compositions of rat sciatic nerve sulfatide. Total lipids were extracted from sciatic nerve. Sulfatides were purified by TLC and fatty acid compositions determined by GC/MS. Average and SD of 3–4 animals are shown, except at day 4, which represents a pool of sciatic nerves from 4 animals. In all samples C18:1 and C24:1 fatty acids were not detectable.
Fig. 6.
Relative contents of 2-hydroxy and non-hydroxy galactolipids of postnatal rat sciatic nerve. The 2-hydroxy (filled circle) and non-hydroxy (open circle) fatty acid contents were expressed as percent total fatty acids in purified GalCer (A) and sulfatide (B).
The developmental changes in sulfatide-associated fatty acids were similar to GalCer-associated fatty acids with some interesting differences (Fig. 5). As in GalCer, there was little 2-hydroxy fatty acids in sulfatide at P4. The increase in 2-hydroxy fatty acid contents in sulfatide was less dramatic than that in GalCer, and non-hydroxy fatty acids were still predominant at P60. The most notable difference was that non-hydroxy C26 content did not increase significantly as in GalCer. Unsaturated fatty acids (C18:1 and C24:1, both hydroxy and non-hydroxy) were not detected in sulfatide as well. Relative abundance of 2-hydroxy fatty acids changed from 4.5% at P4 to 34% at P30, and remained at the same level at P60 (Fig. 6B). These percentages are comparable to TLC-based measurements in mice (22).
FA2H is required for the synthesis of 2-hydroxy GalCer
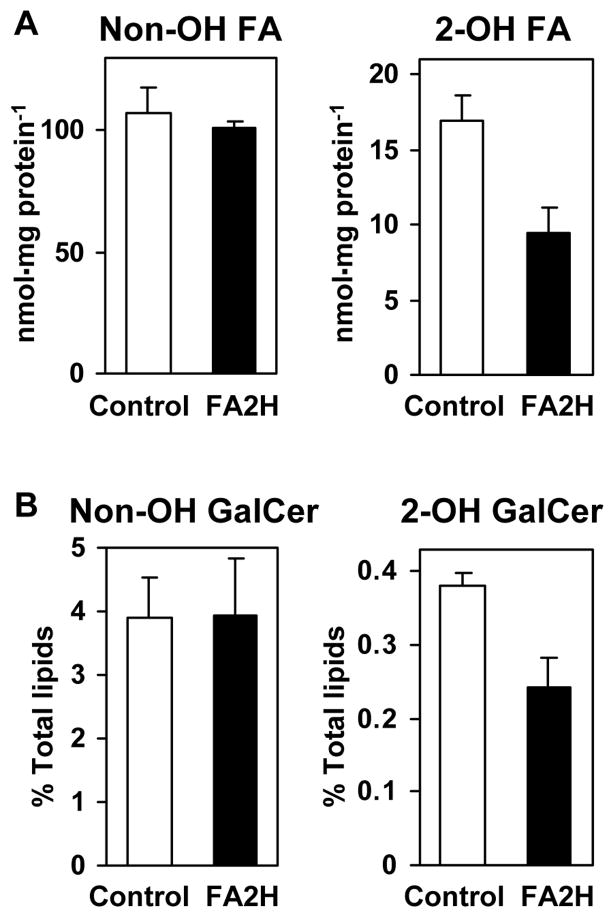
The data shown above indicate a strong correlation between FA2H expression/activity, and the increase in 2-hydroxy galactolipid contents in postnatal rat sciatic nerve. To provide direct evidence for the involvement of FA2H in 2-hydroxy galactolipid synthesis, FA2H-directed RNA interference (RNAi) was performed in the cell line D6P2T. Originally isolated from a Schwannoma, D6P2T cells maintain many characteristics of Schwann cells, including synthesis of 2-hydroxy galactolipids and myelin proteins (18,23). For stable RNAi, D6P2T cells were transfected with FA2H-specific short-hairpin RNA (shRNA) expression plasmid or a control shRNA expression plasmid. The retroviral elements within the plasmid vector facilitate efficient integration into the host genome, conferring puromycin resistance to the cells. By culturing transfected cells in the presence of puromycin for 2 weeks, polyclonal, stably transfected cells were obtained. FA2H shRNA expression resulted in an approximately 40% reduction in total cellular 2-hydroxy fatty acids with no effect on non-hydroxy fatty acids (Fig. 7A). The effects on total 2-hydroxy fatty acids were very similar to the effects seen with FA2H siRNA transfection (Fig. 8). Therefore, the reduction of 2-hydroxy fatty acids is not likely an artifact caused by the integration of the plasmid into the genome or by the selection with puromycin. Subsequently, cellular lipids were metabolically labeled with [14C] acetate for 48 hr to measure GalCer produced in these cells. As shown in Fig. 7B, 2-hydroxy GalCer was reduced 36% by FA2H shRNA expression with no effect on non-hydroxy GalCer. These data provide direct evidence that FA2H is required for the formation of 2-hydroxy GalCer.
