Abstract
An efficient chemoenzymatic method for the construction of homogeneous N-glycoproteins was described that explores the transglycosylation activity of the endo-β-N-acetylglucosaminidase from Arthrobacter protophormiae (Endo-A) with synthetic sugar oxazolines as the donor substrates. First, an array of large oligosaccharide oxazolines were synthesized and evaluated as substrates for the Endo-A catalyzed transglycosylation using ribonuclease B as a model system. The experimental results showed that Endo-A could tolerate modifications at the outer mannose residues of the Man3GlcNAc-oxazoline core, thus allowing introduction of large oligosaccharide ligands into a protein and meanwhile preserves the natural, core N-pentasaccharide (Man3GlcNAc2) structure in the resulting glycoprotein upon transglycosylation. In addition to ligands for galectins and mannose-binding lectins, azido functionality could be readily introduced at the N-pentasaccharide (Man3GlcNAc2) core using azido-containing Man3GlcNAc oxazoline as the donor substrate. The introduction of azido functionality permits further site-specific modifications of the resulting glycoproteins, as demonstrated by the successful attachment of two copies of αGal epitopes to ribonuclease B. This study reveals a broad substrate specificity of Endo-A for transglycosylation, and the chemoenzymatic method described here points to a new avenue for a quick access to various homogeneous N-glycoproteins for structure-activity relationship studies and for biomedical applications.
Keywords: Enzymatic glycosylation, Homogeneous glycoprotein, Endoglycosidase, Carbohydrate oxazoline derivative, Click chemistry
Introduction
Protein glycosylation is a ubiquitous posttranslational modification capable of transforming a protein’s properties in different ways. It is known that glycosylation can profoundly impact a protein’s folding, stability, and intracellular trafficking.1 On the other hand, the covalently-linked oligosaccharides of glycoproteins can serve as specific ligands for lectins and receptors on cell surface, thus, directly participating in many important cellular communication processes, such as cell adhesion, cell differentiation, pathogen-host interaction, development, and immune responses.2 However, a detailed understanding of the functions of glycoproteins is often hindered by the structural micro-heterogeneity caused by the diverse patterns of glycosylation. In most cases, natural and recombinant glycoproteins are produced as mixtures of glycoforms that have the same polypeptide backbone but differ in the pendant sugar chains. As pure glycoforms are extremely difficult to isolate from natural and recombinant sources with current techniques, synthetic natural and unnatural homogeneous glycopeptides and glycoproteins are urgently needed for both structural/functional studies and biomedical applications. The last decade has witnessed a tremendous progress in this field, and many chemical, enzymatic, and bioengineering methods have been explored in order to overcome a series of technical obstacles in the road toward the ultimate assembly of homogeneous glycoproteins carrying defined oligosaccharides.3 In particular, the exploration of various chemical and enzymatic ligation methods, including native chemical ligation, auxiliary-assisted ligation, expressed protein ligation, as well as the use of glyco-enzymes for sugar chain elongation in glycoprotein synthesis, have significantly expanded our synthetic repertoire.4–7
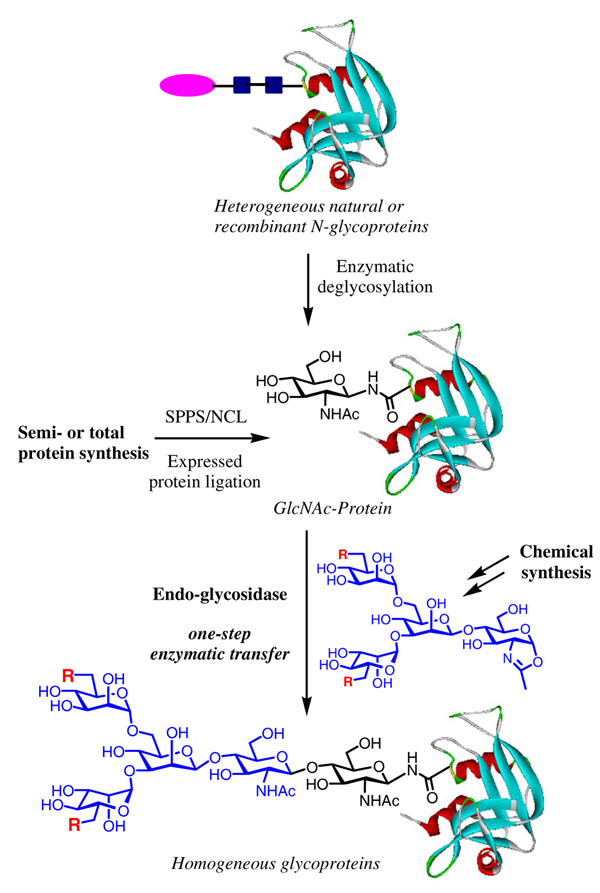
The chemoenzymatic method based on the transglycosylation activity of endo-β-N-acetylglucosaminidase (ENGases) has attracted much attention in this field in recent years.7 ENGases are a class of hydrolytic enzymes that remove N-glycans from glycoproteins by hydrolyzing the β-1,4-glycosidic bond in the N,N’-diacetylchitobiose core. But a few ENGases, including the Endo-M from Mucor hiemali8 and Endo-A from Arthrobactor protophormiae, 9–11 possess transgycosylation activity, that is, the ability to transfer the released oligosaccharide moiety to an acceptor such as a N-acetylglucosamine (GlcNAc)-containing peptide to form a new homogeneous glycopeptide. A big advantage of the endoglycosidase-based method is its high convergence, as the enzyme is able to attach a large intact oligosaccharide to a GlcNAc-polypeptide in a single step and in a regio- and stereo-specific manner to form a homogeneous glycopeptide or glycoprotein in natural glycosidic linkages, without the need for any protecting groups.7 To address the relatively low transglycosylation yield, the limitation of the use of only natural N-glycans as donor substrate, and the problem of product hydrolysis, we and other groups have recently explored synthetic sugar oxazolines as donor substrates for transglycosylation, with a focus on its application for glycopeptide synthesis.6, 7, 12, 13 The highly activated synthetic sugar oxazoline can be regarded as a mimic of the presumed oxazolinium ion intermediate generated by a substrate-assisted catalytic mechanism. It was demonstrated that oligosaccharide oxazoline corresponding to the N-glycan core Man3GlcNAc, i.e., Manα1,3(Manα1,6)Manyβ1,4GlcNAc and some of its truncated and selectively modified forms, were able to serve as donor substrates for transglycosylation. Interestingly, the resulting glycopeptides that carry truncated or modified N-glycans were poor substrates for hydrolysis because of the structural modifications, resulting in accumulation of the glycopeptide product. Moreover, we have also reported a preliminary study showing that this approach could be extended to glycoprotein synthesis and glycosylation engineering without loss of the transglycosylation efficiency.6 In addition to the truncated or modified N-glycans, full-size natural N-glycan such as Man9GlcNAc2 might also be introduced into a GlcNAc-protein by a novel glycosynthase, EndoM-N175A mutant, that we have recently reported.14 EndoM-N175A can take the high-mannose sugar oxazoline as substrate for transglycosylation, but lacks the ability to hydrolyze the resulting natural N-glycopeptide because of the mutation. These studies implicate a great potential of the endoglycosidase-based method for synthesizing both modified and natural N-glycoproteins. We describe in this paper the expansion of this chemoenzymatic approach to the synthesis of an array of homogeneous N-glycoproteins carrying defined oligosaccharide ligands potentially useful for biological recognition. This chemoenzymatic approach involves two key steps, as depicted in Figure 1. The first step is to prepare a GlcNAc-protein, which can be achieved by selective de-glycosylation of a natural or recombinant glycoprotein by an endo-enzyme such as Endo-H to remove the heterogeneous N-glycan, leaving only the innermost GlcNAc residue attached to the Asn at the glycosylation site. Alternatively, a GlcNAc-protein can be synthesized by modern chemical protein synthesis techniques, in which the stable Asn-linked GlcNAc can be incorporated at any desired site(s) during the synthesis. The second step is the regio- and stereo-specific enzymatic ligation of a pre-assembled oligosaccharide moiety (in the form of the activated oxazoline) to the GlcNAc-protein by the endoglycosidase-catalyzed transglycosylation. We have previously shown that Endo-A could tolerate modifications on the outer mannose residues of the Man3GlcNAc oxazoline.6, 13 Moreover, unnatural modification on the outer residues of the N-glycan core also retarded the enzymatic hydrolysis of the glycoprotein product, thus permitting accumulation of the transglycosylation product.6, 13 Therefore, to introduce specific oligosaccharide ligands into a protein, we have decided to glue the ligands at the outer residues of the Man3GlcNAc oxazoline core, so that a native core N-pentasaccharide (Man3GlcNAc2) at the glycosylation site would be generated after transglycosylation. The preservation of a natural core N-glycan structure may be important for maintaining the native conformations of the glycoproteins upon ligand introduction, as the N-glycan core, especially the inner most chitobiose (GlcNAcβ1,4GlcNAc) moiety, plays a primary role in impacting a protein’s conformations.15
Figure 1.
A chemoenzymatic strategy for N-glycoprotein synthesis
Results and Discussion
Design and Synthesis of Novel Oligosaccharide Oxazolines as Donor Substrates
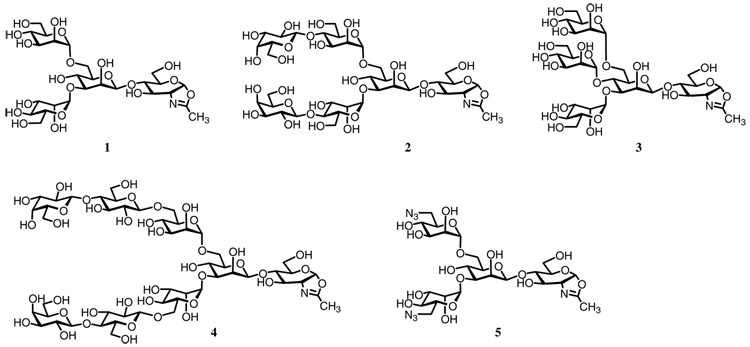
We have previously synthesized several oligosaccharide oxazolines corresponding to the N-glycan core, such as oxazolines 1 and 2 (Figure 2), and evaluated them as substrates of Endo-A for transglycosylation.6, 13 We have found that Endo-A could tolerate certain modifications on the outer mannose residues of Man3GlcNAc-oxazoline and even the Manβ1,4GlcNAc-oxazoline core. These results imply a broad substrate specificity for the oxazoline substrates and suggest that the outer residues might tolerate various modifications, a property ideal for introducing various carbohydrate ligands. To further examine the substrate specificity of Endo-A for oxazolines modified with functional groups and/or large carbohydrate ligands, we have designed three novel oligosaccharide oxazolines 3–5 (Figure 2), which will allow the introduction of specific carbohydrate ligands into proteins if proven as substrates for ENGases. For oxazoline 3, an additional mannose residue was added at the bisecting location of the N-glycan core Man3GlcNAc. If this oxazoline is a substrate for Endo-A, then a novel oligomannose ligand can be introduced into a protein, which may be useful for targeting the protein to specific cells, e.g., macrophages and dendritic cells that express mannose receptors or DC-SIGN.16 Oligosaccharide oxazoline 4 carries two lactose moieties β-1,6-linked to the α-mannoside residues in the Man3GlcNAc core. Lactose moiety is known to be recognized by various lectins such as PNA and galectins,17 and can serve as a substrate for various glycosyltransferases like α1,3-galactosyltransferase and sialyltransferases. The incorporation of two lactose moieties on the core N-glycan is likely to enhance the avidity of the ligand to various lectins.18 In oligosaccharide oxazoline 5, two azido groups were introduced at the 6-positions of the terminal mannose residues in the Man3GlcNAc core. Azido group is one of the most fascinating functional groups in bioconjugate chemistry because of its small size and its chemo-selective reactivity. Thus, once it is introduced into a protein, a variety of functional groups could be site-specifically and chemo-selectively attached through orthogonal Staudinger ligation or the Huisgen 1,3-dipolar cycloaddition.19
Figure 2.
