Abstract
The non-receptor protein-tyrosine phosphatases (PTPs) 1B and T-cell phosphatase (TCPTP) have been implicated as negative regulators of multiple signaling pathways including receptor-tyrosine kinases. We have identified PTP1B and TCPTP as negative regulators of the hepatocyte growth factor receptor, the Met receptor-tyrosine kinase. In vivo, loss of PTP1B or TCPTP enhances hepatocyte growth factor-mediated phosphorylation of Met. Using substrate trapping mutants of PTP1B or TCPTP, we have demonstrated that both phosphatases interact with Met and that these interactions require phosphorylation of twin tyrosines (Tyr-1234/1235) in the activation loop of the Met kinase domain. Using confocal microscopy, we show that trapping mutants of both PTP1B and the endoplasmic reticulum-targeted TCPTP isoform, TC48, colocalize with Met and that activation of Met enables the nuclear-localized isoform of TCPTP, TC45, to exit the nucleus. Using small interfering RNA against PTP1B and TCPTP, we demonstrate that phosphorylation of Tyr-1234/1235 in the activation loop of the Met receptor is elevated in the absence of either PTP1B or TCPTP and further elevated upon loss of both phosphatases. This enhanced phosphorylation of Met corresponds to enhanced biological activity and cellular invasion. Our data demonstrate that PTP1B and TCPTP play distinct and non-redundant roles in the regulation of the Met receptor-tyrosine kinase.
The receptor for hepatocyte growth factor/scatter factor, Met, controls a program of epithelial growth and remodeling through the coordination of cell proliferation and survival, cell migration, and epithelial morphogenesis (1). This process is important both during embryogenesis and for organ regeneration in the adult (2-4). In response to HGF5 binding, the catalytic kinase activity of the Met receptor is elevated, leading to intermolecular autophosphorylation and recruitment of signaling molecules. These, through a series of protein-protein interactions, transduce the extracellular signal to the interior of the cell. Although numerous studies have addressed mechanisms resulting in elevation of Met RTK activity (5), little is known about the mechanisms involved in down-regulation of the Met receptor.
Biological consequences resulting from RTK activation are determined by the duration, intensity, and specificity of the signals activated downstream of the receptor. Regulation of signals occurs on multiple levels, including the RTK itself. Ligand activation of the Met receptor promotes internalization of the receptor into the endocytic pathway with subsequent degradation in the lysosome (6). Termination of RTK signaling has been correlated with receptor dephosphorylation, degradation, or sequestration from the cytoplasm (5). In addition to receptor internalization, trafficking, and degradation in the lysosome as mechanisms of down-regulation, the Met receptor has been identified as a substrate for several PTPs. The receptor PTP LAR (leukocyte antigen-related) targets the Met receptor in confluent cells. This interaction is initiated after cell-cell contact and suppresses the mitogenic response of primary rat hepatocytes, resulting in contact inhibition (7). Another receptor PTP, DEP-1, has been proposed to negatively regulate Met RTK signaling via dephosphorylation of the carboxyl-terminal tyrosine residues in Met that are involved in substrate binding (8). Although DEP-1 suppresses Met signaling, it does not dephosphorylate the tyrosine residues in the activation loop (Tyr-1234/1235) that are required for Met catalytic activity. Because Met continues to signal after internalization (9), other PTPs are likely to be involved in the suppression and/or down-regulation of Met receptor catalytic activity.
We have recently established that phosphorylation of Met is elevated in PTP1B-null mice after treatment with Fas ligand, suggesting that the Met receptor may be a target for this phosphatase (10). PTP1B is a non-receptor tyrosine phosphatase that has been shown to either genetically or biochemically interact with multiple RTKs, including the insulin, insulin-like growth factor, EGF, and PDGFβ receptors (11). PTP1B possesses a phosphatase catalytic domain and a proline-rich domain. It interacts with and dephosphorylates multiple proteins including p130Cas, p62Dok, Jak2, and Tyk2 (12-17). At its carboxyl terminus, it contains an endoplasmic reticulum (ER)-targeting signal that anchors PTP1B to the cytoplasmic face of the ER (18, 19). The role of PTP1B in the down-regulation of the insulin receptor (IR) has been extensively studied. Liver and muscle from PTP1B knock-out (KO) mice show hyperphosphorylation of the IR upon stimulation with insulin. These mice are resistant to weight gain caused by a high fat diet, demonstrating that loss of PTP1B has physiological consequences in vivo (20, 21).
Recent studies show that internalization of the EGF and PDGF receptors is required for their colocalization with PTP1B (22), supporting a role for PTP1B in the dephosphorylation and down-modulation of internalized RTKs. The mechanism by which PTP1B interacts with its substrates has been elucidated. Biochemical studies have demonstrated that the affinity of PTP1B for a substrate containing twin tyrosines is 70-fold higher than for a substrate in which one tyrosine is substituted with phenylalanine (23). Moreover, using peptides corresponding to the activation loop of the kinase domain of the IR, the catalytic domain of PTP1B has been shown to associate with the phosphorylated twin tyrosines present in this sequence (23). Additionally, recent studies have shown that PTP1B docks on the kinase domain of the IR in a region opposite to the activation loop that contains these tyrosine residues (24).
A given RTK can be the substrate of several phosphatases, emphasizing the importance of this mechanism. For example, the EGF receptor is thought to be negatively down-regulated by PTPΩ, LAR, SHP-1, PTP1B, and TCPTP (25-28), and the IR has been shown to associate with and be dephosphorylated by PTP1B (20, 21, 29), TCPTP (30, 31), and PTPε (32), among others.
PTP1B is most closely related to the non-receptor TCPTP, which is expressed in all tissues and at all stages of development (33). TCPTP is alternatively spliced, yielding two isoforms that differ in their non-catalytic carboxyl termini: the ER-localized 48 kDa form (TC48) and the nuclear-localized 45-kDa form (TC45) (34). Both TC48 and TC45 are present in humans (35), but only TC45 has been identified in mouse (36). TCPTP can regulate signaling pathways by dephosphorylating its substrates, which include the EGF (37), PDGF-β (38, 39) and insulin receptors (30, 31, 40), JAK1 and JAK3 (41), p52Shc (37), STAT1 (42), and Src (43). Although PTP1B and TCPTP are closely related and are structurally similar (44), they possess differential specificities for recognition of substrates (30, 43). Moreover, siRNA against TCPTP or PTP1B in vivo has demonstrated that PTP1B and TCPTP do not act additively on the IR in mouse liver (45).
The interaction of a wild-type (WT) phosphatase with its substrate is transient. Substrate trapping mutants of PTPs have been engineered in which a single aspartic acid residue in the catalytic domain is replaced with alanine (D/A mutants) (27). These proteins are capable of binding to PTP substrates but are slow to catalyze the dephosphorylation reaction and, thus, form a stable intermediate with the substrate (46). Hence, such mutants compete when co-expressed with WT PTPs. In this study we demonstrate a role for PTP1B and TCPTP in the dephosphorylation of the Met RTK and show that substrate trapping mutants of either PTP1B or TCPTP (TC48) are recruited to an internalized Met receptor. Moreover, Met activation promotes nuclear exit of TCPTP (TC45) and recruitment to the membrane, indicating a role for each phosphatase in the dephosphorylation and modulation of the signal from the Met receptor.