Fig. 7.
FA2H-directed RNAi reduces cellular 2-hydroxy fatty acids and 2-hydroxy GalCer. D6P2T cells were transfected with control shRNA or FA2H shRNA expression plasmids. Transfected cells were selected for puromycin resistance (1 μg/ml) for 2 weeks. (A) Total non-hydroxy and 2-hydroxy fatty acids were measured by GC/MS following hydrolysis of all cellular lipids. (B) Cells were metabolically labeled with [14C] acetate for 48 hr. Non-hydroxy and 2-hydroxy GalCer were isolated by TLC and radioactivity was measured by liquid scintillation counting. Mean and SD of triplicate samples are shown.
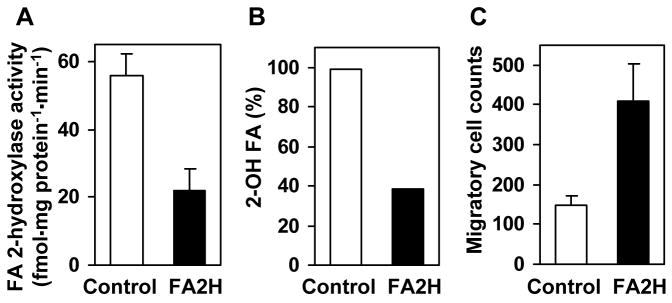
Fig. 8.
FA2H-directed RNAi enhances D6P2T cell migration. Cells were transfected with control or FA2H siRNA and harvested after 72 hr. (A) Fatty acid 2-hydroxylase activities in whole cell lysates. The mean and range of two measurements are shown. (B) Total 2-hydroxy fatty acids were measured by GC/MS following hydrolysis of all cellular lipids. The combined quantities of the three major 2-hydroxy fatty acids (2-hydroxy C16, C18, C24) are shown. (C) Transfected cells (50,000 per insert) were placed in cell culture inserts and allowed to migrate for 4 hr before staining. The mean and SD of triplicate cell countings are shown.
2-Hydroxy lipids may play a role in regulation of cell migration
Enhanced expression of surface lipid antigens present in myelinating cells, such as GalCer, is a key feature of cAMP-mediated Schwann cell differentiation (17). Our results further indicate that these changes in myelin lipid contents also correlate with enhanced expression of FA2H in differentiated Schwann cells (Fig. 2). Because 2-hydroxy lipids likely have a role in the tight packing of lipids required for membrane compaction during myelin sheath formation, a possible consequence of altered levels of FA2H expression would be a change in cell membrane fluidity and therefore cell migration capability. We therefore tested whether FA2H-directed RNAi alters migratory processes in D6P2T cells. Transfection of FA2H siRNA decreased FA2H activity by 60% compared to transfection of control siRNA (Fig. 8A). Accordingly, total 2-hydroxy fatty acids were decreased approximately 60% compared to control (Fig. 8B). As mentioned above, these results are in close agreement with results obtained with shRNA expression (Fig. 7A). Surprisingly, we found that cell migration in response to serum was enhanced approximately 2.8-fold with FA2H siRNA treatment compared to control siRNA-transfected cells (Fig. 8C). Migration of cells treated with transfection reagent without siRNA was indistinguishable from control siRNA-treated cells. Very similar enhancement of cell migration was also observed with FA2H shRNA expression (data not shown). These observations suggest that, in addition to their structural role in the compaction of myelin membranes, 2-hydroxy lipids may also greatly influence the migratory properties of Schwann cells.