Synthetic oligosaccharide oxazolines
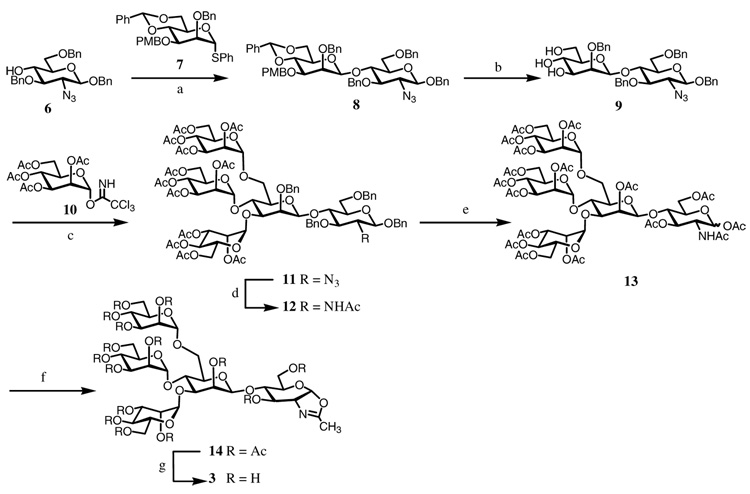
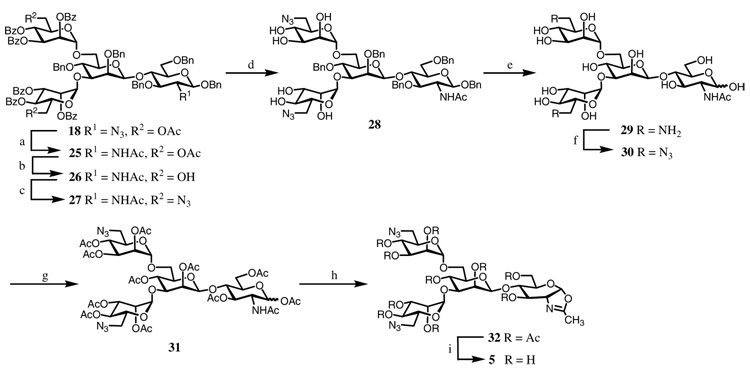
The synthesis of oxazoline 3 was summarized in Scheme 1. Disaccharide 8 was prepared via direct β-mannosylation using the Crich method.20 The 2-azido-2-deoxy derivative 621 was chosen as the acceptor since it was demonstrated to be a better glycosyl acceptor in the direct β-mannosylation reaction than the corresponding 2-phthalimido or 2-N-acetyl derivative.20 Thus, reaction of 6 and glycosyl donor 722 using a mixture of BSP/TTBP/Tf2O as the promoter gave the disaccharide 8 in 67% yield. The 4,6-O-benzyliden group and the 3-O-p-methoxybenzyl (PMB) group of 8 were removed by treatment with TFA to give the triol derivative 9. Glycosylation of 9 with an excess (8 molar equivalent) of mannosyl trichloroacetimidate (10)23 under the catalysis of TMSOTf gave the pentasaccharide derivative 11 in 79% yield. The newly attached mannosyl residues were confirmed to be in the desired α-O-glycosidic linkages by NMR analysis. Next, the 2-azide group in 11 was converted to acetamido group by treatment with thioacetic acid to give 12. The O-benzyl groups were then removed by hydrogenation, and the resulting hydroxyl groups were acetylated to provide the fully acetylated derivative 13. Oxazoline ring formation was achieved by treatment with TMSBr, BF3·OEt2, and 2,4,6-collidine to provide the oxazoline derivative 14 in 67% yield. Finally, de-O-acetylation with a catalytic amount of MeONa in MeOH afforded the pentasaccharide oxazoline 3 in quantitative yield (Scheme 1).
Scheme 1.
Synthesis of oligosaccharide oxazoline 3
Reagents and conditions: (a) BSP, TTBP, Tf2O, CH2Cl2, 67%; (b) TFA, CH2Cl2, 85%; (c) TMSOTf, CH2Cl2, 79%; (d) AcSH, 86%; (e) (i) Pd(OH)2-C, H2, MeOH; (ii) Ac2O, pyridine, 90% (2 steps); (f) TMSBr, BF3·OEt2, 2,4,6-collidine, CH2Cl2, 67%; (g) MeONa, MeOH, quant.
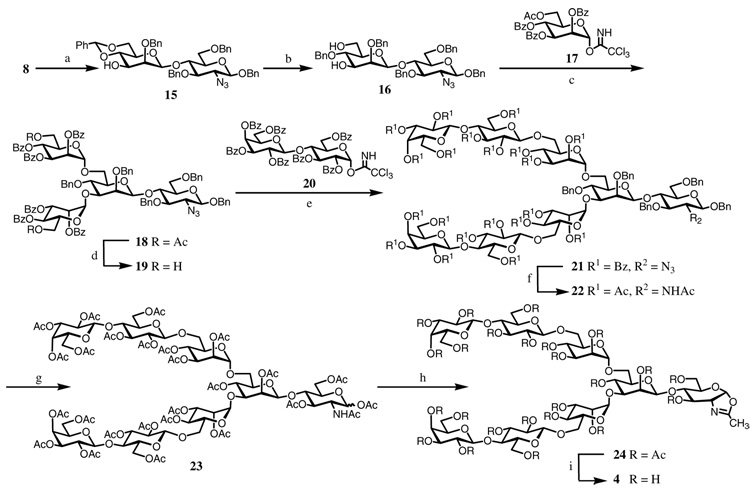
The synthesis of sugar oxazoline 4 was summarized in Scheme 2. Selective removal of the PMB group in disaccharide 8 with DDQ, followed by regio-selective opening of the 4,6-O-benzylidene with Cu(OTf)2 and BH3 in THF24 to give the diol 16 in 58% yield in two steps. Double glycosylation of diol 16 with 6-O-acetyl-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl trichloroacetimidate 1725 provided the tetrasaccharide 18 in excellent yield. Selective removal of the two O-acetyl groups in 18 by mild acidic hydrolysis (acetic chloride in MeOH and DCM) afforded the diol 19. This compound can serve as a key intermediate for introducing various functional groups at the 6-positions of the α-mannoside residues. To introduce two lactose moieties at the N-glycan core, compound 19 was glycosylated with the lactose glycosyl donor 20,26 giving the octasaccharide derivative 21. Compound 21 was then changed to the per-acetylated derivative 23 through several steps of protecting group manipulations. Oxazoline formation was carried out by treatment of 23 with TMSBr and BF3·Et2O to give the octasaccharide derivative 24 (35%). The relatively low yield of 24 was mainly due to the slow reaction, as the starting material 23 was partially recovered after reaction for 3 days. De-O-acetylation of 24 with MeONa/MeOH gave the octasaccharide oxazoline 4 in quantitative yield.
Scheme 2.
Synthesis of sugar oxazoline 4
Reagents and conditions: (a) DDQ, CH2Cl2, H2O, 80%; (b) Cu(OTf)2, BH3·THF, THF, 73%; (c) TMSOTf, CH2Cl2, 96%; (d) AcCl, CH2Cl2, MeOH, 90%; (e) TMSOTf, CH2Cl2, 65%; (f) (i) MeONa, CH2Cl2, MeOH; (ii) Ac2O, pyridine; (iii) AcSH, pyridine, CHCl3, 79% (3 steps); (g) (i) Pd(OH)2-C, H2, MeOH; (ii) Ac2O, pyridine, 77% (2 steps); (h) TMSBr, BF3·OEt2, 2,4,6-collidine, CH2ClCH2Cl, 35%; (i) MeONa, MeOH, quant.
The synthesis of azido-containing oxazoline 5 was carried out using the tetrasaccharide derivative 18 as the starting material (Scheme 3). The 2-azide group of 18 was converted to acetamido group by treatment with thioacetic acid to give 25. The 6-O-acetyl group was then selectively removed by using mild acidic hydrolysis without affecting the benzoyl groups to give the diol 26. Tosylation of 26, followed by azide substitution, provided compound 27 in 84% yield, with two azido moieties attached at the 6-positions of the terminal α-mannosyl residues. Removal of the benzoyl groups gave compound 28. Since there was no efficient method to selectively de-protect the benzyl groups without affecting the azido functionality in oligosaccharide synthesis, we decided to apply a two-step conversion to restore the azido-functionality. First, the benzyl groups in 28 were removed by catalytic hydrogenation. Under this condition, the azido groups were simultaneously reduced to amino groups to give compound 29. Then the amino groups in 29 were changed back to azido functionality by the copper-catalyzed diazo transfer reaction27 to form the azido derivative 30. The reaction was monitored by ESI-MS and the conversion proceeded efficiently to give the diazido compound, which was isolated as the per-acetylated derivative 31 in 61% yield in 3 steps. Treatment of 31 with TMSBr, BF3·OEt2, and 2,4,6-collidine gave the oxazoline derivative 32 in 52% yield. Finally, de-O-acetylation with MeONa/MeOH afforded the azido-containing sugar oxazoline 5 in quantitative yield (Scheme 3).
Scheme 3.
Synthesis of sugar oxazoline 5
Reagents and conditions: (a) AcSH, pyridine, CHCl3, 85%, (b) AcCl, CH2Cl2, MeOH, 72%; (c) (i) TsCl, pyridine; (ii) NaN3, DMF, 84% (2 steps); (d) MeONa, MeOH, 85%; (e) Pd(OH)2-C, H2, MeOH; (f) TfN3, K2CO3, CuSO4, CH2Cl2, MeOH, H2O; (g) Ac2O, pyridine, 61% (3 steps); (h) TMSBr, BF3·OEt2, 2,4,6-collidine, CH2Cl2, 52%; (i) MeONa, MeOH, quant.
Enzymatic Transglycosylation to Form Homogeneous Glycoproteins
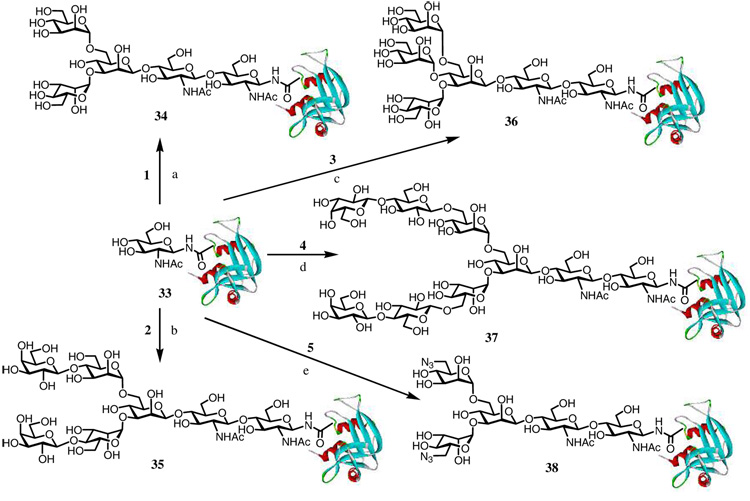
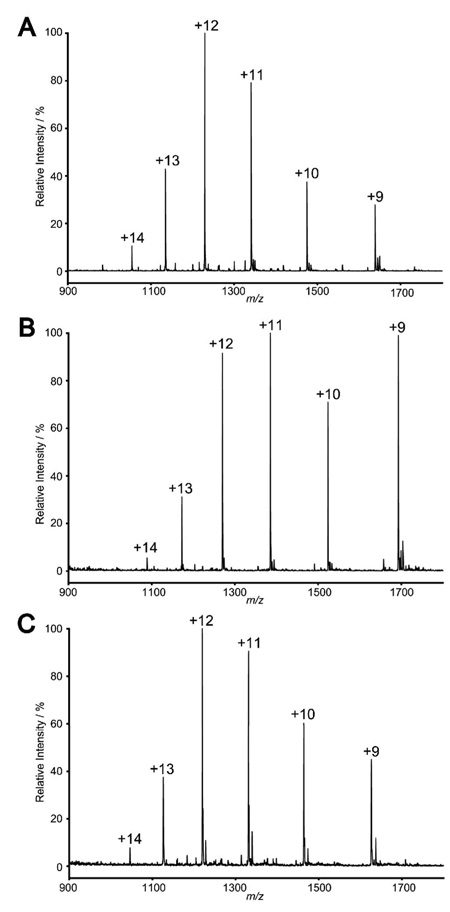
To examine the efficiency of the ENGase-catalyzed transglycosylation for glycoprotein synthesis using pre-assembled oligosaccharide oxazolines as the donor substrates, we have chosen bovine ribonuclease B (RB) as a model system that has been used by us and others for testing new synthetic strategy.5, 6, 9 Bovine RB is a small natural glycoprotein that consists of 124 amino acids. It carries heterogeneous high-mannose type oligosaccharides (Man5–9) attached at the single glycosylation site, Asn-34. As demonstrated in our previous communication,6 the heterogeneous N-glycans on native RB were removed by treatment with Endo-H to provide the homogeneous GlcNAc-protein 33, in which only the innermost GlcNAc residue of the N-glycan was left at the Asn-34 site. This GlcNAc-containing protein (GlcNAc-RB) was used as the acceptor for examining the transglycosylation with the synthetic oligosaccharide oxazolines. We have previously demonstrated that the oxazoline 1 corresponding to the N-glycan Man3GlcNAc core and the oxazoline 2, which has two galactose residues attached at the 4-positions of the outer mannose residues of the core, were excellent substrates for Endo-A catalyzed transglycosylation with GlcNAc-RB to form the glycoproteins 34 and 35.6 Thus the Endo-A catalyzed transglycosylation with oxazolines 3–5 were examined in the similar way in a phosphate buffer (50 mM, pH 6.5) at 23 °C. It was found that sugar oxazoline 3, which carries a bisecting mannose at the 4-position of the core β-mannose residue, could serve as a donor substrate for Endo-A to react with GlcNAc-RB (33) to give a new, homogeneous glycoprotein 36, in which a novel oligomannose glycan was introduced (Scheme 4). This newly formed glycoprotein 36 was eluted slightly earlier than GlcNAc-RB under an appropriate reverse phase HPLC (RP-HPLC) condition (see experimental section) and was purified by RP-HPLC in 71% yield. The ESI-MS spectrum of the glycoprotein 36 (Figure 3A) clearly indicates its homogeneity. Deconvolution of the ESI-MS of 36 gave a molecular mass of 14740 Da, which is in good agreement with the calculated mass (14745 Da) of glycoprotein 36.
Scheme 4.
enzymatic transglycosylation
Reagents and conditions: (a) Endo-A, phosphate buffer (50 mM, pH 6.5), 82%; (b) Endo-A, phosphate buffer (50 mM, pH 6.5), 96%; (c) Endo-A, phosphate buffer (50 mM, pH 6.5), 71%; (d) Endo-A, phosphate buffer (50 mM, pH 6.5), 38%; (e) Endo-A, phosphate buffer (50 mM, pH 6.5), 89%;
Figure 3.
The ESI mass spectra of the synthetic glycoproteins. A, glycoprotein 36; B, glycoprotein 37; and C, glycoprotein 38. Peaks are labeled according to the corresponding charge states.