EXPERIMENTAL PROCEDURES
DNA Constructs and siRNA—GFP-TC48 or GFP-TC45 WT and D/A constructs were created by cloning TC45 or TC48 WT or D/A into the BglII and HindIII sites of the pEGFPC2 vector (Clontech, Mountain View, CA). PTP1B WT and D/A was inserted into the BglII and EcoRI sites of the pEGFPC2 vector. TC48 was a kind gift from N. Tonks. Tpr-Met and its various mutants, Met and Met-Y1003F, have been described previously (9, 47). siRNA duplexes against the PTP1B target sequence CACGTGGGTATTTAATAAGAA and TCPTP target sequence CACAAAGAAGTTACATCTTAA were obtained from Qiagen (Mississauga, ON, Canada) and transfected into HeLa cells for 48 h using Hiperfect (Qiagen) following the manufacturer's instructions.
Cell Culture and DNA Transfections—All cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and antibiotics. For expression of cDNAs, 1 × 106 HEK 293 cells were seeded 24 h before transient transfection using Lipofectamine Plus (Invitrogen), carried out following the manufacturer's instructions. Media was replaced 3 h post-transfection, and cells were lysed 24 h posttransfection in lysis buffer (50 mm HEPES, 150 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 1% Triton X-100, 10% glycerol) lacking sodium vanadate unless otherwise indicated in the figure legends. 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mm phenylmethylsulfonyl fluoride were added to the lysis buffer before use.
Antibodies and Reagents—A rabbit polyclonal antibody raised against a peptide from the carboxyl terminus of human (48) or mouse (49) Met was used for anti-Met immunoprecipitations and Western blots, and anti-Met p-Tyr-1234/35, p-Tyr-1349, p-Tyr-100, and p-Tyr-1003, anti-pJNK, and JNK were purchased from Cell Signaling Technologies (Pickering, ON, Canada). Anti-Met B2 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), anti-p-Tyr-20 was from Berkeley Antibody Co., Inc./Bio-Can (Mississauga, ON, Canada), and anti-Myc 9E10, anti-EEA1, and anti-GFP were from Upstate Biotechnologies Inc. (Lake Placid, NY). An antibody against the extracellular region of Met (anti-Met AF276) was purchased from R&D Systems (Minneapolis, MN).
Immunoprecipitation and Western Blotting—500 μg of HEK 293 cell lysates were used for each immunoprecipitation. The antibody was allowed to bind for 1 h at 4 °C, after which 10 μl of protein A or protein G-Sepharose beads were added to collect immune complexes. Beads were washed twice in lysis buffer, then resuspended in 20 μl of Laemmli sample buffer and boiled for 10 min. Supernatants were separated on 10% SDS-PAGE gels. Proteins were then transferred onto nitrocellulose membranes (Hybond-C, Amersham Biosciences, Baie d'Urfe, Québec, Canada), which were blocked with 3% bovine serum albumin and probed with the appropriate antibody. Horseradish peroxidase-linked secondary antibodies (Amersham Biosciences) indicating the positions of target proteins were visualized by enhanced chemiluminescence (Amersham Biosciences). Odyssey blocking buffer, IRDye800 anti-rabbit, and Cy5.5 anti-mouse secondary antibodies for use with the Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE) were purchased from Rockland (Gilbertsville, PA). Analyses using the Odyssey infrared imaging system were performed according to the manufacturer's instructions.
Animal Experiments—WT and PTP1B-null mice are a hybrid of 129S/v and BALB/c backgrounds (20). 4-6-week-old mice were injected with 15 nm HGF via the hepatic portal vein, and livers were excised at the times indicated. All experiments were carried out in accordance with approved animal use protocols (McGill University). Whole livers were lysed in a buffer containing 50 mm Tris, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 2 mm EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, and 1 mm sodium vanadate. The Met receptor was immunoprecipitated from 3 mg of protein lysate using the mouse-specific anti-Met antibody (49). Blots were probed with antibodies as indicated in figure legends and quantified using the NIH ImageJ software. Each experiment was carried out a minimum of three times.
Confocal Immunofluorescence Microscopy—2 × 104 HeLa cells or 1 × 105 T47D cells were seeded on glass coverslips (Bellco Glass Inc. Vineland, NJ) in 24-well plates (Nalgene NUNC, Rochester, NY) and transfected with the indicated DNA using Lipofectamine Plus (Invitrogen) following the manufacturer's instructions 16 h post-plating. 16 h later, cells were serum-starved for 2 h either with or without cycloheximide (0.1 mg/ml) before HGF treatment (1.5 nm) as indicated. Coverslips were washed once with PBS and then fixed in 2% paraformaldehyde (PFA; Fisher) in PBS for 20 min. Coverslips were then washed 4 times in PBS, and residual PFA was removed by three 5-min washes with 100 mm glycine/PBS. Cells were permeabilized with 0.3% Triton X-100/PBS and blocked for 30 min with blocking buffer (5% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS). Coverslips were incubated with primary and secondary antibodies diluted in blocking buffer for 1 h and 40 min, respectively, at room temperature. Coverslips were washed four times in IF buffer (0.5% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS) between primary and secondary antibodies. Coverslips were mounted with Immumount (Thermo-Shandon, Pittsburgh, PA). Confocal images were taken using a Zeiss 510 Meta laser scanning confocal microscope (Carl Zeiss Canada Ltd, Toronto, ON, Canada) with a 100× objective. Image analysis was carried out using the LSM 5 image browser (Empix Imaging, Mississauga, ON, Canada).
Invasion Assays—3 × 104 HeLa cells were seeded directly onto 6.5-mm Corning Costar transwells coated with 100 μg/cm2 Matrigel (BD Biosciences). Complete media was added to both the top and bottom wells, and cells were incubated at 37 °C overnight. For HGF stimulations, 0.15 nm HGF was added to the bottom wells. After the overnight incubation, cells on both sides of the transwells were fixed using formalin phosphate for 20 min at room temperature. After washing with PBS, cells were stained with 0.1% crystal violet in 20% methanol for 20 min at room temperature. Cells on the top layer were scraped, and membranes were left to dry overnight. Images were captured using a Retiga 1300 digital camera (QIMAGING, Burnaby, BC, Canada) and a Zeiss AxioVert 135 microscope (Carl Zeiss Canada Ltd.). Imaging of these assays was carried out with a 10× objective, and images were analyzed using the ImageScope software (Aperio Technologies, Vista, CA). Each experiment was carried out a minimum of three times.
RESULTS
Loss of PTP1B Results in Increased Levels of Met Phosphorylation—To test if Met is a physiological substrate for PTP1B, PTP1B-null mice were injected via the hepatic portal vein with 15 nm HGF, the ligand for the Met receptor. Livers were harvested at the times indicated, and proteins from lysates were subjected to immunoprecipitation using an antibody specific to murine Met protein. Increase in phosphorylation of Met was normalized to the levels of Met receptor as evaluated by densitometry (Fig. 1). Notably, we observe no significant difference in basal tyrosine phosphorylation of the Met receptor in livers from WT or PTP1B-null mice (Fig. 1A). However, in response to HGF, tyrosine phosphorylation of the Met receptor was enhanced significantly in PTP1B-null animals when compared with livers from WT animals (Fig. 1, A and B). Using general anti-phosphotyrosine antibodies (p-Tyr-100), we observe that the overall tyrosine phosphorylation of Met in PTP1B-null animals is 4-fold higher than Met phosphorylation in WT animals when stimulated with HGF for similar time periods (Fig. 1, A and B).