DISCUSSION
Here we provide evidence that FA2H is required for the formation of 2-hydroxy galactolipids in rat PNS myelin, and that 2-hydroxy lipids may have a regulatory role in cell migration. Our data show that: 1) FA2H expression in rat sciatic nerve is upregulated during developmental myelination; 2) FA2H is expressed in cultured Schwann cells and is highly upregulated in response to differentiation signals, such as cAMP; 3) the fatty acid compositions of rat sciatic nerve galactolipids change dramatically during myelination such that increasing proportions of 2-hydroxy fatty acids and very-long-chain fatty acids are incorporated; 4) synthesis of 2-hydroxy GalCer in D6P2T Schwannoma cells is reduced by expression of FA2H shRNA; 5) cell migration is enhanced when cellular 2-hydroxy lipids were reduced as a consequence of FA2H siRNA or shRNA. Altogether, these data demonstrate the direct link between FA2H activity and the synthesis of 2-hydroxy galactolipids in the PNS. The data also indicate that 2-hydroxy lipids are not merely structural lipids of myelin as previously thought, but may also regulate cell migration.
Previous studies showed that FA2H is highly expressed in human brain (24) and upregulated during myelination in mouse brain (15,25). FA2H catalyzes 2-hydroxylation of free fatty acids to provide precursors of myelin 2-hydroxy galactolipids (15). Biochemical characteristics of FA2H indicate that it is the same enzyme as the rat brain fatty acid α-hydroxylase, studied extensively by Kishimoto and colleagues [for review see (26)]. Previously, fatty acid α-hydroxylase activities could not be detected in any rat tissues outside of the brain (27), and a question remained as to whether the same enzyme was responsible for the synthesis of myelin 2-hydroxy galactolipids in the PNS. The new method to measure fatty acid 2-hydroxylase activity provided improved sensitivity and enabled us to characterize FA2H in most biological samples, including tissue culture cells (20) and cultured human keratinocytes (28). To our knowledge, this is the first report on fatty acid 2-hydroxylase in the sciatic nerve. The data shown here indicate that the synthesis of 2-hydroxy galactolipids in PNS myelin is FA2H-dependent, as in the CNS.
In both CNS and PNS, 2-hydroxy fatty acid contents in galactolipids are very low at the onset of myelination and rapidly increase during developmental myelination. Fatty acid chain length also changes from long-chain (C16–C20) to very-long-chain (>C20) fatty acids during myelination. Currently, the physiological significance of these transitions is not fully understood. Previous biophysical studies indicate that the 2-hydroxyl group of ceramide-linked N-acyl chains can facilitate lipid packing via additional hydrogen bonds. In addition, longer acyl chains would enhance hydrophobic interaction among lipids. It is therefore conceivable that the transition of the N-acyl chains of myelin galactolipids may confer progressively higher membrane rigidity during the course of myelination, which may be necessary for proper organization of myelin proteins and myelin compaction. The recent findings by Fewou et al. (14) are consistent with this hypothesis. In CGT-transgenic mice, total myelin galactolipid levels were not significantly altered, but the proportion of 2-hydroxy GalCer was reduced compared to wild type mice. Myelin of these mice was unstable and uncompacted, and the mice developed progressive hindlimb paralysis and demyelination (14). These findings indicate that fatty acid compositions of galactolipids are critical for myelin stability.
The biochemical basis of the increase in 2-hydroxy galactolipids during myelination is now explained, at least in part, by the upregulation of FA2H in oligodendrocytes and Schwann cells. The mechanism for the developmental changes in fatty acid chain length is less clear. In the ceramide biosynthetic pathway, several reactions are catalyzed by multiple enzyme isoforms. For instance, there are at least six isoforms of fatty acid elongases, encoded by Elovl1-6, each of which shows distinct tissue-specific expression (29). Similarly, there are at least six isoforms of dihydroceramide synthases (Lass/CerS) with distinct substrate specificities (30). The isoforms of these enzymes involved in the synthesis of myelin galactolipid are unknown. The remarkable diversity and developmental transition of galactolipid molecular species during myelination are likely achieved by coordinated regulation of specific Elovl gene(s) and Lass/CerS gene(s) along with FA2H.