The oxazoline 4 was also able to serve as a donor substrate of Endo-A for transglycosylation, but the enzymatic reaction proceeded in a much slower path than the enzymatic reactions with oxazolines 1, 2, and 3. After 3 days of reaction, the newly formed glycoprotein 37 was isolated by RP-HPLC in 38% yield and the unreacted starting material GlcNAc-RB (33) was recovered. Deconvolution of the ESI-MS of 37 (Figure 3B) gave a molecular mass of 15229 Da, which agreed well with the calculated MS (15231 Da) of glycoprotein 37. The relatively low reactivity of the octasaccharide oxazoline 4 in comparison with the core tetrasaccharide oxazoline 1 and the hexasaccharide oxazoline 2 might be attributed to the unnatural modification on the 6-positions of the α-mannosyl residues and the relatively large size of the lactose moieties. Consistent with this assumption, the sugar oxazoline 5, which has two small azido groups attached at the 6-position of the outer mannose residues, was found to be an excellent substrate for Endo-A. Thus, transglycosylation of GlcNAc-RB (33) with sugar oxazoline 5 under the catalysis of Endo-A proceeded efficiently to give the glycoprotein 38 in 89% yield (Scheme 4). Again, deconvolution of the ESI-MS of 38 (Figure 3C) gave a molecular mass of 14630 Da, indicating the attachment of the azido-oligosaccharide moiety to the GlcNAc-RB to form glycoprotein 38 (calculated molecular mass, 14619 Da).
Ligand Introduction via Huisgen 1,3-Dipolar Cycloaddition
The efficient chemoenzymatic introduction into proteins of a core N-pentasaccharide carrying azido functionality opens an avenue to further site-specific functionalization of the protein, as the azido group can serve as a unique tag for introducing various functional groups through highly chemo-selective bioorthogonal reactions.19 These include the Staudinger ligation in which the azide reacts with a functionalized phosphine to form a stable amide bond,28 and the azide-alkyne [3 + 2] cycloaddition to form a stable triazole linkage under catalysis.29 To demonstrate usefulness of the combination of the chemoenzymatic transglycosylation and the “Click chemistry”, we selected αGal epitope as a ligand to introduce into RB via the transglycosylation product glycoprotein 38. For the purpose, an alkyne-functionalized αGal trisaccharide epitope 41 was prepared using the amino-containing trisaccharide derivative 39 as the starting material (Scheme 5), which we have previously synthesized for HIV-1 immuno-targeting purpose.30 To incorporate αGal epitopes into glycoprotein 38 that contains two azido groups, we examined several catalytic methods for the 1,3-dipolar cycloaddition. It was found that the cycloaddition reaction between glycoprotein 38 and the alkyne-oligosaccharide 41 proceeded most efficiently under the catalysis of CuSO4/l-ascorbic acid in the presence of bathophenanthroline disulfonic acid (to serve as a ligand for the catalyst).31 Thus, when an excess of 41 was used, an esentially quantitative conversion of the azido-protein 38 to the new glycoprotein 42 was fulfilled after 12 h at room temperature, as monitored by HPLC and ESI-MS. After the reaction, the resulting glycoprotein 42 was easily isolated in 87% yield by RP-HPLC, in which two copies of the αGal epitope were introduced through the 1,3-dipolar cycloaddition. The purity and identity of the new glycoprotein 42 were revealed by SDS-PAGE and ESI-MS analysis (Figure 4). The SDS gel of 42 showed a single band at 18 kDa, which is about 2 kDa larger than the glycoprotein 38, implicating the introduction of two αGal epitopes (Figure 4A). Deconvolution of its ESI-MS (Figure 4B) gave a molecular mass of 15971 Da, which matched well with the calculated mass (M = 15976 Da) of glycoprotein 42. It should be pointed out that the addition of the bathophenanthroline ligand in the reaction mixture is essential for the success of cycloaddition, as an inefficient reaction was observed in the absence of bathophenanthroline disulfonic acid, giving ca. 28% of a mono-substituted glycoprotein and less than 5% of the double-substituted glycoprotein 42 after 24 h reaction (confirmed by ESI-MS analysis). On the other hand, we have also observed that the use of CuSO4/copper wire catalyzed reaction32 resulted in precipitation of the protein. There are precedents that certain catalytic conditions for the Huisgen 1,3-dipolar azide-alkyne cycloaddition would lead to precipitation of protein substrates.33 Thus, a choice of an appropriate cycloaddition condition is critical for the success of ligation particularly when it is applied to protein-based reactants. The recognition of the αGal epitopes (an array of oligosaccharides with terminal Galα1,3Gal moieties) on cells of most animals such as pigs by natural human anti-αGal antibodies is mainly responsible for the hyperacute rejection in xenotransplantation.34 A successful introduction of αGal epitopes into proteins under physiological pH and temperature in a site-specific manner may find interesting applications such as for potential immuno-targeting.30
Scheme 5.
Synthesis of alkyne-functionalized αGal epitope
Reagents and conditions: (a) NaHCO3, MeOH, MeCN, H2O, 79%.
Figure 4.
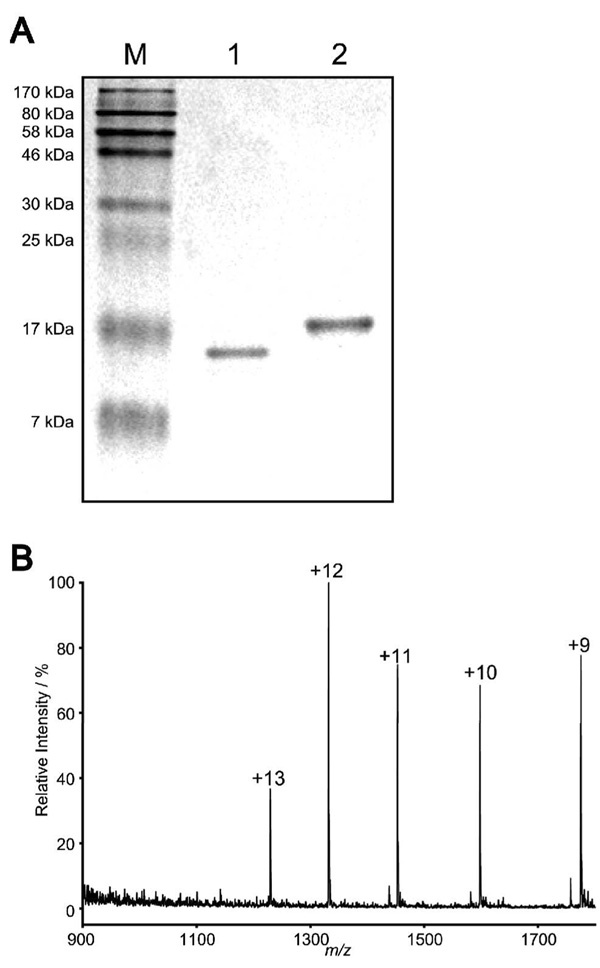
SDA-PAGE and ESI-MS analysis of the αGal-incorporated glycoprotein product (42) generated by “Click” chemistry. A, Coomassie blue-stained SDS-PAGE gel (Lane M is protein marker with sizes on the left; Lane 1 is the starting material, glycoprotein 38, and lane 2 is the purified product, glycoprotein 42); B, The ESI mass spectrum of glycoprotein 42. Charge states are labeled.
Binding of the Synthetic Glycoproteins with Respective Lectins
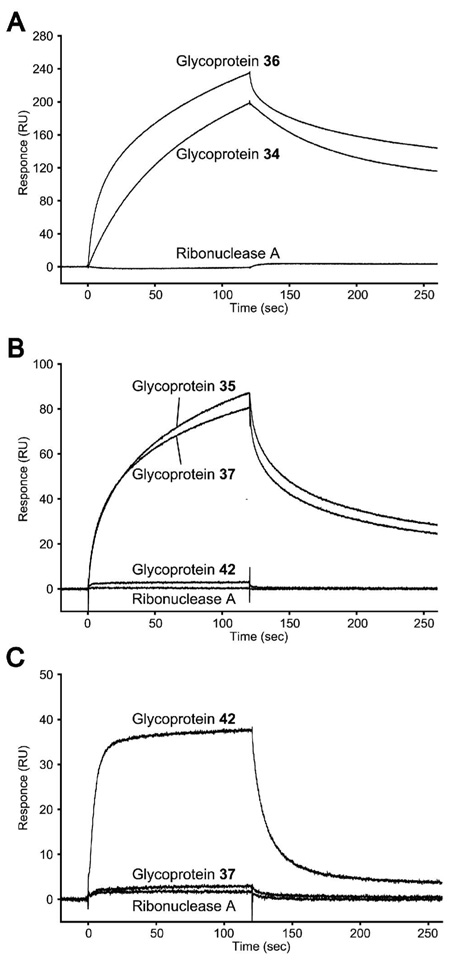
The introduction of specific oligosaccharide ligands into a protein or glycoprotein would enable various useful applications on the basis of ligand-receptor interactions. Thus, we have performed a preliminary study on the binding of the synthetic ligand-containing glycoproteins with respective lectin and antibody, using surface plasmon resonance (SPR) technology. It was found that the oligomannose-containing glycoproteins 34 and 36 could be efficiently recognized by ConA, a lectin that binds terminal α-mannosyl residues.35 Interestingly, the glycoprotein 36 exhibited a slightly better affinity to ConA than glycoprotein 34, implicating a positive contribution of the additional bisecting mannose residue. But the enhancement was not significant. In contrast, the ribonuclease A that shares the same sequence as RB but lacks the N-glycan did not bind to ConA at all (Figure 5A). For the terminal galactose-containing glycoproteins, it was observed that the glycoproteins 35 and 37 that carried the Galβ1,4Man and Galβ1,4Glc (lactose) moieties, respectively, could efficiently bind to PNA, a lectin that recognizes terminal β-linked galactiose moiety.35 The two glycoproteins showed very similar affinity to lectin PNA. However, the αGal-containing glycoprotein 42, as well as the ribonuclease A that does not contain glycan did not bind to PNA (Figure 5B). These results confirm the importance of the anomeric configuration of the carbohydrate ligands in lectin recognition. To detect the binding of αGal-glycoprotein 42 to human anti-αGal antibodies, the whole human IgG that contains about 1–2% of anti-αGal antibodies were immobilized on chips and the antibody chips were used for probing the synthetic glycoproteins. As expected, the αGal-containing glycoprotein 42 could be readily recognized by the immobilized whole human IgG that contains a small fraction of anti-αGal antibodies, whereas the lactose-containing glycoprotein 37 and ribonuclease A did not react to the immobilized human IgG (Figure 5C).
Figure 5.
SPR sensorgrams of the binding between respective synthetic glycoproteins and immobilized lectin or human serum antibody. A, binding to immobilized ConA; B, binding to immobilized PNA; and C, binding to immobilized whole IgG-type antibodies from human serum. For comparison, respective glycoprotein or ribonuclease A was injected onto the sensor chip surface at a concentration of 1.0 µM.
Conclusion
An array of large oligosaccharide oxazolines were synthesized and evaluated as substrates for the Endo-A catalyzed transglycosylation for the synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands. The results suggest that Endo-A could tolerate modifications at the outer mannose residues of the Man3GlcNAc-oxazoline core. Thus a broad substrate specificity of the enzyme was revealed, which allows introduction of large oligosaccharide ligands into a protein and meanwhile preserves the natural N-pentasaccharide (Man3GlcNAc2) core upon transglycosylation. In particular, the incorporation of the azido functionality at the N-pentasaccharide (Man3GlcNAc2) core permits further structural modifications of the resulting glycoproteins, as demonstrated by the successful ligation of two copies of αGal epitopes to ribonuclease B. This novel chemoenzymatic approach opens a new avenue to various homogeneous N-glycoproteins that will be useful for basic research and biomedical applications.
Experimental Section
Materials
Endo-β-N-acetylglucosaminidase from Arthrobacter protophormiae (Endo-A) was overproduced in E. coli following the reported procedure.10 ConA, PNA, and IgG from normal human serum were purchased from Sigma (St. Louis, MO).
General procedures
TLC was performed using Silica-gel on aluminum plates (Sigma-Aldrich). Flash column chromatography was performed on silica gel 60 (230–400 mesh). SDS-PAGE was performed using 18% (w/v) gel. NMR spectra were recorded on JEOL ECX 400 MHz spectrometer. The chemical shifts were assigned in ppm. The ESI-MS spectra were measured on a micromass ZQ-4000 single quadruple mass spectrometer. Analytical RP-HPLC was performed on a Waters 626 HPLC instrument with a Symmetry300™ C18 column (5.0 µm, 4.6 × 250 mm) at 40°C. The column was eluted with a linear gradient 23–29% MeCN containing 0.1% TFA for 30 min at the flow rate of 1.0 mL/min. Preparative HPLC was performed with a Waters 600 HPLC instrument of a Waters C18 column (Symmetry 300, 19 × 300 mm). The column was eluted with a suitable gradient of MeCN–H2O containing 0.1% TFA at a flow rate of 12 mL / min.