FIGURE 1.
In response to HGF, Met is hyperphosphorylated in livers from PTP1B-null mice versus WT mice. A, PTP1B WT or null mice were injected with 15 nm HGF via the hepatic portal vein at the times indicated. Protein extracts prepared from livers of the injected mice were subjected to immunoprecipitation using an anti-mouse Met antibody and probed with the anti-phosphotyrosine antibody, p-Tyr-100, or phosphotyrosine antibodies directed against specific tyrosines of the Met receptor (p-Tyr-1003, p-Tyr-1365, or p-Tyr-1234/1235). Each time point represents an individual mouse. B, densitometric analysis of the data presented in A measured by NIH ImageJ and normalized against total Met levels from three independent replicates. C, protein lysates from livers of mice injected with HGF for times indicated probed with anti-pJNK, JNK, pERK, or total ERK antibodies. D, densitometric analysis of the p54 JNK blot shown in C compared against total protein levels. The error bars represent S.E. Each experiment was performed a minimum of three times.
To provide insight into the specificity of enhanced Met phosphorylation in PTP1B-null animals, we utilized Met tyrosine-phosphorylation specific antibodies. Upon binding HGF, Met is autophosphorylated on tyrosine 1234 and 1235 in the activation loop of the Met catalytic domain (47). In response to HGF, phosphorylation of these tyrosines is required for activation of Met kinase and subsequent phosphorylation of other sites in the Met receptor, including Tyr-1003 in the juxtamembrane domain and Tyr-1003 and -1356 in the Met carboxyl terminus (47, 50-52). Utilizing phosphotyrosine-specific antibodies to compare phosphorylation of Tyr-1234/1235, Tyr-1003 or Tyr-1365 of Met in response to HGF in livers from WT and PTP1B-null mice, we observed an overall increase in phosphorylation of these tyrosines of 4-6-fold when compared with lysates of livers from WT mice (Fig. 1, A and B). In response to HGF, phosphorylation of tyrosines 1234/35 is elevated in PTP1B-null mice when compared with WT mice, demonstrating for the first time in vivo that the phosphorylation state of Tyr-1234/1235, required for the catalytic activity of the Met receptor, is regulated by PTP1B (Fig. 1, A and B). As expected, enhanced phosphorylation of Tyr-1234/1235 coincides with enhanced phosphorylation of non-catalytic tyrosines, Tyr-1003 in the juxtamembrane domain and Tyr-1365 in the carboxyl terminus (Fig. 1, A and B). To examine whether loss of PTP1B also impacts on downstream signaling pathways, the engagement of the JNK and ERK pathways were studied using phosphospecific antibodies. Consistent with enhanced phosphorylation of Met, increased phosphorylation of the Met-activated pathway components ERK1/2 in HGF-injected livers from KO versus WT mice was observed (Fig. 1C). Interestingly, we saw no induction of p46 Jnk1, whereas p54 Jnk2 was elevated in PTP1B KO livers after HGF portal vein injection when compared with WT (Fig. 1, C and D), consistent with an increase in Jnk2/3 activation post-HGF stimulation in rat hepatoma cells (53). These results are consistent with the Met receptor being a physiological substrate for PTP1B in vivo, which regulates its catalytic activity and subsequent downstream signaling.
Dephosphorylation of Met in Response to HGF—Previous data from our laboratory and others has shown that down-regulation of Met can take place via internalization followed by degradation of the receptor (6, 9). To study the role of dephosphorylation in down-regulation of the Met receptor, the endogenous Met receptor in HeLa cells was stimulated with HGF at 4 °C for 60 min, and activation of the receptor as visualized through anti-phosphotyrosine Western blotting was followed during a chase in medium lacking HGF at 37 °C. Under these conditions, loss of tyrosine phosphorylation (t½) of the receptor was detectable as early as 15 min post-chase, whereas Met itself was stable (Fig. 2, A and B). These data demonstrate that under these conditions Met is significantly dephosphorylated before degradation.
FIGURE 2.
Dephosphorylation precedes Met degradation. A, HeLa cells were stimulated with HGF at 4 °C for 60 min and chased at 37 °C for times indicated. Protein extracts were subjected to immunoprecipitation using the human anti-Met antibody and probed with an anti-p-Tyr-20 and anti-Met antibody simultaneously. Western blots were analyzed using the Odyssey imaging system (Licor) and then probed for actin as loading control. B, densitometric analysis of data presented in Fig. 2A measured by NIH ImageJ program.
Knockdown of TCPTP or PTP1B Results in Increased Phosphorylation of the Met RTK—To establish if PTP1B and its closely related enzyme TCPTP dephosphorylate the Met RTK, Met phosphorylation was examined in HeLa cells transfected with scrambled or siRNA designed to target either PTP1B, TCPTP, or both TCPTP and PTP1B (Fig. 3). Upon stimulation with HGF, Met tyrosine phosphorylation was elevated in cells in which the levels of either PTP1B or TCPTP were knocked down by specific siRNA but not in cells transfected with scrambled siRNA (Fig. 3A). Met tyrosine phosphorylation increased up to 2-fold in the presence of siRNA for either PTP1B or TCPTP alone (Fig. 3B). Notably, when both PTP1B and TCPTP are targeted together, up to 3-fold higher tyrosine phosphorylation of Met was observed, suggesting an additive role for these phosphatases in the regulation of tyrosine phosphorylation of Met (Fig. 3, A and B).
FIGURE 3.
Met is hyperphosphorylated in response to HGF in the absence of either PTP1B or TCPTP. A, HeLa cells were transfected with scrambled, PTP1B, TCPTP, or TCPTP + PTP1B siRNA and stimulated with HGF for the times indicated. Proteins from lysates were immunoprecipitated with the anti-human Met antibody and immunoblotted for either Met p-Tyr-1234/1235 or total Met. Protein lysates were also probed for TCPTP and PTP1B. B, phosphorylated Met/total Met quantitation of three independent replicates. The error bars represent S.E.
Knockdown of TCPTP or PTP1B Results in Enhanced HGF-dependent Cell Invasion—To study the physiological relevance of the Met-PTP1B/TCPTP interaction, HeLa cells transfected with scrambled or siRNA designed to target PTP1B, TCPTP, or both TCPTP and PTP1B were trypsinized and seeded on Matrigel in the presence or absence of HGF. The HGF-dependent invasion of HeLa cells treated with siRNA to PTP1B or TCPTP was 1.3 and 1.6-fold higher, respectively, than that of cells treated with scrambled siRNA alone (Fig. 4). These data represent an average of four independent experiments. Moreover, knockdown of both phosphatases results in enhanced invasion (1.8-fold) supporting a role for TCPTP and PTP1B in Met dephosphorylation and negative regulation of Met biological activity.
FIGURE 4.
Loss of PTP1B or TCPTP increases the invasive potential of cells in response to HGF. PTP1B, TCPTP, and TCPTP and PTP1B or scrambled siRNA-treated cells were seeded on Matrigel in the absence or presence of HGF. A, representative images of invading cells post-fixing and staining, treated as indicated. B, quantitative representation of number of invading cells. The experiment was performed a minimum of three times, and statistical analysis was performed using Students t test. Error bars represent S.E. NS, not significant; UT, untransfected.