2-Hydroxy fatty acids are highly abundant in the nervous tissue as components of myelin galactolipids. A number of non-neural tissues also contain 2-hydroxy fatty acids as components of various sphingolipids, though at much lower concentrations. Examples include ceramide in the epidermis (31), sphingomyelin in kidney, intestine, spermatozoa (32–34), and various glycosphingolipids in liver and intestine (35–37). In all of these tissues and organs, physiological roles of 2-hydroxy lipids are not understood beyond a hypothetical role as structural lipids that confer physical stability to the membrane. Recently, we have demonstrated that FA2H-dependent synthesis of 2-hydroxy ceramide/GlcCer is required for proper formation and secretion of lipid lamellar bodies in differentiating human keratinocytes, providing the first evidence that 2-hydroxy lipids may regulate complex cellular processes (28). The current study also presents an intriguing possibility that 2-hydroxy lipids greatly influence cell migration. This may be due to their ability to alter membrane fluidity. Alternatively, 2-hydroxy lipids may influence the lipid/protein organization within sphingolipid- and cholesterol-rich microdomains, or lipid rafts. Lipid rafts play critical roles in neural development (38), and a number of myelin proteins involved in cell signaling and adhesion are associated with lipid rafts [for example (39)]. Interestingly, raft-association of Plp, the major myelin protein in the CNS, is disrupted in the absence of GalCer, indicating that myelin galactolipids play a major role in proper organization of myelin proteins in lipid rafts (40). It is conceivable that 2-hydroxy lipids influence lipid raft constituents, thereby modulating cell adhesion and migratory properties. Our findings warrant further study of the regulation of cell migration by 2-hydroxy lipids.
Supplementary Material
Acknowledgments
We thank Dr. Julie Chao for the generous gift of rat tissues used in preliminary studies and Sayuri Rendon for assistance with cell cultures. This work was supported in part by a National Institutes of Health Grant RR-17677.
Footnotes
The abbreviations used are: CNS, central nervous system; PNS, peripheral nervous system; GalCer, galactosylceramide; CGT, UDP-galactose:ceramide galactosyltransferase; GlcCer, glucosylceramide; DMEM, Dulbecco’s modified Eagle’s Medium; FBS, fetal bovine serum; P, post natal days; PCR, polymerase chain reaction; PBS, phosphate-buffered saline; PMSF, phenylmethylsulphonylfluoride; GC/MS, gas chromatography/mass spectrometry; TLC, thin layer chromatography.
References
- 1.Norton WT, Cammer W. In: Myelin. Morell P, editor. Plenum; New York: 1984. pp. 147–195. [Google Scholar]
- 2.Hoshi M, Williams M, Kishimoto Y. Characterization of brain cerebrosides at early stages of development in the rat. J Neurochem. 1973;21:709–712. doi: 10.1111/j.1471-4159.1973.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto Y, Radin NS. Isolation and determination methods for brain cerebrosides, hydroxy fatty acids, and unsaturated and saturated fatty acids. J Lipid Res. 1959;1:72–78. [Google Scholar]
- 4.Boggs JM, Koshy KM, Rangaraj G. Influence of structural modifications on the phase behavior of semi- synthetic cerebroside sulfate. Biochim Biophys Acta. 1988;938:361–372. doi: 10.1016/0005-2736(88)90134-4. [DOI] [PubMed] [Google Scholar]
- 5.Lofgren H, Pascher I. Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem Phys Lipids. 1977;20:273–284. doi: 10.1016/0009-3084(77)90068-8. [DOI] [PubMed] [Google Scholar]
- 6.Pascher I. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim Biophys Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- 7.Stewart RJ, Boggs JM. A carbohydrate-carbohydrate interaction between galactosylceramide-containing liposomes and cerebroside sulfate-containing liposomes: Dependence on the glycolipid ceramide composition. Biochemistry. 1993;32:10666–10674. doi: 10.1021/bi00091a017. [DOI] [PubMed] [Google Scholar]
- 8.Morell P, Radin NS. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 1969;8:506–512. doi: 10.1021/bi00830a008. [DOI] [PubMed] [Google Scholar]
- 9.Bosio A, Binczek E, Stoffel W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc Natl Acad Sci U S A. 1996;93:13280–13285. doi: 10.1073/pnas.93.23.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: Normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 11.Coetzee T, Suzuki K, Popko B. New perspectives on the function of myelin galactolipids. Trends Neurosci. 1998;21:126–130. doi: 10.1016/s0166-2236(97)01178-8. [DOI] [PubMed] [Google Scholar]
- 12.Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 2002;1573:406–413. doi: 10.1016/s0304-4165(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 13.Stoffel W, Bosio A. Myelin glycolipids and their functions. Curr Opin Neurobiol. 1997;7:654–661. doi: 10.1016/s0959-4388(97)80085-2. [DOI] [PubMed] [Google Scholar]