Benzyl 2-O-benzyl-4,6-O-benzylidene-3-O-p-methoxybenzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (8)
A mixture of phenyl 2-O-benzyl-4,6-O-benzylidene-3-O-p-methoxybenzyl-1-thio-α-d-mannopyranoside 722 (450 mg, 0.79 mmol), BSP (182 mg, 0.87 mmol), TTBP (329 mg, 1.58 mmol), and activated 3 Å molecular sieves (3.46 g) in CH2Cl2 (16 mL) was stirred for 30 min at −60 °C under an argon atmosphere. Then Tf2O (159 µL, 0.95 mmol) was added at this temperature. After 5 min, a solution of benzyl 2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside 621 (250 mg 0.53 mmol) in CH2Cl2 (2.6 mL) was added and the mixture was stirred at −60 °C for 2 h. The mixture was filtered through a Celite pad. The filtrate was poured into saturated NaHCO3, and extracted with CH2Cl2. The organic layer was washed with brine, dried over MgSO4, and concentrated. The residue was subjected to silica gel column chromatography (hexanes/EtOAc, 8:1) to afford 8 (329 mg, 67%) as a colorless syrup. 1H NMR (400 MHz, CDCl3, TMS) δ 7.49–6.83 (m, 29H), 5.51 (s, 1H), 5.05 (d, 1H, J = 10.6 Hz), 4.92 (d, 1H, J = 11.9 Hz), 4.84 (d, 1H, J = 11.9 Hz), 4.77 (d, 1H, J = 11.9 Hz), 4.69 (d, 1H, J = 11.9 Hz), 4.67 (d, 1H, J = 11.9 Hz), 4.62 (d, 2H, J = 11.4 Hz), 4.52 (d, 1H, J = 11.9 Hz), 4.49 (s, 1H), 4.40 (d, 1H, J = 11.9 Hz), 4.28 (d, 1H, J = 8.2 Hz), 4.10–4.03 (m, 2H), 3.96 (t, 1H, J = 9.4 Hz), 3.78 (s, 3H), 3.69 (br d, 1H, J = 2.7 Hz), 3.63 (dd, 1H, J = 11.3, 2.1 Hz), 3.56–3.45 (m, 3H), 3.40 (dd, 1H, J = 9.9, 3.1 Hz), 3.32 (t, 1H, J = 9.2 Hz), 3.26 (m, 1H), 3.08 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 159.10, 138.48, 138.42. 137.60, 137.57, 136.76, 130.50, 129.01, 128.80, 128.49, 128.43, 128.26, 128.11, 128.07, 127.97, 127.94, 127.89, 127.76, 127.49, 126.04, 113.67, 101.42, 101.28, 100.38, 81.47, 78.61, 77.90, 77.31, 77.00, 75.11, 75.04, 74.72, 73.54, 72.29, 70.79, 68.40, 68.29, 67.32, 65.68, 55.21.
Benzyl 2-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (9)
To a stirred solution of compound 8 (80 mg, 85 µmol) in CH2Cl2 (4 mL) was added TFA (0.75 mL) at −20 °C. The mixture was stirred for 30 min at this temperature, then for 1 h at 0 °C. MeOH (2 mL) and CH2Cl2 (20 mL) were added to the reaction mixture, and the mixture was sequentially washed with saturated NaHCO3 and brine and then dried over MgSO4. After removal of the solvent, the residue was purified by chromatography on a silica gel column (hexanes/EtOAc, 2:3) to yield 9 (53 mg, 85%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.41–7.24 (m, 20H), 5.02 (d, 1H, J = 10.5 Hz), 4.94 (d, 1H, J = 11.5 Hz), 4.94 (d, 1H, J = 11.9 Hz), 4.71 (d, 1H, J = 11.9 Hz), 4.71–4.65 (m, 2H), 4.54 (d, 1H, J = 11.5 Hz), 4.50–4.47 (m, 2H), 4.31 (d, 1H, J = 8.3 Hz), 3.95 (t, 1H, J = 9.4 Hz), 3.73 (dd, 1H, J = 11.2, 2.0 Hz), 3.67 (dd, 1H, J = 11.0, 3.6 Hz), 3.62 (m, 1H), 3.57 (br d, 1H, J = 3.7 Hz), 3.52–3.48 (m, 2H), 3.39–3.30 (m, 3H), 3.19 (br s, 1H), 3.01 (m, 1H), 2.45 (br s, 1H), 2.30 (br s, 1H), 1.80 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 138.21, 137.98, 137.39, 136.70, 128.55, 128.42, 128.27, 128.06, 128.02, 127.91, 127.82, 127.65, 127.41, 100.96, 100.31, 81.35, 77.95, 77.08, 75.56, 75.25, 75.03, 74.79, 73.84, 73.65, 70.76, 68.96, 68.12, 65.72, 62.16.
Benzyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→4)]-{[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)]}-2-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (11)
A solution of compound 9 (37 mg, 50 µmol) and 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl tricholoroacetimidate 1023 (199 mg, 0.40 mmol) in CH2Cl2 (4 ml) containing activated 4 Å molecular sieves (240 mg) was stirred under an atmosphere of argon at room temperature for 1 h. After cooling to −40 °C, a solution of TMSOTf in CH2Cl2 (1 M, 81 µL, 81 µmol) was added and the resulting mixture was stirred at room temperature for 24 h. The mixture was filtered through a Celite pad, poured into saturated NaHCO3, and extracted with CH2Cl2. The organic layer was washed with brine, dried over MgSO4, and filtered. The filtrate was concentrated and the residue was subjected to flash silica gel column chromatography (hexanes/EtOAc, 1:1) to provide 11 (69 mg, 79%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.44–7.18 (m, 20 H), 5.32–5.19 (m, 8H), 5.14–5.10 (m, 3H), 4.96 (s, 1H), 4.94–4.90 (m, 2H), 4.88 (d, 1H, J = 10.5 Hz), 4.78 (d, 1H, J = 11.9 Hz), 4.70–4.64 (m, 2H), 4.51 (d, 1H, J = 12.3 Hz), 4.46 (d, 1H, J = 11.9 Hz), 4.46 (s, 1H), 4.34 (d, 1H, J = 8.3 Hz), 4.30 (m, 1H), 4.19–4.13 (m, 2H), 4.08 (dd, 1H, J = 11.9, 1.8 Hz), 4.00–3.86 (m, 5H), 3.84 (d, 1H, J = 2.8 Hz), 3.79–3.69 (m, 5H), 3.55 (t, 1H, J = 9.0 Hz), 3.44–3.29 (m, 4H), 3.18 (m, 1H), 2.16, 2.12, 2.10, 2.10, 2.06, 2.04, 2.02, 2.01, 2.00, 1.98, 1.94, 1.89 (12s, 36 H); 13C NMR (100 MHz, CDCl3) δ 170.71, 170.49, 170.28, 169.97, 169.83, 169.71, 169.69, 169.62, 169.56, 169.49, 138.18, 138.03, 137.62, 136.72, 128.76, 128.38, 128.32, 128.24, 128.05, 127.99, 127.91, 127.53, 127.33, 126.47, 100.44, 99.94, 99.36, 97.62, 83.19, 80.98, 78.02, 77.20, 76.03, 75.78, 74.78, 74.69, 73.83, 73.71, 70.71, 69.65, 69.58, 69.28, 69.17, 68.95, 68.74, 68.50, 68.42, 68.34, 68.27, 66.08, 65.95, 65.65, 65.43, 62.53, 62.48, 61.80, 20.84, 20.78, 20.73, 20.65, 20.61, 20.56.
Benzyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→4)]-{[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)]}-2-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (12)
Compound 11 (59 mg, 35 µmol) was treated with thioacetic acid (2 mL) for 24 h at room temperature. The reaction mixture was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (hexanes/EtOAc, 2:3) to give 12 (52 mg, 86%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.43–7.18 (m, 20H), 6.06 (d, 1H, J = 7.8 Hz), 5.35–5.17 (m, 9H), 5.13 (d, 1H, J = 12.3 Hz), 5.09 (d, 1H, J = 1.8 Hz), 4.99 (d, 1H, J = 7.8 Hz), 4.90–4.84 (m, 3H), 4.77 (d, 1H, J = 12.4 Hz), 4.59–4.52 (m, 4H), 4.43 (d, 1H, J = 12.4 Hz), 4.32 (dd, 1H, J = 12.3, 5.5 Hz), 4.27 (dd, 1H, J = 12.9, 3.7 Hz), 4.09–3.96 (m, 6H), 3.92–3.57 (m, 10H), 3.48 (m, 1H), 3.31 (m, 1H), 3.21 (m, 1H), 2.16, 2.12, 2.09, 2.08, 2.05, 2.03, 2.02, 1.99, 1.97, 1.94, 1.90, 1.83 (12s, 39H); 13C NMR (100 MHz, CDCl3) δ 170.93, 170.38, 170.31, 170.27, 169.92, 169.80, 169.77, 169.70, 169.62, 169.55, 169.46, 138.56, 138.12, 137.84, 137.55, 128.73, 128.35, 128.28, 128.20, 128.07, 127.70, 127.60, 127.50, 127.25, 126.49, 99.86, 99.62, 99.43, 99.28, 97.00, 82.75, 77.87, 77.63, 77.20, 76.83, 75.99, 74.74, 74.31, 73.72, 73.68, 73.55, 70.71, 69.54, 69.52, 69.43, 69.39, 69.36, 68.97, 68.69, 68.45, 68.33, 67.57, 66.08, 66.04, 65.38, 62.56, 62.47, 62.18, 56.49, 23.34, 20.82, 20.77, 20.76, 20.71, 20.66, 20.63, 20.59, 20.56.
2,3,4,6-Tetra-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→4)]-{[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)]}-2-O-acetyl-β-d-mannopyranosyl-(1→4)-2-acetamido-1,3,6-tri-O-acetyl-2-deoxy-d-glucopyranose (13)
To a solution of 12 (52 mg, 30 µmol) in MeOH (3 mL) was added 20% palladium(II) hydroxide on activated carbon (30 mg). The mixture was vigorously stirred at room temperature under a hydrogen atmosphere overnight. The mixture was filtered through a Celite pad and the filtrate was concentrated in vacuo. The residue was dissolved in pyridine (4 mL) and Ac2O (4 mL) was added. The mixture was stirred at room temperature overnight and then was concentrated. The residue was then dissolved in CH2Cl2, and the solution was washed sequentially with 1 M HCl, saturated NaHCO3, and brine. The organic layer was dried over MgSO4 and concentrated. Silica gel column chromatography (EtOAc) of the residue afforded 13 (41 mg, 90%, α/β = 79/21) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS, selected signals) δ 6.06 (d, 0.79H, J = 3.2 Hz), 5.65 (d, 0.21H, J = 8.3 Hz), 2.22–1.93 (m, 51H); 13C NMR (100 MHz, CDCl3, selected signals) δ 171.07, 170.65, 170.62, 170.40, 170.24, 170.07, 170.03, 169.86, 169.77, 169.70, 169.62, 169.57, 169.48, 169.22, 169.05, 98.33, 97.17, 97.03, 96.30, 92.23, 90.64, 22.84, 22.66, 20.88, 20.83, 20.68, 20.61, 20.50, 20.44, 20.32; ESI-MS: Calcd. for C64H88NO42 [M+H]+ 1542.48, Found: 1542.58.
2-Methyl-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→4)]-{[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)]}-2-O-acetyl-β-d-mannopyranosyl-(1→4)-3,6-di-O-acetyl-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (14)
To a solution of 13 (214 mg, 0.14 mmol) in CH2Cl2 (21 mL) mL) containing activated 4 Å molecular sieves (1.7 g) were added 2,4,6-collidine (365 µl, 2.8 mmol), TMSBr (357 µl, 2.8 mmol), and BF3·OEt2 (347 µl, 2.8 mmol). The reaction mixture was stirred at room temperature for 24 h. The mixture was diluted with CH2Cl2, filtered through a Celite pad, washed sequentially with saturated NaHCO3 and brine, dried over MgSO4, filtered, and concentrated. The residue was chromatographed (hexanes/EtOAc, 1:1 to 1:4) to give 14 (137 mg, 67%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 5.91 (d, 1H, J = 7.4 Hz), 5.55 (d, 1H, J = 2.3 Hz), 5.36–5.16 (m, 10H), 5.10 (d, 1H, J = 1.0 Hz), 5.02 (s, 1H), 4.98 (d, 1H, J = 0.9 Hz), 4.79 (s, 1H), 4.37–3.89 (m, 16H), 3.67 (m, 1H), 3.60 (d, 1H, J = 8.7 Hz), 3.41 (m, 1H), 2.20, 2.15, 2.12, 2.12, 2.11, 2.11, 2.10, 2.09, 2.09, 2.05, 2.04 (11s, 33H), 2.03 (d, 3H), 2.01, 1.98, 1.96, 1.96 (4s, 12H); 13C NMR (100 MHz, CDCl3) δ 170.56, 170.47, 170.34, 169.75, 169.70, 169.67, 169.64, 169.58, 169.52, 169.40, 165.86, 99.55, 99.05, 98.93, 98.11, 78.33, 77.20, 76.45, 74.71, 74.00, 70.18, 69.52, 69.49, 69.39, 69.32, 69.18, 69.05, 68.94, 68.49, 68.36, 67.81, 67.18, 65.98, 65.81, 65.58, 64.43, 63.39, 62.36, 62.15, 62.01, 20.87, 20.80, 20.70, 20.62, 20.58, 20.54, 13.49; ESI-MS: Calcd. for C62H84NO40 [M+H]+ 1482.46, Found: 1482.37.
2-Methyl-[α-d-mannopyranosyl-(1→3)-[α-d-mannopyranosyl-(1→4)]-{[α-d-mannopyranosyl-(1→6)]}-β-d-mannopyranosyl-(1→4)-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (3)
To a solution of 14 (98 mg, 66 µmol) in MeOH (6 mL) was added MeONa in MeOH (0.5 M, 13.2 µL, 6.6 µmol). After stirring at room temperature overnight, the reaction mixture was concentrated to dryness. The residue was dissolved in water and lyophilized to give the oxazoline 3 (56 mg, quantitative). 1H NMR (400 MHz, D2O) δ 5.96 (d, 1H, J = 7.4 Hz), 5.00 (d, 1H, J = 1.4 Hz), 4.94 (s, 1H), 4.88 (d, 1H, J = 1.9 Hz), 4.60 (s, 1H), 4.23 (dd, 1H, J = 3.0, 1.6 Hz), 4.08–4.05 (m, 2H), 3.91–3.48 (m, 26H), 3.27 (m, 1H), 1.94 (d, 3H, J = 1.8 Hz); 13C NMR (100 MHz, D2O) δ 168.67, 102.92, 101.94, 101.02, 100.07, 99.97, 82.39, 77.92, 73.91, 73.75, 73.55, 72.86, 70.97, 70.54, 70.40, 70.20, 69.97, 69.17, 66.84, 66.50, 66.34, 65.25, 61.77, 61.09, 60.99, 60.94, 13.01; ESI-MS: Calcd. for C32H54NO25 [M+H]+ 852.30, Found: 852.40.