TCPTP or PTP1B (D/A) Substrate Trapping Mutants Interact with the Met RTK—PTPs contain a conserved aspartate that is required as a proton acceptor in the phosphate hydrolysis reaction. Substitution of this residue with alanine (D/A) results in a PTP that can form complexes with tyrosine-phosphorylated proteins in a cellular context (27). These mutants, termed trapping mutants, have provided a powerful mechanism to identify putative substrates for tyrosine phosphatases (54, 55). To establish if PTP1B or TCPTP can interact with the Met RTK as a cellular substrate and if they interact with similar tyrosines in the Met RTK, GFP fusion proteins of PTP1B, TC45 or TC48 WT or D/A (trapping mutants) were co-expressed with a constitutively active variant of the Met RTK (Tpr-Met) (56, 57). The trapping mutants, but not WT PTP1B or either isoform of WT TCPTP, were able to associate with Tpr-Met as evident from their coimmunoprecipitation (Fig. 5A). To establish if association of either phosphatase with Met is dependent on the ability of the catalytic domain of TC45, TC48, or PTP1B to interact with Met, the ability of Met to be trapped by these phosphatases was tested in the presence of sodium orthovanadate, a potent nonspecific phosphatase inhibitor that binds to the phosphatase catalytic domain (58). The addition of sodium orthovanadate abrogated the ability of TC45, TC48, and PTP1B (D/A) to coimmunoprecipitate with Tpr-Met, indicating that this interaction is specific to the ability of the phosphatase to bind to its substrate via the phosphatase catalytic domain (Fig. 5B).
FIGURE 5.
Met stably interacts with substrate trapping mutants of PTP1B and TCPTP. Extracts prepared from HEK 293 cells co-expressing Tpr-Met and either GFP-tagged WT (WT) PTP1B (1B), and TCPTP (TC45 or TC48) or their respective trapping mutants (DA) were immunoprecipitated using an anti-GFP antibody. A, immunoprecipitates of either PTP1B, TC45, or TC48 were probed for the presence of Met, phosphotyrosine (p-Tyr-100), and GFP. Immunoblot analysis of whole cell lysates (WCL) was performed with antibodies against Met and GFP to examine levels of Met, PTP1B, TC45, and TC48. B, immunoprecipitates of either PTP1B, TC45, or TC48 from cells lysed in the presence or absence of 1 mm sodium orthovanadate (NaV) were probed for the presence of Met, GFP, and overall phosphotyrosine (p-Tyr-100). Immunoblot analysis of WCL was performed with antibodies against Met, GFP, and p-Tyr-100 to examine levels of Met, phosphatases (PTP1B, TC45, TC48), and phosphotyrosine. Immunoblot analysis of WCL with actin was used as the loading control.
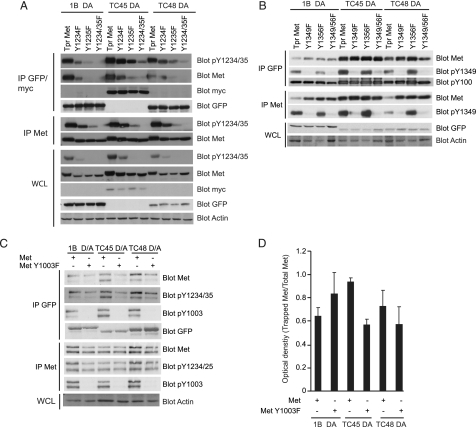
Interaction of PTP1B and TCPTP with Met Requires Activation Loop Tyrosines—Crystallographic analysis of PTP1B in complex with a peptide corresponding to the tandem tyrosines present in the activation loop of the IR revealed that PTP1B engages both of these tyrosines, one at the PTP1B catalytic pocket and the second at an adjacent p-Tyr binding pocket (23). The second site is conserved in TCPTP (44). Similarly, analysis of the crystal structure of the catalytic domain of TCPTP shows a high degree of structural similarity to PTP1B (44). Both TCPTP and PTP1B recognize the twin tyrosines of the IR in vivo (30, 31); hence, they may interact with analogous tandem tyrosines (located at Tyr-1234 and Tyr-1235) in the Met receptor. To test this, the ability of Tpr-Met or Tpr-Met carrying phenylalanine substitutions of Tyr-1234, Tyr-1235, or Tyr-1234/1235 in the activation loop to coimmunoprecipitate with TC45, TC48, or PTP1B (D/A) mutants was examined after transient co-transfections. Coimmunoprecipitation of tyrosine-phosphorylated Met was monitored with antibodies specific for phosphotyrosines (p-Tyr-100), and the Met-specific p-Tyr-1234/1235. Coimmunoprecipitation of tyrosine-phosphorylated Met with TCPTP (D/A) or PTP1B (D/A) decreased significantly when either Tyr-1234 or Tyr-1235 was substituted with phenylalanine (Fig. 6A). Moreover, substitution of both tyrosine residues additionally decreased the ability of both TCPTP (D/A) and PTP1B (D/A) mutants to coimmunoprecipitate with Met (Fig. 6A). Consistent with studies of the IR (30), these data demonstrate a requirement for Tyr-1234 and Tyr-1235 in the Met receptor activation loop for interaction with PTP1B and TCPTP.
FIGURE 6.
Interaction of Met with PTP1B or TCPTP requires tyrosines Tyr-1234 and Tyr-1235 of the Met receptor. A, protein lysates from HEK 293 cells co-expressing a combination of either GFP-tagged trapping mutants of PTP1B or TC48 or Myc-tagged TC45 and 1234 and/or 1235 twin tyrosine substitutions of Tpr-Met were subjected to immunoprecipitation using an anti-GFP or anti-Myc antibody as indicated, and immunoblot analysis was performed on both the immunoprecipitates and the corresponding WCL with antibodies as indicated. B, immunoprecipitation was performed with anti-GFP antibody, and blots were probed with antibodies specific for p-Tyr-100, Met, Met p-Tyr-1349, and GFP in lysates of cells expressing GFP-tagged PTP1B, TC45 or TC48, and Met mutants as indicated. Met receptor was immunoprecipitated, and immunoblot analysis was performed with antibodies raised against p-Tyr-100, Met, or Met p-Tyr-1349. WCL were probed with an antibody against GFP to examine levels of phosphatase present in each lane. C, immunoprecipitations were performed with anti-GFP and anti-Met antibodies in lysates of cells expressing GFP-tagged phosphatases and Met mutants where indicated, and immunoblot analysis was performed with antibodies against Met, Met p-Tyr-1234/1235, Met p-Tyr-1003, and GFP. D, densitometric analysis of amount of Met trapped by PTP1B, TC45, or TC48. Analysis was performed using NIH ImageJ. Results shown are the average of three independent experiments. The error bars in D represent S.E. Immunoblot analysis of WCL with actin was used as a loading control.