- 14.Fewou SN, Bussow H, Schaeren-Wiemers N, Vanier MT, Macklin WB, Gieselmann V, Eckhardt M. Reversal of non-hydroxy: Alpha-hydroxy galactosylceramide ratio and unstable myelin in transgenic mice overexpressing udp-galactose: Ceramide galactosyltransferase. J Neurochem. 2005;94:469–481. doi: 10.1111/j.1471-4159.2005.03221.x. [DOI] [PubMed] [Google Scholar]
- 15.Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. Fa2h-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res. 2006;47:2772–2780. doi: 10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11:2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobue G, Shuman S, Pleasure D. Schwann cell responses to cyclic amp: Proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986;362:23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- 18.Bansal R, Pfeiffer SE. Regulated galactolipid synthesis and cell surface expression in schwann cell line d6p2t. J Neurochem. 1987;49:1902–1911. doi: 10.1111/j.1471-4159.1987.tb02453.x. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Alderson NL, Walla MD, Hama H. A novel method for measurement of in vitro fatty acid 2-hydroxylase activity in biological samples by gas chromatography-mass spectrometry. J Lipid Res. 2005;46:1569–1575. doi: 10.1194/jlr.D500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 22.Heape A, Juguelin H, Fabre M, Boiron F, Cassagne C. A quantitative developmental study of the peripheral nerve lipid composition during myelinogenesis in normal and trembler mice. Brain Res. 1986;390:181–189. doi: 10.1016/s0006-8993(86)80226-8. [DOI] [PubMed] [Google Scholar]
- 23.Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497–508. doi: 10.1002/jnr.10327. [DOI] [PubMed] [Google Scholar]
- 24.Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human fa2h gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- 25.Eckhardt M, Yaghootfam A, Fewou SN, Zoller I, Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem J. 2005;388:245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishimoto Y, Akanuma H, Singh I. Fatty acid alpha-hydroxylation and its relation to myelination. Mol Cell Biochem. 1979;28:93–105. doi: 10.1007/BF00223361. [DOI] [PubMed] [Google Scholar]
- 27.Hoshi M, Kishimoto Y. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J Biol Chem. 1973;248:4123–4130. [PubMed] [Google Scholar]
- 28.Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, Elias PM, Holleran WM. Fatty acid 2-hydroxylase, encoded by fa2h, accounts for differentiation-associated increase in 2-oh ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–13219. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- 29.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do lasses (longevity assurance genes) become cers (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 31.Downing DT. Lipid and protein structures in the permeability barrier of mammalian epidermis. J Lipid Res. 1992;33:301–313. [PubMed] [Google Scholar]
- 32.Breimer ME. Distribution of molecular species of sphingomyelins in different parts of bovine digestive tract. J Lipid Res. 1975;16:189–194. [PubMed] [Google Scholar]
- 33.Breimer ME, Karlsson KA, Samuelsson BE. Presence of phytosphingosine combined with 2-hydroxy fatty acids in sphingomyelins of bovine kidney and intestinal mucosa. Lipids. 1975;10:17–19. doi: 10.1007/BF02532188. [DOI] [PubMed] [Google Scholar]
- 34.Robinson BS, Johnson DW, Poulos A. Novel molecular species of sphingomyelin containing 2-hydroxylated polyenoic very-long-chain fatty acids in mammalian testes and spermatozoa. J Biol Chem. 1992;267:1746–1751. [PubMed] [Google Scholar]
- 35.Bouhours JF, Bouhours D, Hansson GC. Developmental changes of glycosphingolipid composition of epithelia of rat digestive tract. Adv Lipid Res. 1993;26:353–372. [PubMed] [Google Scholar]
- 36.Dahiya R, Brown MD, Brasitus TA. Distribution of glycosphingolipids of monkey small and large intestinal mucosa. Lipids. 1986;21:107–111. doi: 10.1007/BF02534429. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson O, Svennerholm L. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J Lipid Res. 1982;23:327–334. [PubMed] [Google Scholar]
- 38.Decker L, Baron W, Ffrench-Constant C. Lipid rafts: Microenvironments for integrin-growth factor interactions in neural development. Biochem Soc Trans. 2004;32:426–430. doi: 10.1042/BST0320426. [DOI] [PubMed] [Google Scholar]
- 39.Arvanitis DN, Min W, Gong Y, Heng YM, Boggs JM. Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin. J Neurochem. 2005;94:1696–1710. doi: 10.1111/j.1471-4159.2005.03331.x. [DOI] [PubMed] [Google Scholar]
- 40.Simons M, Kramer EM, Thiele C, Stoffel W, Trotter J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol. 2000;151:143–154. doi: 10.1083/jcb.151.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.