Benzyl 2-O-benzyl-4,6-O-benzylidene-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (15)
To a solution of 8 (2.08 g, 2.22 mmol) in a mixture of CH2Cl2/H2O (17.5/1, 74 mL) was added DDQ (1.16 g, 5.11 mmol) at 0 °C. After 30 min, the reaction mixture was warmed to room temperature and further stirred for 1 h. The reaction mixture was diluted with CH2Cl2, washed with saturated NaHCO3 and brine, and dried over MgSO4. Concentration and purification by column chromatography on silica gel (hexanes/EtOAc, 10:1 to 3:1) provided 15 (1.46 g, 80%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.47–7.25 (m, 25H), 5.44 (s, 1H), 5.05 (d, 1H, J = 10.1 Hz), 4.94 (d, 1H, J = 11.9 Hz), 4.92 (d, 1H, J = 11.4 Hz), 4.73 (d, 1H, J = 12.3 Hz), 4.69 (d, 1H, J = 12.4 Hz), 4.66–4.61 (m, 2H), 4.58 (s, 1H), 4.47 (d, 1H, J = 11.9 Hz), 4.30 (d, 1H, J = 7.8 Hz), 4.07 (dd, 1H, J = 10.3, 4.8 Hz), 4.01 (t, 1H, J = 9.2 Hz), 3.73–3.64 (m, 4H), 3.56–3.44 (m, 3H), 3.35–3.30 (m, 2H), 3.08 (m, 1H), 2.32 (d, 1H, J = 8.7 Hz); 13C NMR (100 MHz, CDCl3) δ 138.36, 138.00, 137.46, 137.16, 136.71, 129.08, 128.59, 128.43, 128.21, 128.15, 128.12, 127.98, 127.94, 127.92, 127.86, 127.55, 126.25, 101.92, 101.57, 100.34, 81.50, 79.02, 78.84, 77.49, 75.77, 75.14, 74.75, 73.68, 70.87, 70.79, 68.35, 68.15, 66.88, 65.67.
Benzyl 2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (16)
A 1 M solution of BH3·THF in THF (6 mL, 6 mmol) was added to compound 15 (980 mg, 1.20 mmol) at room temperature. The mixture was stirred for 5 min, and copper(II) trifluoromethanesulfonate (43 mg, 0.119 mmol) was added to the solution. After stirring for 5 h, the mixture was cooled down to 0 °C, and the reaction was quenched by sequential additions of Et3N (167 µL, 1.2 mmol) and MeOH (2.2 mL). The resulting mixture was concentrated under reduced pressure followed by co-evaporation with MeOH. The residue was purified by flash column chromatography (hexanes/EtOAc, 3:1) on silica gel to give 16 (718 mg, 73%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.42–7.25 (m, 25H), 5.05 (d, 1H, J = 10.5 Hz), 4.95–4.92 (m, 2H), 4.81 (d, 1H, J = 11.0 Hz), 4.71–4.66 (m, 3H), 4.59 (d, 1H, J = 11.4 Hz), 4.57 (d, 1H, J = 11.0 Hz), 4.49 (d, 1H, J = 12.4 Hz), 4.50 (s, 1H), 4.31 (d, 1H, J= 8.2 Hz), 3.94 (t, 1H, J = 9.4 Hz), 3.71 (dd, 1H, J= 11.0, 1.9 Hz), 3.66 (dd, 1H, J = 11.0, 3.2 Hz), 3.65–3.57 (m, 2H), 3.52–3.42 (m, 3H), 3.38–3.30 (m, 3H), 3.05 (m, 1 H), 2.28 (m, 1 H), 1.59 (m, 1H); 13C NMR (100MHz, CDCl3) δ 138.35, 138.20, 138.05, 137.40, 136.73, 128.55, 128.50, 128.43, 128.38, 128.33, 128.05, 127.94, 127.89, 127.77, 127.65, 127.19, 100.97, 100.32, 81.37, 78.32, 77.17, 76.40, 75.25, 74.92, 74.82, 74.64, 74.07, 73.67, 70.77, 68.12, 65.80, 62.02.
Benzyl 6-O-acetyl-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[6-O-acetyl-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (18)
A solution of compound 16 (76 mg, 93 µmol) and 2,3,4-tri-O-acetyl-6-O-benzoyl-α-d-mannopyranosyl tricholoroacetimidate 1725 (315 mg, 464 µmol) in CH2Cl2 (3.9 ml) containing activated 4 Å molecular sieves (469 mg) was stirred under an atmosphere of argon at room temperature for 15 min. After cooling to −40 °C, a solution of TMSOTf in CH2Cl2 (1 M, 46 µL, 46 µmol) was added and the resulting mixture was stirred at room temperature overnight. The mixture was filtered through a Celite pad. The filtrate was poured into saturated NaHCO3, and extracted with CH2Cl2. The organic layer was washed with brine, dried over MgSO4, and filtered. The filtrate was concentrated and the residue was subjected to flash silica gel column chromatography (hexanes/EtOAc, 5:1 to 3:1) to provide 18 (165 mg, 96%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.11–7.13 (m, 55H), 5.98–5.87 (m, 3H), 5.84–5.79 (m, 2H), 5.54 (d, 1H, J = 1.9 Hz), 5.37 (s, 1H), 5.20 (d, 1H, J = 12.3 Hz), 5.85–5.06 (m, 2H), 4.99 (d, 1H, J = 10.5 Hz), 4.92 (d, 1H, J = 11.9 Hz), 4.77 (d, 1H, J = 12.4 Hz), 4.73–4.64 (m, 3H), 4.60 (s, 1H), 4.52 (d, 1H, J = 11.9 Hz), 4.35 (d, 1H, J = 8.2 Hz), 4.29–4.23 (m, 2H), 4.18–4.11 (m, 3H), 4.06–4.00 (m, 2H), 3.94 (m, 1H), 3.85–3.61 (m, 6H), 3.44 (m, 1H), 3.40–3.26 (m, 3H), 2.06 (s, 3H), 2.00 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.69, 170.36, 165.45, 165.40, 165.33, 165.09, 164.97, 138.52, 138.47, 137.72, 137.45, 136.93, 133.58, 133.44, 133.38, 133.23, 133.19, 132.90, 129.82, 129.80, 129.76, 129.70, 129.44, 129.22, 129.14, 129.02, 128.77, 128.57, 128.54, 128.51, 128.46, 128.34, 128.29, 128.25, 128.12, 127.99, 127.93, 127.83, 127.46, 127.35, 126.98, 100.78, 100.34, 99.84, 97.89, 83.24, 81.02, 78.33, 76.14, 75.67, 75.49, 74.95, 74.65, 74.39, 74.30, 73.69, 70.85, 70.35, 70.01, 69.94, 69.67, 69.17, 68.94, 68.60, 68.25, 66.75, 66.41, 65.36, 62.78, 62.27, 20.67, 20.58.
Benzyl 2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (19)
To a solution of 18 (124 mg, 67 µmol) in 1:2 (v/v) CH2Cl2–MeOH (12 mL) was added acetyl chloride (0.4 mL). After stirring overnight, the mixture was concentrated under reduced pressure, and the residue was purified by flash silica gel column chromatography (hexanes/EtOAc, 3:1 to 1:1) to give 19 (107 mg, 90%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.08–7.12 (m, 55H), 6.04 (dd, 1H, J = 10.1, 3.2 Hz), 5.89 (dd, 1H, J = 10.1, 3.2 Hz), 5.82 (dd, 1H, J = 3.5, 1.7 Hz), 5.79–5.73 (m, 2H), 5.58 (dd, 1H, J = 3.2, 1.8 Hz), 5.39 (d, 1H, J = 1.4 Hz), 5.15 (d, 1H, J = 1.8 Hz), 5.13 (d, 1H, J = 12.4 Hz), 5.07–5.01 (m, 2H), 4.95 (d, 1H, J = 11.9 Hz), 4.77 (d, 1H, J = 11.0 Hz), 4.72 (d, 1H, J = 9.6 Hz), 4.69–4.65 (m, 2H), 4.62 (s, 1H), 4.51 (d, 1H, J = 12.4 Hz), 4.33 (d, 1H, J = 8.2 Hz), 4.28 (t, 1H, J = 9.4 Hz), 3.97 (m, 1H), 3.90–3.86 (m, 2H), 3.82 (t, 1H, J = 9.4 Hz), 3.73–3.54 (m, 8H), 3.48–3.27 (m, 5H), 2.85 (m, 1H), 2.40 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 166.39, 166.25, 165.36, 165.33, 165.20, 138.48, 138.44, 137.58, 137.49, 136.99, 133.65, 133.54, 133.33, 133.18, 132.87, 129.88, 129.83, 129.67, 129.34, 129.22, 129.15, 129.06, 128.93, 128.59, 128.55, 128.49, 128.44, 128.39, 128.36, 128.28, 128.24, 128.17, 128.07, 128.03, 127.95, 127.81, 127.75, 127.71, 127.42, 127.34, 127.21, 100.74, 100.43, 99.86, 97.78, 82.62, 81.23, 78.06, 76.12, 75.67, 75.44, 74.80, 74.70, 74.38, 74.33, 73.79, 71.56, 71.23, 70.78, 70.53, 70.35, 69.80, 69.40, 68.11, 67.98, 67.26, 67.12, 65.32, 61.33, 61.04.
Benzyl 2,3,4,6-tetra-O-benzoyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-benzoyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-azido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (21)
A mixture of compound 19 (24 mg, 14 µmol) and 2,3,4,6-tetra-O-benzoyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-α-d-glucopyranosyl trichloroacetimidate 2026 (83 mg, 68 µmol) in CH2Cl2 (1.1 ml) containing activated 4 Å molecular sieves (132 mg) was stirred under an atmosphere of argon at −40 °C for 30 min. A solution of TMSOTf in CH2Cl2 (1 M, 6.8 µL, 6.8 µmol) was added and the resulting mixture was stirred at room temperature overnight. The mixture was filtered through a Celite pad. The filtrate was poured into saturated NaHCO3, and extracted with CH2Cl2. The organic layer was washed with brine, dried over MgSO4, and filtered. The filtrate was concentrated and the residue was subjected to flash silica gel column chromatography (hexanes/EtOAc, 3:1 to 1:1) to provide 21 (34 mg, 65%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.11–6.85 (m, 125H), 5.98–5.51 (m, 14H), 5.33–5.29 (m, 2H), 5.05–4.35 (m, 23H), 4.20–3.34 (m, 26H), 3.16 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 165.74, 165.67, 165.59, 165.47, 165.33, 165.27, 165.24, 165.16, 165.09, 164.99, 164.96, 164.68, 138.84, 138.81, 138.31, 137.81, 137.10, 133.44, 133.30, 133.22, 133.17, 133.00, 132.92, 132.84, 132.70, 129.91, 129.84, 129.75, 129.60, 129.53, 129.40, 129.34, 129.28, 129.21, 129.17, 129.12, 128.86, 128.82, 128.74, 128.68, 128.65, 128.49, 128.36, 128.32, 128.20, 128.16, 128.12, 128.10, 128.00, 127.95, 127.79, 127.71, 127.49, 127.38, 127.10, 127.04, 126.88, 102.09, 101.44, 100.93, 100.84, 100.31, 98.92, 97.47, 81.19, 80.53, 78.46, 77.20, 76.18, 75.85, 75.01, 74.93, 74.79, 74.44, 74.05, 73.28, 72.91, 72.80, 72.72, 71.80, 71.61, 71.54, 71.26, 70.93, 70.72, 70.13, 70.04, 69.76, 69.54, 69.34, 68.91, 68.32, 67.43, 67.17, 67.05, 66.96, 66.86, 65.61, 62.67, 62.36, 60.99, 60.37.
Benzyl 2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (22)
Compound 21 (148 mg, 38 µmol) was dissolved in 1:1 (v/v) CH2Cl2–MeOH (8 mL), and a solution of 0.5 M MeONa in MeOH (0.4 mL, 0.2 mmol) was added. After being stirred at room temperature for overnight, the solution was neutralized with Dowex 50W (H+), filtered, and concentrated. The residue was dissolved in dry pyridine (5 mL) and treated with Ac2O (5 mL) at room temperature overnight. The mixture was evaporated to dryness under reduced pressure, and the residue was diluted with CH2Cl2, washed sequentially with 1 M HCl, saturated NaHCO3 and brine, dried over MgSO4, filtered, and concentrated. To the residue were added CHCl3 (1 mL), pyridine (1 mL), and thioacetic acid (2 mL), and the mixture was stirred at room temperature for 48 h. Evaporation of the solvent under reduced pressure followed by silica gel column chromatography (hexanes/EtOAc, 2:5 to 1:3) to yield 22 (80 mg, 79%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 7.43–7.18 (m, 25H), 6.11 (d, 1H, J = 7.8 Hz), 5.35–4.33 (m, 38H), 4.13–3.41 (m, 26H), 3.35 (m, 1H), 3.25 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 170.25, 170.20, 170.15, 170.06, 169.96, 169.73, 169.65, 169.57, 169.55, 169.46, 168.92, 138.75, 138.30, 138.21, 137.64, 137.49, 128.33, 128.31, 128.17, 127.99, 127.94, 127.69, 127.61, 127.52, 127.46, 127.23, 101.21, 100.92, 100.62, 100.26, 99.64, 98.49, 97.12, 79.61, 78.44, 77.11, 76.65, 76.14, 76.06, 75.49, 75.37, 75.06, 74.96, 74.59, 73.19, 72.73, 72.60, 72.25, 72.16, 71.24, 71.05, 70.90, 70.85, 70.53, 70.49, 70.15, 69.73, 69.52, 69.46, 69.15, 69.00, 68.97, 68.73, 68.57, 67.30, 66.87, 66.49, 66.38, 65.83, 65.38, 61.79, 60.67, 60.51, 52.70, 23.04, 20.72, 20.54, 20.42.