Our data establish that tyrosines within the activation loop of Met are required for interaction with PTP1B. However, because substitution of these residues decreases the kinase activity of the receptor (47), other tyrosine residues will exhibit decreased phosphorylation. To determine whether tyrosine residues outside the kinase domain and within the carboxyl terminus of Met, previously shown to play important roles in Met signaling, are targets for PTP1B or TCPTP, we performed further coimmunoprecipitation experiments. When PTP1B D/A or TCPTP D/A mutants were coexpressed with Tpr-Met mutants Y1349F, Y1356F, or Y1349/56F, we observed no significant decrease in the ability of PTP1B D/A or TCPTP D/A to coimmunoprecipitate with the Met mutants (Fig. 6B). Previous studies have suggested that Met-associated phosphatase activity requires Tyr-1003, a residue located in the juxtamembrane domain that is absent in Tpr-Met (59, 60). To determine whether TCPTP or PTP1B interacts with the full-length Met RTK, the ability of WT Met or the mutant Met-Y1003F to coimmunoprecipitate with the D/A mutants of each PTP was examined after transient transfection. Tyrosine phosphorylation of Met was monitored utilizing antibodies specific to Met Tyr-1234/1235 and Met Tyr-1003. Both WT Met and Met-Y1003F coimmunoprecipitated with D/A mutants of PTP1B and TCPTP (Fig. 6C), although the interaction of Met-Y1003F was decreased when compared with WT Met with TCPTP (Fig. 6, C and D). The small decrease in trapping of Y1003F by TCPTP suggests that Tyr-1003 may be involved in the interaction of TCPTP with Met and is consistent with previous studies demonstrating a decrease in phosphatase recruitment by this mutant (59). Taken together, our results demonstrate that tyrosines 1234 and 1235 of Met are essential for the interaction of Met with TCPTP and PTP1B, whereas Tyr-1003 may play an accessory role.
PTP1B D/A Trapping Mutant Colocalizes with an Activated Met Receptor—The ability of PTP1B to act as a phosphatase for the Met receptor in vivo in PTP1B KO mice (Fig. 1) and in HeLa cells (Fig. 3) and that of PTP1B D/A trapping mutants to coimmunoprecipitate with Met (Figs. 5 and 6) prompted us to examine the subcellular location at which this interaction occurs. PTP1B possesses an ER-targeting signal and localizes to the ER (22, 61). A role for PTP1B has been proposed in the dephosphorylation of RTKs after stimulation (18) as well as from the synthetic pathway (62). To examine the colocalization of Met and PTP1B in the absence of stimulation, HeLa cells were transfected with GFP-tagged WT or the substrate trapping mutant of PTP1B (D/A), and their localization was analyzed by confocal microscopy. In the absence of HGF, we observed localization of PTP1B D/A, but not WT PTP1B (data not shown), with the endogenous Met receptor in a perinuclear structure (Fig. 7A). To determine whether this localization is maintained in the absence of de novo synthesis of Met, HeLa cells were pretreated with cycloheximide, resulting in loss of Met from the pool of the synthetic pathway (Fig. 7B). Under these conditions the PTP1B D/A trapping mutant failed to localize with the unstimulated Met receptor. This is in agreement with the ability of PTP1B D/A to engage with receptors within the synthetic pathway (62). Significantly, after stimulation with HGF, PTP1B D/A, but not the GFP vector or PTP1B WT, was observed in peripheral puncta with the Met receptor at 5′ post-stimulation (Fig. 7B and supplemental Fig. 1), providing further evidence that in response to HGF, PTP1B is recruited to the Met receptor in the endosomal pathway.
FIGURE 7.
Localization of Met with PTP1B D/A. A, HeLa cells were seeded on coverslips and transfected with GFP-PTP1B D/A. B, 16 h post-transfection, cells were treated with cycloheximide (0.1 mg/ml) for 2 h and stimulated with 1.5 nm HGF at 37 °C for the indicated times. A and B, coverslips were fixed in 2% PFA and stained with Met AF276 and EEA1 antibodies (B only). Confocal images were taken with a 100× objective and 1× zoom. Yellow staining represents colocalization between endogenous Met (red) and TC48 (green) or EEA1 (green or purple, as shown). The scale bar is as indicated in the figure.
TC45 D/A Accumulates in the Cytoplasm after Ligand Stimulation of the Met Receptor—Under basal conditions TC45 is localized to the nucleus, but in response to stimuli, it can be released into the cytoplasm (31, 37, 63, 64). This can be visualized with the D/A trapping mutant, which when released from the nucleus is thought to be retained in the cytoplasm through its ability to interact directly with its substrates (31, 37). TC45 D/A is released from the nucleus in response to insulin and EGF stimulation of cells (31, 37). To establish if TC45 is released from the nucleus in response to HGF stimulation of the Met receptor, HeLa cells (which express endogenous Met) were transfected with an Myc-tagged TC45 D/A trapping mutant and stimulated with HGF. Under serum-starved conditions, TC45 D/A is localized to the nucleus as expected (Fig. 8A). Release of TC45 D/A from the nucleus was observed by 15 min of post-stimulation with HGF (Fig. 8A), demonstrating that nuclear exit of TCPTP is modulated after activation of the Met receptor.
FIGURE 8.
Met activation causes nuclear exit of TC45. A, HeLa cells were seeded on coverslips and transfected with Myc-TC45 D/A. 16 h post-transfection, cells were stimulated with 1.5 nm HGF at 37 °C for the indicated times. Coverslips were fixed in 2% PFA and stained with anti-Met AF276 or anti-Myc 9E10 antibodies. Confocal images were taken with a 100× objective and 1× zoom. Yellow staining represents colocalization between endogenous Met (red) and Myc-TC45 D/A (green). B, T47D cells were plated on coverslips and transiently transfected with Myc-TC45 and either WT Met or Met K1110A (kinase dead). 16 h post-transfection, coverslips were fixed in 2% PFA and stained with Met AF276 or Myc 9E10 antibodies. Confocal images were taken with a 100× objective and 1× zoom. Yellow staining represents colocalization between Met (red) and Myc-TC45 D/A (green). The scale bar is indicated in the figure.
To further study the requirement for Met activation in the nuclear exit of TC45 D/A, T47D cells, which possess undetectable levels of endogenous Met, were transfected with either WT Met or its kinase dead variant (K1110A). Met, when transiently overexpressed, is active in the absence of its ligand (48, 65). In the presence of active Met, TC45 D/A was localized in the cytoplasm and colocalizes with the active Met receptor, whereas in the presence of a kinase dead Met receptor (65), TC45 D/A remains within the nucleus, and Met is localized to the plasma membrane (Fig. 8B). These data further demonstrate the requirement for Met activation to mediate the nuclear exit of TCPTP and retention of the D/A variant in the cytosol.
TC48, like PTP1B, is an ER-targeted phosphatase (34). It has been shown to interact with the insulin and EGF receptors after stimulation (31, 37), but its role in targeting the RTK synthetic pathway has not been studied. To examine this, HeLa cells transiently transfected with the TC48 D/A trapping mutant were pretreated with cycloheximide to deplete the synthetic pool of Met receptor. As observed for PTP1B D/A in HeLa cells, in the absence of cycloheximide (Fig. 7A), TC48 D/A localizes with Met in a perinuclear compartment (Fig. 9A). However, in the presence of cycloheximide where Met is no longer present in the synthetic pathway, this colocalization is abrogated, and instead, under these conditions, TC48 shows colocalization with Met only after stimulation with HGF (Fig. 9B and supplemental Fig. 1). The GFP vector- and GFP TC48 WT-expressing cells do not show any colocalization with Met (Fig. 9B and supplemental Fig. 1).