2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-acetyl-β-d-mannopyranosyl-(1→4)-2-acetamido-1,3,6-tri-O-acetyl-2-deoxy-d-glucopyranose (23)
To a solution of 22 (37 mg, 14 µmol) in 25:30:1 (v/v/v) CH2Cl2–MeOH–AcOH (1.2 mL) was added 20% palladium(II) hydroxide on activated carbon (37 mg). The reaction mixture was vigorously stirred at room temperature under hydrogen atmosphere for 24h. The mixture was filtered through Celite and the filtrate was concentrated in vacuo. Pyridine (5 mL) and Ac2O (5 mL) were added, and the mixture was stirred at room temperature overnight. The mixture was concentrated, diluted with CH2Cl2, and washed sequentially with 1 M HCl, saturated NaHCO3, and brine. The organic layer was dried over MgSO4 and concentrated. Silica gel column chromatography (CH2Cl2/MeOH, 40:1) of the residue afforded 23 (26 mg, 77%, α/β = 90/10) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS, selected signals) δ 6.04 (d, 0.90H, J = 3.6 Hz), 5.75 (d, 0.10H, J = 9.2 Hz), 5.61 (d, 0.90H, J = 9.2 Hz), 5.56 (d, 0.10H, J = 8.7 Hz), 2.18–1.91 (m, 78H); 13C NMR (100 MHz, CDCl3, for the major α-anomer) δ 171.17, 170.60, 170.12, 170.09, 170.00, 169.93, 169.82, 169.64, 169.49, 169.39, 169.29, 168.91, 168.79, 168.72, 101.18, 100.84, 100.73, 100.55, 97.05, 96.59, 96.26, 90.55, 75.95, 75.71, 72.58, 72.35, 72.28, 71.73, 71.07, 71.01, 70.79, 70.73, 70.39, 70.21, 69.73, 69.49, 69.34, 69.20, 68.91, 67.71, 67.29, 66.55, 66.39, 65.84, 65.37, 61.74, 61.52, 60.56, 60.37, 50.18, 22.70, 20.56, 20.39, 20.28; ESI-MS: Calcd. for C100H136NO66 [M+H]+ 2406.73, Found: 2307.06.
2-Methyl-[2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→3)-[2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-3,6-di-O-acetyl-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (24)
In a manner similar to the preparation of the oxazoline derivative 14, compound 23 (10 mg, 4.3 µmol) was converted to 24 (3.5 mg, 35%). 1H NMR (400 MHz, CDCl3, TMS) δ 5.92 (d, 1H, J = 6.8 Hz), 5.55 (t, 1H, J = 3.2 Hz), 5.36–4.88 (m, 20H), 4.83 (d, 1H, J = 1.4 Hz), 4.52–4.44 (m, 5H), 4.38 (d, 1H, J = 7.7 Hz), 4.26–3.43 (m, 26H), 3.37 (m, 1H), 2.22–1.94 (m, 75H); 13C NMR (100 MHz, CDCl3) δ 170.80, 170.53, 170.31, 170.11, 170.00, 169.91, 169.68, 169.63, 169.57, 169.50, 169.41, 169.04, 166.03, 101.00, 100.97, 100.90, 100.82, 99.67, 98.93, 97.81, 97.21, 76.17, 75.97, 74.19, 72.68, 72.57, 72.26, 71.29, 71.24, 70.96, 70.56, 70.18, 70.10, 69.93, 69.59, 69.24, 69.11, 69.03, 68.57, 68.45, 67.91, 67.73, 66.99, 66.54, 65.99, 64.83, 63.71, 61.84, 60.73, 60.60, 20.74, 20.57, 20.45, 13.80; ESI-MS: Calcd. for C98H132NO64 [M+H]+ 2346.71, Found: 2346.65.
2-Methyl-[β-d-galactopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)-α-d-mannopyranosyl-(1→3)-[β-d-galactopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)-α-d-mannopyranosyl-(1→6)]-β-d-mannopyranosyl-(1→4)-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (4)
In the same manner as described for the conversion of 14 to 3, de-O-acetylation of compound 24 (3.5 mg, 1.5 µmol) with a catalytic amount of MeONa in MeOH gave compound 4 (2.0 mg) in a quantitative yield. 1H NMR (400 MHz, D2O) δ 5.98 (d, 1H, J = 7.3 Hz), 4.97 (s, 1H), 4.83 (s, 1H), 4.63 (s, 1H), 4.43 (d, 1H, J = 7.7 Hz), 4.42 (d, 1H, J = 7.8 Hz), 4.33 (d, 1H, J = 7.8 Hz), 4.32 (d, 1H, J = 7.3 Hz), 4.28 (m, 1H), 4.09–3.18 (m, 47H), 1.97 (s, 3H); 13C NMR (100 MHz, D2O) δ 168.62, 102.98, 102.67, 102.56, 102.54, 101.34, 99.92, 99.80, 81.12, 78.50, 78.42, 77.79, 75.39, 74.82, 74.79, 74.29, 74.23, 73.05, 72.86, 72.55, 72.27, 71.69, 70.99, 70.42, 70.24, 70.00, 69.85, 69.07, 68.78, 68.58, 66.51, 66.43, 65.83, 65.69, 65.12, 62.51, 61.89, 61.06, 60.98, 60.11, 13.10; ESI-MS: Calcd. for C50H84NO40 [M+H]+ 1338.46, Found: 1338.85.
Benzyl 6-O-acetyl-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[6-O-acetyl-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (25)
To a solution of 18 (200 mg, 0.108 mmol) in CHCl3 (2 mL) was consecutively added pyridine (2 mL) and thioacetic acid (2 mL) at room temperature. After stirring for 48 h, the mixture was concentrated and purified by flash column chromatography on silica gel to afford 25 (172 mg, 85%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.13–7.13 (m, 55H), 5.99–5.90 (m, 3H), 5.82–5.79 (m, 2H), 5.61 (t, 1H, J = 2.3 Hz), 5.36 (d, 1H, J = 0.9 Hz), 5.28–5.25 (m, 2H), 5.22 (d, 1H, J = 12.8 Hz), 5.07 (d, 1H, J = 11.0 Hz), 5.02 (d, 1H, J = 7.8 Hz), 4.87 (d, 1H, J = 11.5 Hz), 4.82 (d, 1H, J = 11.9 Hz), 4.76 (d, 1H, J = 12.4 Hz), 4.70 (d, 1H, J = 11.0 Hz), 4.66 (s, 1H), 4.65 (d, 1H, J = 11.9 Hz), 4.55 (d, 1H, J = 10.9 Hz), 4.51 (d, 1H, J = 11.9 Hz), 4.46 (d, 1H, J = 11.9 Hz), 4.27–4.08 (m, 8H), 3.94 (d, 1H, J = 3.2 Hz), 3.85–3.72 (m, 4H), 3.71–3.66 (m, 2H), 3.47–3.38 (m, 2H), 3.35 (m, 1H), 2.09 (s, 3H), 1.99 (s, 3H), 1.58 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.75, 170.31, 170.04, 165.55, 165.44, 165.39, 165.32, 165.10, 164.77, 138.80, 138.54, 137.95, 137.73, 137.44, 133.55, 133.42, 133.37, 133.29, 133.15, 133.07, 129.81, 129.75, 129.68, 129.61, 129.20, 129.00, 128.96, 128.80, 128.75, 128.55, 128.50, 128.46, 128.39, 128.34, 128.30, 128.26, 128.19, 128.12, 128.08, 127.95, 127.78, 127.60, 127.50, 127.41, 127.31, 126.95, 100.28, 99.82, 99.32, 97.33, 83.11, 78.30, 77.20, 77.15, 75.50, 75.38, 74.82, 74.52, 74.26, 73.56, 73.21, 70.98, 70.34, 70.15, 70.10, 69.69, 69.19, 69.11, 68.80, 68.26, 66.70, 66.49, 62.82, 62.40, 56.62, 23.28, 20.67, 20.56.
Benzyl 2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (26)
The title compound was prepared from 25 (85 mg, 46 µmol) in a manner similar to that described for 19. Flash silica gel column chromatography (hexanes/EtOAc, 1:1 to 2:3) of the crude product afforded 26 (59 mg, 72%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.13–7.08 (m, 55H), 6.05 (dd, 1H, J = 10.1, 3.2 Hz), 5.92 (t, 1H, J = 10.1 Hz), 5.85–5.81 (m, 3H), 5.61 (t, 1H, J = 2.6 Hz), 5.41 (d, 1H, J = 1.4 Hz), 5.37 (d, 1H, J = 1.4 Hz), 5.14 (d, 1H, J = 12.4 Hz), 5.04 (d, 1H, J = 11.0 Hz), 5.03 (d, 1H, J = 7.3 Hz), 4.90 (d, 1H, J = 11.9 Hz), 4.88 (d, 1H, J = 11.0 Hz), 4.77 (d, 1H, J = 12.4 Hz), 4.69–4.63 (m, 3H), 4.49 (d, 1H, J = 10.9 Hz), 4.48 (d, 1H, J = 12.4 Hz), 4.47 (d, 1H, J = 11.9 Hz), 4.31 (t, 1H, J = 8.5 Hz), 4.21 (t, 1H, J = 8.7 Hz), 4.07 (m, 1H), 3.97 (m, 1H), 3.88–3.70 (m, 7H), 3.65–3.55 (m, 4H), 3.49 (m, 1H), 3.37 (m, 1H), 3.30–3.21 (m, 2H), 2.38 (dd, 1H, J = 7.8, 6.0 Hz), 1.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.16, 166.35, 165.76, 165.54, 165.39, 165.19, 164.84, 138.74, 138.35, 137.78, 137.55, 137.37, 133.63, 133.51, 133.17, 132.96, 129.84, 129.70, 129.64, 129.11, 129.02, 128.94, 128.56, 128.50, 128.42, 128.33, 128.26, 128.20, 127.98, 127.94, 127.80, 127.57, 127.45, 127.27, 100.44, 99.77, 99.08, 97.56, 82.29, 78.06, 76.67, 76.60, 75.96, 75.50, 74.83, 74.44, 74.25, 73.61, 73.34, 71.53, 71.47, 70.99, 70.49, 70.36, 70.23, 69.42, 68.74, 68.40, 66.99, 61.23, 60.92, 56.73, 23.18.
Benzyl 6-Azido-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→3)-[6-Azido-2,3,4-tri-O-benzoyl-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (27)
To a solution of 26 (304 mg, 0.171 mmol) in pyridine (2 mL) was added TsCl (195 mg, 1.02 mmol). The mixture was stirred overnight at room temperature. The reaction mixture was then concentrated, diluted with CH2Cl2, and washed sequentially with 1 M HCl, saturated NaHCO3, and brine. The organic layer was dried over MgSO4. After removal of solvent, the tosylated crude product was dissolved in DMF (5 mL) and NaN3 (222 mg, 3.41 mmol) was added. The reaction solution was stirred at 80 °C overnight. After removal of DMF, the residue was poured into brine and extracted with EtOAc. The organic layer was dried over MgSO4 and concentrated. Silica gel column chromatography (hexanes/EtOAc, 2:1 to 1:1) of the residue afforded 27 (261 mg, 84%) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS) δ 8.09–7.16 (m, 55H), 5.95 (dd, 1H, J = 10.3, 3.5 Hz), 5.85–5.76 (m, 4H), 5.64 (s, 1H), 5.44 (d, 1H, J = 7.8 Hz), 5.40 (s, 1H), 5.21–5.18 (m, 2H), 5.05 (d, 1H, J = 11.0 Hz), 5.00 (d, 1H, J = 7.8 Hz), 4.92–4.86 (m, 2H), 4.87 (d, 1H, J = 11.9 Hz), 4.76 (s, 1H), 4.71 (d, 1H, J = 11.0 Hz), 4.64–4.59 (m, 2H), 4.53 (d, 1H, J = 12.3 Hz), 4.49 (d, 1H, J = 11.9 Hz), 4.19–4.10 (m, 2H), 4.02 (d, 1H, J = 2.8 Hz), 3.91–3.73 (m, 6H), 3.59–3.35 (m, 5H), 3.29–3.24 (m, 2H), 1.61–1.57 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 170.00, 165.49, 165.47, 165.26, 165.10, 164.95, 138.88, 138.51, 138.05, 137.81, 137.45, 133.56, 133.51, 133.37, 133.14, 133.04, 129.82, 129.76, 129.63, 129.60, 129.32, 129.06, 128.94, 128.92, 128.81, 128.71, 128.54, 128.44, 128.24, 128.13, 128.11, 128.04, 127.99, 127.87, 127.77, 127.64, 127.56, 127.42, 127.26, 100.47, 99.70, 99.36, 97.43, 82.14, 78.06, 77.14, 76.69, 75.42, 75.04, 74.91, 74.37, 73.37, 73.00, 70.90, 70.79, 70.27, 70.02, 69.97, 69.93, 69.42, 69.20, 67.98, 67.56, 67.50, 55.96, 51.19, 50.88, 23.28.