FIGURE 9.
Localization of Met with TC48 D/A. A, HeLa cells were plated on coverslips and transfected with GFP-TC48 D/A. Yellow staining represents colocalization between endogenous Met (red) and TC48 (green). B, 16 h post-transfection cells were treated with cycloheximide (0.1 mg/ml) for 2 h and stimulated with 1.5 nm HGF at 37 °C for the indicated times. A and B, coverslips were fixed in 2% PFA and stained with Met AF276 and EEA1 antibody (B only). Confocal images were taken with a 100× objective and 1× zoom. Yellow staining represents colocalization between endogenous Met (red) and TC48 (green) or EEA1 (green or purple). The Scale bar is indicated in the figure.
DISCUSSION
A common theme in the regulation of RTKs via dephosphorylation is the involvement of multiple phosphatases, which specifically target different residues phosphorylated during RTK activation (66). Although several PTPs have been associated with Met dephosphorylation, to date none has been shown to act as a negative regulator of the major autophosphorylation sites of the Met receptor catalytic domain (Tyr-1234/1235). Here we show that two closely related enzymes of the PTP family, PTP1B and TCPTP, target the Met RTK. Our results demonstrate that Met is a physiological target for these enzymes and that they engage with the twin tyrosine residues (1234/35) within the activation loop of Met. Both PTP1B and TCPTP colocalize with endogenous Met after ligand-induced Met activation (Figs. 7 and 9). In addition, targeted loss of PTP1B or TCPTP leads to increased HGF-dependent phosphorylation of Met and increased cell invasion in response to HGF (Figs. 1 and 3).
We demonstrate enhanced Met phosphorylation in PTP1B-null animals and elevated downstream signaling in response to HGF (Fig. 1). This is similar to what was observed for the IR, where livers from PTP1B-null mice exhibited higher IR phosphorylation than livers from PTP1B-WT mice in response to insulin (20). However, unlike the IR, there was no difference in basal phosphorylation of the Met receptor in the absence of PTP1B (Fig. 1). These data provide additional evidence that PTP1B is not the only phosphatase negatively regulating the Met receptor. Supporting this hypothesis, we have shown that knockdown of either TCPTP or PTP1B leads to higher tyrosine phosphorylation of the activated Met receptor and that knockdown of both phosphatases results in an additive increase in Met tyrosine phosphorylation in response to stimulation (Fig. 3). In agreement with the observed increase in Met tyrosine phosphorylation, pathways downstream of Met, i.e. pERK and pJNK2/3, are more highly phosphorylated in livers from PTP1B-null mice in response to HGF (Fig. 1). This is consistent with the observed elevation of pERK downstream from insulin, EGF, and PDGF RTKs (30, 67) in PTP1B-null MEFs. However, because no increase was observed in basal phosphotyrosine levels of Met, our data indicate that other PTPs may be involved.
PTPs selectively recognize specific tyrosines on their target proteins. Here we show that neither TCPTP (D/A) nor PTP1B (D/A) mutants is able to trap a Met mutant protein in which the twin tyrosines located within the activation loop of the kinase domain (Tyr-1234/1235) are substituted with phenylalanine residues (Fig. 5). Phosphorylation of Tyr-1234/1235 is required for full catalytic activity of Met for phosphorylation of tyrosines outside the catalytic domain (47). Our data, therefore, demonstrates that both TCPTP and PTP1B can interact with the activated Met receptor. Hence, the mechanism through which TCPTP and PTP1B bind to Met may be similar to the interaction of PTP1B with the IR, where PTP1B interacts with the dual tyrosines in the activation loop of the receptor (23, 30, 31, 37, 63). In support of this, tyrosine to phenylalanine substitution mutants outside the kinase domain (Tyr-1003/1349/1356) retained the ability to bind trapping mutants of either PTP1B or TCPTP (Fig. 5, B-D). This is in contrast to DEP-1 receptor PTP, which specifically dephosphorylates Met Tyr-1349 (8). However, the ability of TCPTP but not PTP1B to trap Met was abrogated by ∼40% when Met Tyr-1003 was substituted with phenylalanine (Fig. 5, C and D), supporting previous reports of a role for Tyr-1003 in interacting with TCPTP (59).
Down-modulation of RTKs via degradation is a widely accepted mechanism by which a receptor signal is regulated. The Met RTK continues to signal upon internalization, demonstrating that the receptor is active within the trafficking compartment, and signal termination has been associated with Met degradation (9). However, we provide evidence supporting dephosphorylation of Met before degradation (Fig. 2), demonstrating that dephosphorylation of Met may modulate Met signaling in the cytoplasm. In support of this, after knockdown of either PTP1B, TCPTP, or both, there is a statistically significant increase in the ability of cells to invade in response to HGF (Fig. 4). Similarly, knockdown of PTP1B resulted in increased Met activation in the presence of the tyrosine phosphatase inhibitor, bpV(phen) (68). In addition, treatment with bpV(phen) delayed wound healing in rabbit corneas (68).
Previous studies have shown that interaction of EGF receptor and PDGF receptor with PTP1B requires internalization of the receptor (22). In agreement with this, we did not observe any colocalization between Met and either PTP1B or TCPTP in the absence of Met stimulation or when cycloheximide was used to block de novo protein synthesis, but we saw colocalization after HGF stimulation (Figs. 7 and 9). TC45, the nuclear isoform of TCPTP, is shown to interact with the insulin and EGF receptors only upon stimulation with ligand (31, 63). Consistent with this, we observed robust translocation of TC45 from the nucleus upon HGF stimulation (Fig. 8A) and its relocation to the vicinity of puncta that contain activated Met after stimulation (Fig. 8B). The release of TC45 from the nucleus into the cytosol in response to HGF was as early as 5 min post-HGF stimulation, suggesting that this is likely the result of Met signaling, independent of Met trafficking (Fig. 8 and data not shown), supporting a role for modulation of the Met signal during trafficking (9).
In this study, we have demonstrated that in addition to DEP-1 and LAR, TCPTP and PTP1B dephosphorylate the Met RTK, colocalize with Met after stimulation, and modulate Met receptor-mediated cellular invasion. These studies provide new evidence of mechanisms of down-regulation of the Met RTK and highlight the need for a fuller understanding of the role of tyrosine phosphatases in this process.
Supplementary Material
Acknowledgments
We thank Dr. Nick Tonks (Cold Spring Harbor Laboratories, NY) for the kind gift of TC48. Confocal images were collected at the McGill University Life Sciences Complex Imaging Facility supported by the Canada Foundation for Innovation.