Benzyl 6-Azido-α-d-mannopyranosyl-(1→3)-[6-Azido-α-d-mannopyranosyl-(1→6)]-2,4-di-O-benzyl-β-d-mannopyranosyl-(1→4)-2-acetamido-3,6-di-O-benzyl-2-deoxy-β-d-glucopyranoside (28)
Compound 27 (262 mg, 0.143 µmol) was dissolved in 1:4 (v/v) CH2Cl2–MeOH (20 mL), and a solution of 0.5 M MeONa in MeOH (0.8 mL, 0.4 mmol) was added. After being stirred at room temperature for 2 h, the solution was neutralized with Dowex 50W (H+), filtered, and concentrated. The residue was subjected to flash silica gel column chromatography (CH2Cl2/MeOH, 10:1) to give 28 (146 mg, 85%) as a white amorphous. 1H NMR (400 MHz, CD3OD, selected signals) δ 7.42–7.14 (m, 25H), 1.80 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 173.40, 140.56, 140.29, 139.49, 139.44, 139.17, 129.77, 129.62, 129.45, 129.41, 129.33, 129.27, 129.22, 129.16, 129.09, 129.04, 128.95, 128.83, 128.75, 128.54, 128.42, 104.16, 101.83, 101.38, 82.54, 81.62, 80.23, 78.08, 76.69, 76.13, 76.10, 76.02, 75.97, 75.46, 74.86, 74.48, 73.54, 72.58, 72.48, 72.32, 71.99, 71.88, 69.90, 69.58, 69.47, 67.51, 56.45, 53.36, 52.79, 23.21. ESI-MS: Calcd. for C61H74N7O19 [M+H]+ 1208.50, Found: 1208.90.
2,3,4-Tri-O-acetyl-6-azido-α-d-mannopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-6-azido-α-d-mannopyranosyl-(1→6)]-2,4-di-O-acetyl-β-d-mannopyranosyl-(1→4)-2-acetamido-1,3,6-tri-O-acetyl-2-deoxy-d-glucopyranose (31)
The title compound was prepared via a three-step procedure: (1) To a solution of 28 (100 mg, 83 µmol) in MeOH (5 mL) was added 20% palladium(II) hydroxide on activated carbon (100 mg). The reaction mixture was vigorously stirred at room temperature under hydrogen atmosphere for 7 h. After addition of H2O (5 mL), the reaction mixture was stirred overnight at room temperature under hydrogen atmosphere. The mixture was filtered through a Celite pad and the filtrate was concentrated in vacuo to give 6-amino-α-d-mannopyranosyl-(1→3)-[6-amino-α-d-mannopyranosyl-(1→6)]-β-d-mannopyranosyl-(1→4)-2-acetamido-2-deoxy-d-glucopyranose 29: ESI-MS: Calcd. for C26H48N3O19 [M+H]+ 706.29, Found: 706.40. Compound 29 was used in the next step without further purification.
(2) Compound 29 was dissolved in H2O (4 mL) and treated with K2CO3 (91 mg, 0.66 mmol) and CuSO4 (6.0 mg, 37 µmol). To the solution was added MeOH (9.4 mL) and a freshly prepared solution of trifluoromethanesulfonyl azide (0.53 mmol)36 in CH2Cl2 (4 mL). After stirring at room temperature for 24 h, the reaction mixture was concentrated to afford crude 6-azido-α-d-mannopyranosyl-(1→3)-[6-amino-α-d-mannopyranosyl-(1→6)]-β-d-mannopyranosyl-(1→4)-2-acetamido-2-deoxy-d-glucopyranose 30: ESI-MS: Calcd. for C26H43KN7O19 [M+K]+ 796.23, Found: 796.36. Compound 30 was used directly in the next step.
(3) Compound 30 was acetylated with acetic anhydride (10 mL) in pyridine (10 mL) at room temperature for overnight. The mixture was concentrated, diluted with CH2Cl2, and washed sequentially with 1 M HCl, saturated NaHCO3, and brine. The organic layer was dried over MgSO4 and concentrated. Silica gel column chromatography (CH2Cl2/MeOH, 60:1) of the residue afforded 31 (61 mg, 61% from 28, α/β = 38/62) as a white amorphous. 1H NMR (400 MHz, CDCl3, TMS, selected signals) δ 6.07 (d, 0.38H, J = 3.6 Hz), 5.96 (d, 0.62H, J = 9.6 Hz), 5.73 (d, 0.38H, J = 9.1 Hz), 5.61 (d, 0.62H, J = 8.7 Hz), 2.22–1.92 (m, 36H); 13C NMR (100 MHz, CDCl3, selected signals) δ 171.09, 170.65, 170.39, 170.36, 170.09, 170.03, 169.99, 169.88, 169.78, 169.61, 169.49, 169.34, 168.91, 168.85, 98.13, 97.65, 97.25, 96.63, 96.55, 96.19, 92.37, 90.40, 22.73, 22.64, 20.71, 20.58, 20.53, 20.48, 20.36; ESI-MS: Calcd. for C48H66N7O30 [M+H]+ 1220.39, Found: 1220.47.
2-Methyl-[2,3,4-tri-O-acetyl-6-azido-α-d-mannopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-6-azido-α-d-mannopyranosyl-(1→6)]-2,4-di-O-acetyl-β-d-mannopyranosyl-(1→4)-3,6-di-O-acetyl-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (32)
Compound 32 (26 mg, 52%) was obtained from 31 (53 mg, 43 µmol) following the procedure described for 14. 1H NMR (400 MHz, CDCl3, TMS) δ 5.92 (d, 1H, J = 6.8 Hz), 5.59 (m, 1H), 5.44 (d, 1H, J = 3.2 Hz), 5.30–5.16 (m, 6H), 5.01–4.99 (m, 2H), 4.83 (s, 1H), 4.81 (s, 1H), 4.27–4.06 (m, 5H), 3.93–3.91 (m, 2H), 3.69–3.62 (m, 3H), 3.45–3.26 (m, 5H), 2.24 (s, 3H), 2.16–2.15 (m, 6H), 2.14, 2.11, 2.09, 2.06 (4s, 12H), 2.05–2.04 (m, 3H), 2.02, 1.99, 1.97 (3s, 9H); 13C NMR (100 MHz, CDCl3) δ 170.72, 170.55, 169.99, 169.87, 169.79, 169.59, 169.56, 169.51, 169.38, 165.96, 99.23, 98.52, 97.33, 76.44, 76.01, 72.66, 70.36, 69.86, 69.78, 69.70, 69.08, 68.69, 67.89, 67.77, 67.38, 66.87, 66.57, 64.57, 63.30, 50.76, 50.60, 20.86, 20.69, 20.53, 13.62; ESI-MS: Calcd. for C46H62N7O28 [M+H]+ 1160.36, Found: 1160.41.
2-Methyl-[6-azido-α-d-mannopyranosyl-(1→3)-[6-azido-α-d-mannopyranosyl-(1→6)]-β-d-mannopyranosyl-(1→4)-1,2-di-deoxy-α-d-glucopyrano]-[2,1-d]-2-oxazoline (5)
Compound 32 (27 mg, 23 µmol) was de-O-acetylated in a manner similar to the preparation of compound 3 to give the title compound (5) (17 mg, quantitative). 1H NMR (400 MHz, D2O) δ 5.97 (d, 1H, J = 7.3 Hz), 4.96 (d, 1H, J = 1.4 Hz), 4.83 (d, 1H, J = 1.4 Hz), 4.64 (s, 1H), 4.27 (dd, 1H, J = 3.0, 1.6 Hz), 4.08 (m, 1H), 4.04 (d, 1H, J = 3.2 Hz), 3.96 (dd, 1H, J = 3.4, 1.6 Hz), 3.91–3.87 (m, 2H), 3.82–3.39 (m, 17H), 3.28 (m, 1H), 1.95 (d, 3H, J = 1.4 Hz); 13C NMR (100 MHz, D2O) δ 168.68, 102.72, 101.44, 99.92, 99.68, 81.05, 77.86, 74.36, 72.20, 71.52, 71.01, 70.46, 70.37, 70.13, 69.97, 69.81, 69.05, 67.70, 67.53, 65.56, 65.11, 61.83, 51.37, 51.16, 13.01; ESI-MS: Calcd. for C26H42N7O18 [M+H]+ 740.26, Found: 740.46.
Glycoprotein 36
A mixture of pentasaccharide oxazoline 3 (0.36 mg, 0.42 µmol) and GlcNAc-RB 336 (1.95 mg, 0.14 µmol) in a phosphate buffer (50 mM, pH 6.5, 75 µl) was incubated at 30 °C with Endo-A (20 mU). The reaction was monitored by analytical HPLC. After 7 h, additional oxazoline 3 (0.36 mg, 0.42 µmol) and Endo-A (20 mU) were added to the solution, and the mixture was incubated at 30 °C. This procedure was repeated twice to push the reaction to completion. The product was then purified by preparative HPLC (23–29% MeCN containing 0.1% TFA in 30 min) to afford the glycoprotein 36 (0.099 µmol, 1.46 mg, 71%). The isolated glycoprotein product was quantified by UV absorbance at 280 nm using a standard solution (at an accurate molar concentration) of GlcNAc-RB as the reference. ESI-MS of 36: calculated, M = 14745; Found, 1638.71 [M+9H]9+, 1475.01 [M+10H]10+, 1341.05 [M+11H]11+, 1229.32 [M+12H]12+, 1134.91 [M+13H]13+, 1053.91 [M+14H]14+.
Glycoprotein 37
Incubation of oxazoline 4 (1.15 mg, 0.86 µmol), GlcNAc-RB 33 (1.0 mg, 0.072 µmol), and Endo-A (20 mU) was performed in the same way as described for the preparation of 36 to give glycoprotein 37 (0.028 µmol, 0.42 mg, 38%). ESI-MS of 37: calculated, M = 15231; Found, 1693.04 [M+9H]9+, 1523.86 [M+10H]10+, 1385.46 [M+11H]11+, 1270.13 [M+12H]12+, 1172.57 [M+13H]13+, 1088.84 [M+14H]14+.
Glycoprotein 38
Glycoprotein 38 was prepared from 5 (0.64 mg, 0.86 µmol) and GlcNAc-RB 33 (1.0 mg, 0.072 µmol), as described in the preparation of 36, to give glycoprotein 38 (0.0643 µmol, 0.94 mg, 89%). ESI-MS of 38: calculated, M = 14619; Found, 1626.58 [M+9H]9+, 1464.00 [M+10H]10+, 1331.10 [M+11H]11+, 1220.23 [M+12H]12+, 1126.47 [M+13H]13+, 1046.13 [M+14H]14+.
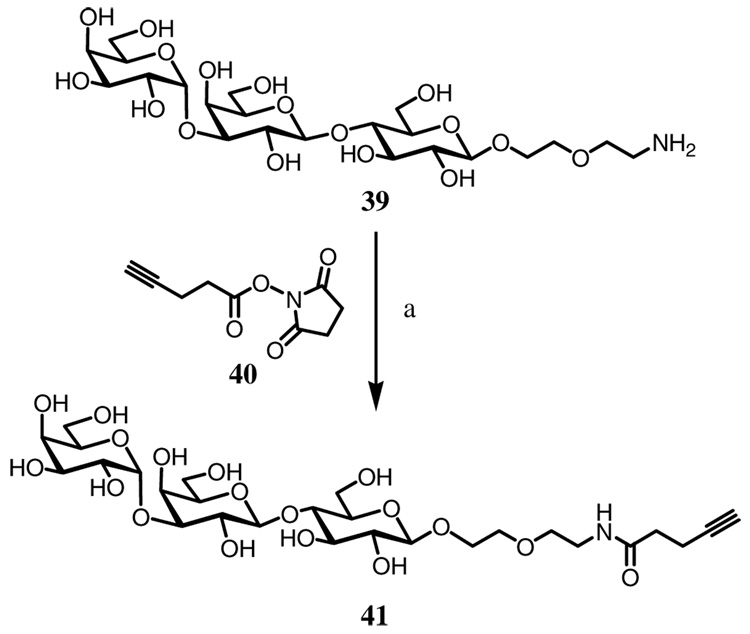
2-[2-(4-Pentynamido)-ethoxy]-ethyl α-d-galactopyranosyl-(1→3)-β-d-galactopyranosyl-(1→4)-β-d-glucopyranoside (41)
To a solution of 2-(2-Aminoethoxy)-ethyl α-d-galactopyranosyl-(1→3)-β-d-galactopyranosyl-(1→4)-β-d-glucopyranoside 3930 (5.0 mg, 8.5 µmol) in aqueous NaHCO3 (0.3 M, 250 µL) and MeOH (50 µL) was added a solution of N-succinimido 4-pentynonate 4037 (2.5 mg, 13 µmol) in MeCN (250 µL). The residue was purified by preparative HPLC (0–10% MeCN in 30 min) to give 41 (4.5 mg, 6.7 µmol, 79%). 1H NMR (400 MHz, D2O) δ 5.03 (d, 1H, J = 3.5 Hz), 4.41–4.39 (m, 2H), 4.09–4.06 (m, 2H), 3.96–3.82 (m, 4H), 3.76–3.46 (m, 16H), 3.32–3.29 (m, 2H), 3.23 (t, 1H, J = 8.3 Hz), 2.41–2.32 (m, 5H); ESI-MS: Calcd. for C27H46NO18 [M+H]+ 672.27, Found: 672.45.