This research was supported by a fellowship from Cancer Research Society, Inc. and the Terry Fox Foundation (National Cancer Institute of Canada) (to V. S.), by the McGill University Health Center and Faculty of Medicine, McGill University (to G. P.), by United States Department of Defense Breast Cancer Research Initiative Grant DAMD17-99-1-9284 (to J. A.), and by a Canadian Institutes of Health Research Doctoral Award and Alexander McFee Memorial Fellowship (to N. D.). This work was also supported by the Cancer Research Society, Inc. and Canadian Institutes of Health Research Operating Grant MOP-62887 (to M. L. T.) and by Canadian Institutes of Health Research operating Grant MOP-1154 (to M. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: HGF, hepatocyte growth factor; RTK, receptor-tyrosine kinase; EGF, epidermal growth factor; PDGF, platelet-derived growth factor; KO, knock out; WT, wild type; GFP, green fluorescent protein; JNK, c-Jun NH2-terminal kinase; PBS, phosphate-buffered saline; ERK, extracellular signal-regulated kinase; PTP, protein-tyrosine phosphatase; TCPTP, T-cell phosphatase; siRNA, small interfering RNA; ER, endoplasmic reticulum; IR, insulin receptor; PFA, paraformaldehyde; WCL, whole cell lysates; IP, immunoprecipitate.
References
- 1.Birchmeier, C., Birchmeier, W., Gherardi, E., and Vande Woude, G. F. (2003) Nat. Rev. Mol. Cell Biol. 4 915-925 [DOI] [PubMed] [Google Scholar]
- 2.Bladt, F., Riethmacher, D., Isenmann, S., Aguzzi, A., and Birchmeier, C. (1995) Nature 376 768-771 [DOI] [PubMed] [Google Scholar]
- 3.Borowiak, M., Garratt, A. N., Wustefeld, T., Strehle, M., Trautwein, C., and Birchmeier, C. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 10608-10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh, C. G., Factor, V. M., Sanchez, A., Uchida, K., Conner, E. A., and Thorgeirsson, S. S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4477-4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peschard, P., and Park, M. (2007) Oncogene 26 1276-1285 [DOI] [PubMed] [Google Scholar]
- 6.Hammond, D. E., Urbe, S., Vande Woude, G. F., and Clague, M. J. (2001) Oncogene 20 2761-2770 [DOI] [PubMed] [Google Scholar]
- 7.Machide, M., Hashigasako, A., Matsumoto, K., and Nakamura, T. (2006) J. Biol. Chem. 281 8765-8772 [DOI] [PubMed] [Google Scholar]
- 8.Palka, H. L., Park, M., and Tonks, N. K. (2003) J. Biol. Chem. 278 5728-5735 [DOI] [PubMed] [Google Scholar]
- 9.Abella, J. V., Peschard, P., Naujokas, M. A., Lin, T., Saucier, C., Urbe, S., and Park, M. (2005) Mol. Cell. Biol. 25 9632-9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangwan, V., Paliouras, G. N., Cheng, A., Dube, N., Tremblay, M. L., and Park, M. (2006) J. Biol. Chem. 281 221-228 [DOI] [PubMed] [Google Scholar]
- 11.Stuible, M., Doody, K. M., and Tremblay, M. L. (2008) Cancer Metastasis Rev. 27 215-230 [DOI] [PubMed] [Google Scholar]
- 12.Cheng, A., Uetani, N., Simoncic, P. D., Chaubey, V. P., Lee-Loy, A., McGlade, C. J., Kennedy, B. P., and Tremblay, M. L. (2002) Dev. Cell 2 497-503 [DOI] [PubMed] [Google Scholar]
- 13.Zabolotny, J. M., Bence-Hanulec, K. K., Stricker-Krongrad, A., Haj, F., Wang, Y., Minokoshi, Y., Kim, Y. B., Elmquist, J. K., Tartaglia, L. A., Kahn, B. B., and Neel, B. G. (2002) Dev. Cell 2 489-495 [DOI] [PubMed] [Google Scholar]
- 14.Cook, W. S., and Unger, R. H. (2002) Dev. Cell 2 385-387 [DOI] [PubMed] [Google Scholar]
- 15.Liu, F., Sells, M. A., and Chernoff, J. (1998) Mol. Cell. Biol. 18 250-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dube, N., Cheng, A., and Tremblay, M. L. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 1834-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers, M. P., Andersen, J. N., Cheng, A., Tremblay, M. L., Horvath, C. M., Parisien, J. P., Salmeen, A., Barford, D., and Tonks, N. K. (2001) J. Biol. Chem. 276 47771-47774 [DOI] [PubMed] [Google Scholar]
- 18.Tonks, N. K. (2003) FEBS Lett. 546 140-148 [DOI] [PubMed] [Google Scholar]
- 19.Cheng, A., Dube, N., Gu, F., and Tremblay, M. L. (2002) Eur. J. Biochem. 269 1050-1059 [DOI] [PubMed] [Google Scholar]
- 20.Elchebly, M., Payette, P., Michaliszyn, E., Cromlish, W., Collins, S., Loy, A. L., Normandin, D., Cheng, A., Himms-Hagen, J., Chan, C. C., Ramachandran, C., Gresser, M. J., Tremblay, M. L., and Kennedy, B. P. (1999) Science 283 1544-1548 [DOI] [PubMed] [Google Scholar]
- 21.Klaman, L. D., Boss, O., Peroni, O. D., Kim, J. K., Martino, J. L., Zabolotny, J. M., Moghal, N., Lubkin, M., Kim, Y. B., Sharpe, A. H., Stricker-Krongrad, A., Shulman, G. I., Neel, B. G., and Kahn, B. B. (2000) Mol. Cell. Biol. 20 5479-5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haj, F. G., Verveer, P. J., Squire, A., Neel, B. G., and Bastiaens, P. I. (2002) Science 295 1708-1711 [DOI] [PubMed] [Google Scholar]
- 23.Salmeen, A., Andersen, J. N., Myers, M. P., Tonks, N. K., and Barford, D. (2000) Mol. Cell 6 1401-1412 [DOI] [PubMed] [Google Scholar]
- 24.Li, S., Depetris, R. S., Barford, D., Chernoff, J., and Hubbard, S. R. (2005) Structure 13 1643-1651 [DOI] [PubMed] [Google Scholar]
- 25.Suarez Pestana, E., Tenev, T., Gross, S., Stoyanov, B., Ogata, M., and Bohmer, F. D. (1999) Oncogene 18 4069-4079 [DOI] [PubMed] [Google Scholar]
- 26.Kulas, D. T., Goldstein, B. J., and Mooney, R. A. (1996) J. Biol. Chem. 271 748-754 [DOI] [PubMed] [Google Scholar]
- 27.Flint, A. J., Tiganis, T., Barford, D., and Tonks, N. K. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 1680-1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenev, T., Keilhack, H., Tomic, S., Stoyanov, B., Stein-Gerlach, M., Lammers, R., Krivtsov, A. V., Ullrich, A., and Bohmer, F. D. (1997) J. Biol. Chem. 272 5966-5973 [DOI] [PubMed] [Google Scholar]
- 29.Seely, B. L., Staubs, P. A., Reichart, D. R., Berhanu, P., Milarski, K. L., Saltiel, A. R., Kusari, J., and Olefsky, J. M. (1996) Diabetes 45 1379-1385 [DOI] [PubMed] [Google Scholar]
- 30.Galic, S., Hauser, C., Kahn, B. B., Haj, F. G., Neel, B. G., Tonks, N. K., and Tiganis, T. (2005) Mol. Cell. Biol. 25 819-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galic, S., Klingler-Hoffmann, M., Fodero-Tavoletti, M. T., Puryer, M. A., Meng, T. C., Tonks, N. K., and Tiganis, T. (2003) Mol. Cell. Biol. 23 2096-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa, Y., Aoki, N., Aoyama, K., Shimizu, H., Shimano, H., Yamada, N., and Miyazaki, H. (2005) Zool. Sci. 22 169-175 [DOI] [PubMed] [Google Scholar]
- 33.Ibarra-Sanchez, M. J., Simoncic, P. D., Nestel, F. R., Duplay, P., Lapp, W. S., and Tremblay, M. L. (2000) Semin. Immunol. 12 379-386 [DOI] [PubMed] [Google Scholar]
- 34.Lorenzen, J. A., Dadabay, C. Y., and Fischer, E. H. (1995) J. Cell Biol. 131 631-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cool, D. E., Tonks, N. K., Charbonneau, H., Walsh, K. A., Fischer, E. H., and Krebs, E. G. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 5257-5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosinger, B., Jr., Tillmann, U., Westphal, H., and Tremblay, M. L. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 499-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiganis, T., Bennett, A. M., Ravichandran, K. S., and Tonks, N. K. (1998) Mol. Cell. Biol. 18 1622-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markova, B., Herrlich, P., Ronnstrand, L., and Bohmer, F. D. (2003) Biochemistry 42 2691-2699 [DOI] [PubMed] [Google Scholar]
- 39.Persson, C., Savenhed, C., Bourdeau, A., Tremblay, M. L., Markova, B., Bohmer, F. D., Haj, F. G., Neel, B. G., Elson, A., Heldin, C. H., Ronnstrand, L., Ostman, A., and Hellberg, C. (2004) Mol. Cell. Biol. 24 2190-2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng, T. C., Buckley, D. A., Galic, S., Tiganis, T., and Tonks, N. K. (2004) J. Biol. Chem. 279 37716-37725 [DOI] [PubMed] [Google Scholar]
- 41.Simoncic, P. D., Lee-Loy, A., Barber, D. L., Tremblay, M. L., and McGlade, C. J. (2002) Curr. Biol. 12 446-453 [DOI] [PubMed] [Google Scholar]
- 42.ten Hoeve, J., de Jesus Ibarra-Sanchez, M., Fu, Y., Zhu, W., Tremblay, M., David, M., and Shuai, K. (2002) Mol. Cell. Biol. 22 5662-5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Vliet, C., Bukczynska, P. E., Puryer, M. A., Sadek, C. M., Shields, B. J., Tremblay, M. L., and Tiganis, T. (2005) Nat. Immunol. 6 253-260 [DOI] [PubMed] [Google Scholar]
- 44.Iversen, L. F., Moller, K. B., Pedersen, A. K., Peters, G. H., Petersen, A. S., Andersen, H. S., Branner, S., Mortensen, S. B., and Moller, N. P. (2002) J. Biol. Chem. 277 19982-19990 [DOI] [PubMed] [Google Scholar]
- 45.Xu, J., Li, L., Hong, J., and Huang, W. (2007) Cell Biol. Int. 31 88-91 [DOI] [PubMed] [Google Scholar]
- 46.Xie, L., Zhang, Y. L., and Zhang, Z. Y. (2002) Biochemistry 41 4032-4039 [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues, G. A., and Park, M. (1994) Oncogene 9 2019-2027 [PubMed] [Google Scholar]
- 48.Rodrigues, G. A., Naujokas, M. A., and Park, M. (1991) Mol. Cell. Biol. 11 2962-2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, X. M., and Park, M. (1993) Dev. Biol. 157 308-320 [DOI] [PubMed] [Google Scholar]
- 50.Ponzetto, C., Bardelli, A., Maina, F., Longati, P., Panayotou, G., Dhand, R., Waterfield, M. D., and Comoglio, P. M. (1993) Mol. Cell. Biol. 13 4600-4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fixman, E. D., Holgado-Madruga, M., Nguyen, L., Kamikura, D. M., Fournier, T. M., Wong, A. J., and Park, M. (1997) J. Biol. Chem. 272 20167-20172 [DOI] [PubMed] [Google Scholar]
- 52.Fixman, E. D., Naujokas, M. A., Rodrigues, G. A., Moran, M. F., and Park, M. (1995) Oncogene 10 237-249 [PubMed] [Google Scholar]
- 53.Yang, H., Magilnick, N., Noureddin, M., Mato, J. M., and Lu, S. C. (2007) J. Cell. Physiol. 210 766-773 [DOI] [PubMed] [Google Scholar]
- 54.Blanchetot, C., Chagnon, M., Dube, N., Halle, M., and Tremblay, M. L. (2005) Methods 35 44-53 [DOI] [PubMed] [Google Scholar]
- 55.Tiganis, T., and Bennett, A. M. (2007) Biochem. J. 402 1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper, C. S., Park, M., Blair, D. G., Tainsky, M. A., Huebner, K., Croce, C. M., and Vande Woude, G. F. (1984) Nature 311 29-33 [DOI] [PubMed] [Google Scholar]
- 57.Park, M., Dean, M., Cooper, C. S., Schmidt, M., O'Brien, S. J., Blair, D. G., and Vande Woude, G. F. (1986) Cell 45 895-904 [DOI] [PubMed] [Google Scholar]
- 58.Huyer, G., Liu, S., Kelly, J., Moffat, J., Payette, P., Kennedy, B., Tsaprailis, G., Gresser, M. J., and Ramachandran, C. (1997) J. Biol. Chem. 272 843-851 [DOI] [PubMed] [Google Scholar]
- 59.Villa-Moruzzi, E., Puntoni, F., Bardelli, A., Vigna, E., De Rosa, S., and Comoglio, P. M. (1998) Biochem. J. 336 235-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen, J. N., Mortensen, O. H., Peters, G. H., Drake, P. G., Iversen, L. F., Olsen, O. H., Jansen, P. G., Andersen, H. S., Tonks, N. K., and Moller, N. P. (2001) Mol. Cell. Biol. 21 7117-7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frangioni, J. V., Beahm, P. H., Shifrin, V., Jost, C. A., and Neel, B. G. (1992) Cell 68 545-560 [DOI] [PubMed] [Google Scholar]
- 62.Schmidt-Arras, D. E., Bohmer, A., Markova, B., Choudhary, C., Serve, H., and Bohmer, F. D. (2005) Mol. Cell. Biol. 25 3690-3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiganis, T., Kemp, B. E., and Tonks, N. K. (1999) J. Biol. Chem. 274 27768-27775 [DOI] [PubMed] [Google Scholar]
- 64.Lam, M. H., Michell, B. J., Fodero-Tavoletti, M. T., Kemp, B. E., Tonks, N. K., and Tiganis, T. (2001) J. Biol. Chem. 276 37700-37707 [DOI] [PubMed] [Google Scholar]
- 65.Zhu, H., Naujokas, M. A., and Park, M. (1994) Cell Growth Differ. 5 359-366 [PubMed] [Google Scholar]
- 66.Ostman, A., Hellberg, C., and Bohmer, F. D. (2006) Nat. Rev. 6 307-320 [DOI] [PubMed] [Google Scholar]
- 67.Haj, F. G., Markova, B., Klaman, L. D., Bohmer, F. D., and Neel, B. G. (2003) J. Biol. Chem. 278 739-744 [DOI] [PubMed] [Google Scholar]
- 68.Kakazu, A., Sharma, G., and Bazan, H. E. (2008) Investig. Ophthalmol. Vis. Sci. 49 2927-2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.