Glycoprotein 42
Compound 41 (37.6 µL, 20 mM in H2O), CuSO4 (9.4 µL, 50 mM in H2O), bathophenanthrolinedisulfonic acid (18.8 µL, 20 mM in H2O), and l-ascorbic acid (9.4 µL, 50 mM in H2O) were added to a solution of glycoprotein 38 (0.55 mg, 0.038 µmol) in Tris buffer (0.17 M, pH 8.0, 113 µ/L). The reaction mixture was incubated at room temperature overnight. The solution was subjected to preparative HPLC purification (23–29% MeCN containing 0.1% TFA in 30 min) to afford the glycoprotein 42 (0.033 µmol, 0.52 mg, 87%). ESI-MS: calculated MS = 15976; Found, 1775.43 [M+9H]9+, 1598.08 [M+10H]10+, 1453.03 [M+11H]11+, 1331.98 [M+12H]12+, 1229.71 [M+13H]13+.
Surface Plasmon Resonance measurements
SPR measurements were performed with a BIACore T100 instrument (GE Healthcare, USA). ConA, PNA, and IgG from human serum were immobilized on CM5 sensor chips in an acetate buffer (10 mM, pH 5.0) using the amine coupling kit provided by the manufacturer. Approximately 4000 resonance units (RU) of proteins were immobilized. Analyses were performed at 25 °C with a flow rate of 30 mL / min using either HBS-P+ buffer (10 mM HEPES, 150 mM NaCl, 0.05% surfactant P20, pH 7.4), for PNA and IgG, or HBS-P+ buffer containing 1 mM CaCl2 and 1 mM MnCl2, for ConA. Injection times for glycoproteins were 2 min followed by 10 min of dissociation. Regeneration was performed using either a 1-min pulse of 100 mM glycine (pH 1.7) stripping buffer, for ConA and IgG, or a 1-min pulse of 0.3 M lactose, for PNA.
Supplementary Material
The 1H- and 13C-NMR spectra of key synthetic compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Scheme 6.
Catalytic 1,3-dipolar cycloaddition to introduce αGal epitope
Reagents and conditions: (a) Tris buffer (0.1 M, pH 8.0), CuSO4, l-ascorbic acid, bathophenanthrolinedisulfonic acid, 87%.
Acknowledgment
We thank Prof. Kaoru Takegawa for providing the pGEX-2T/Endo-A plasmid that was used for expression the Endo-A enzyme, Dr. Bing Li for providing compound 35 that was included in the binding studies, and Dr. Cishan Li for helpful discussions. This work was supported by the National Institutes of Health (NIH grant R01 GM080374).
References
- 1.(a) Imperiali B, O'Connor SE. Curr. Opin. Chem. Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]; (b) Sola RJ, Rodriguez-Martinez JA, Griebenow K. Cell Mol Life Sci. 2007;64:2133–2152. doi: 10.1007/s00018-007-6551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Helenius A, Aebi M. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]; (d) Petrescu AJ, Wormald MR, Dwek RA. Curr. Opin. Struct. Biol. 2006;16:600–607. doi: 10.1016/j.sbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.(a) Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dwek RA. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]; (c) Dwek RA, Butters TD, Platt FM, Zitzmann N. Nat. Rev. Drug. Discov. 2002;1:65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]; (d) Haltiwanger RS, Lowe JB. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]; (e) Dube DH, Bertozzi CR. Nat. Rev. Drug. Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 3.(a) Koeller KM, Wong CH. Nat. Biotechnol. 2000;18:835–841. doi: 10.1038/78435. [DOI] [PubMed] [Google Scholar]; (b) Seitz O. ChemBioChem. 2000;1:214–246. doi: 10.1002/1439-7633(20001117)1:4<214::AID-CBIC214>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]; (b) Herzner H, Reipen T, Schultz M, Kunz H. Chem. Rev. 2000;100:4495–4538. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]; (c) Hang HC, Bertozzi CR. Acc. Chem. Res. 2001;34:727–736. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]; (d) Davis BG. Chem. Rev. 2002;102:579–601. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]; (e) Grogan MJ, Pratt MR, Marcaurelle LA, Bertozzi CR. Annu. Rev. Biochem. 2002;71:593–634. doi: 10.1146/annurev.biochem.71.110601.135334. [DOI] [PubMed] [Google Scholar]; (f) Wong CH. J. Org. Chem. 2005;70:4219–4225. doi: 10.1021/jo050278f. [DOI] [PubMed] [Google Scholar]; (g) Guo Z, Shao N. Med. Res. Rev. 2005;25:655–678. doi: 10.1002/med.20033. [DOI] [PubMed] [Google Scholar]; (h) Pratt MR, Bertozzi CR. Chem. Soc. Rev. 2005;34:58–68. doi: 10.1039/b400593g. [DOI] [PubMed] [Google Scholar]; (i) Liu L, Bennett CS, Wong CH. Chem. Commun. 2006:21–33. doi: 10.1039/b513165k. [DOI] [PubMed] [Google Scholar]; (j) Buskas T, Ingale S, Boons GJ. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]; (k) Brik A, Ficht S, Wong CH. Curr. Opin. Chem. Biol. 2006;10:638–644. doi: 10.1016/j.cbpa.2006.10.003. [DOI] [PubMed] [Google Scholar]; (l) Bennett CS, Wong CH. Chem. Soc. Rev. 2007;36:1227–1238. doi: 10.1039/b617709c. [DOI] [PubMed] [Google Scholar]; (m) Wildt S, Gerngross TU. Nat. Rev. Microbiol. 2005;3:119–128. doi: 10.1038/nrmicro1087. [DOI] [PubMed] [Google Scholar]; (n) Yu H, Chen X. Org. Biomol. Chem. 2007;5:865–872. doi: 10.1039/b700034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:6576–6578. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]; (b) Macmillan D, Bertozzi CR. Angew. Chem. Int. Ed. 2004;43:1355–1359. doi: 10.1002/anie.200352673. [DOI] [PubMed] [Google Scholar]; (c) Hojo H, Matsumoto Y, Nakahara Y, Ito E, Suzuki Y, Suzuki M, Suzuki A. J. Am. Chem. Soc. 2005;127:13720–13725. doi: 10.1021/ja053711b. [DOI] [PubMed] [Google Scholar]; (d) Wu B, Chen J, Warren JD, Chen G, Hua Z, Danishefsky SJ. Angew. Chem. Int. Ed. 2006;45:4116–4125. doi: 10.1002/anie.200600538. [DOI] [PubMed] [Google Scholar]; (e) Brik A, Ficht S, Yang YY, Bennett CS, Wong CH. J. Am. Chem. Soc. 2006;128:15026–15033. doi: 10.1021/ja065601q. [DOI] [PubMed] [Google Scholar]; (f) Payne RJ, Ficht S, Tang S, Brik A, Yang YY, Case DA, Wong CH. J. Am. Chem. Soc. 2007;129:13527–13536. doi: 10.1021/ja073653p. [DOI] [PubMed] [Google Scholar]; (g) Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. J. Am. Chem. Soc. 2008;130:501–510. doi: 10.1021/ja072543f. [DOI] [PubMed] [Google Scholar]
- 5.Witte K, Sears P, Martin R, Wong CH. J. Am. Chem. Soc. 1997;119:2114–2118. [Google Scholar]
- 6.Li B, Song H, Hauser S, Wang LX. Org. Lett. 2006;8:3081–3084. doi: 10.1021/ol061056m. [DOI] [PubMed] [Google Scholar]
- 7.Wang LX. Carbohydr. Res. 2008;343:1509–1522. doi: 10.1016/j.carres.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Yamamoto K, Kadowaki S, Watanabe J, Kumagai H. Biochem. Biophys. Res. Commun. 1994;203:244–252. doi: 10.1006/bbrc.1994.2174. [DOI] [PubMed] [Google Scholar]; (b) Haneda K, Inazu T, Yamamoto K, Kumagai H, Nakahara Y, Kobata A. Carbohydr. Res. 1996;292:61–70. doi: 10.1016/s0008-6215(96)91025-3. [DOI] [PubMed] [Google Scholar]; (c) Mizuno M, Haneda K, Iguchi R, Muramoto I, Kawakami T, Aimoto S, Yamamoto K, Inazu T. J. Am. Chem. Soc. 1999;121:284–290. [Google Scholar]; (d) Yamamoto K. J. Biosci. Bioeng. 2001;92:493–501. doi: 10.1263/jbb.92.493. [DOI] [PubMed] [Google Scholar]; (e) Fujita K, Yamamoto K. Biochim. Biophys. Acta. 2006;1760:1631–1635. doi: 10.1016/j.bbagen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Takegawa K, Tabuchi M, Yamaguchi S, Kondo A, Kato I, Iwahara S. J. Biol. Chem. 1995;270:3094–3099. doi: 10.1074/jbc.270.7.3094. [DOI] [PubMed] [Google Scholar]
- 10.Fujita K, Tanaka N, Sano M, Kato I, Asada Y, Takegawa K. Biochem. Biophys. Res. Commun. 2000;267:134–138. doi: 10.1006/bbrc.1999.1963. [DOI] [PubMed] [Google Scholar]
- 11.(a) Wang LX, Fan JQ, Lee YC. Tetrahedron Lett. 1996;37:1975–1978. [Google Scholar]; (b) Wang LX, Tang M, Suzuki T, Kitajima K, Inoue Y, Inoue S, Fan JQ, Lee YC. J. Am. Chem. Soc. 1997;119:11137–11146. [Google Scholar]; (c) Singh S, Ni J, Wang LX. Bioorg. Med. Chem. Lett. 2003;13:327–330. doi: 10.1016/s0960-894x(02)01025-9. [DOI] [PubMed] [Google Scholar]; (d) Li H, Singh S, Zeng Y, Song H, Wang LX. Bioorg. Med. Chem. Lett. 2005;15:895–898. doi: 10.1016/j.bmcl.2004.12.066. [DOI] [PubMed] [Google Scholar]; (e) Wang LX, Song H, Liu S, Lu H, Jiang S, Ni J, Li H. ChemBioChem. 2005;6:1068–1074. doi: 10.1002/cbic.200400440. [DOI] [PubMed] [Google Scholar]
- 12.(a) Fujita M, Shoda S, Haneda K, Inazu T, Takegawa K, Yamamoto K. Biochim. Biophys. Acta. 2001;1528:9–14. doi: 10.1016/s0304-4165(01)00164-7. [DOI] [PubMed] [Google Scholar]; (b) Li H, Li B, Song H, Breydo L, Baskakov IV, Wang LX. J. Org. Chem. 2005;70:9990–9996. doi: 10.1021/jo051729z. [DOI] [PubMed] [Google Scholar]; (c) Rising TW, Claridge TD, Moir JW, Fairbanks AJ. ChemBioChem. 2006;7:1177–1180. doi: 10.1002/cbic.200600183. [DOI] [PubMed] [Google Scholar]; (d) Rising TW, Claridge TD, Davies N, Gamblin DP, Moir JW, Fairbanks AJ. Carbohydr. Res. 2006;341:1574–1596. doi: 10.1016/j.carres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.(a) Li B, Zeng Y, Hauser S, Song H, Wang LX. J. Am. Chem. Soc. 2005;127:9692–9693. doi: 10.1021/ja051715a. [DOI] [PubMed] [Google Scholar]; (b) Zeng Y, Wang J, Li B, Hauser S, Li H, Wang LX. Chem. Eur. J. 2006;12:3355–3364. doi: 10.1002/chem.200501196. [DOI] [PubMed] [Google Scholar]
- 14.Umekawa M, Huang W, Li B, Fujita K, Ashida H, Wang LX, Yamamoto K. J. Biol. Chem. 2008;283:4469–4479. doi: 10.1074/jbc.M707137200. [DOI] [PubMed] [Google Scholar]
- 15.(a) Andreotti AH, Kahne D. J. Am. Chem. Soc. 1993;115:3352–3353. [Google Scholar]; (b) Imperiali B, Rickert KW. Proc. Natl. Acad. Sci. U.S.A. 1995;92:97–101. doi: 10.1073/pnas.92.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liang R, Andreotti AH, Kahne D. J. Am. Chem. Soc. 1995;117:10395–10396. [Google Scholar]; (d) O'Conner SE, Imperiali B. Chem. Biol. 1998;5:427–437. doi: 10.1016/s1074-5521(98)90159-4. [DOI] [PubMed] [Google Scholar]; (e) O'Connor SE, Pohlmann J, Imperiali B, Saskiawan I, Yamamoto K. J. Am. Chem. Soc. 2001;123:6187–6188. doi: 10.1021/ja010094s. [DOI] [PubMed] [Google Scholar]
- 16.McGreal EP, Miller JL, Gordon S. Curr. Opin. Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer CF, Miceli MC, Baum LG. Curr. Opin. Struct. Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee RT, Lee YC. Glycoconj. J. 2000;17:543–551. doi: 10.1023/a:1011070425430. [DOI] [PubMed] [Google Scholar]
- 19.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 20.Crich D, Dudkin V. J. Am. Chem. Soc. 2001;123:6819–6825. doi: 10.1021/ja010086b. [DOI] [PubMed] [Google Scholar]
- 21.Singh L, Seifert J. Tetrahedron Lett. 2001;42:3133–3136. [Google Scholar]
- 22.Crich D, Li W, Li H. J. Am. Chem. Soc. 2004;126:15081–15086. doi: 10.1021/ja0471931. [DOI] [PubMed] [Google Scholar]
- 23.Ren T, Liu D. Tetrahedron Lett. 1999;40:7621–7625. [Google Scholar]
- 24.Shie CR, Tzeng ZH, Kulkarni SS, Uang BJ, Hsu CY, Hung SC. Angew. Chem. Int. Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 25.Heng L, Ning J, Kong F. Carbohydr. Res. 2001;331:431–437. doi: 10.1016/s0008-6215(01)00059-3. [DOI] [PubMed] [Google Scholar]
- 26.Boons GJPH, van Delft FL, van der Klein PAM, van der Marel GA, van Boom JH. Tetrahedron. 1992;48:885–904. [Google Scholar]
- 27.Nyffeler PT, Liang CH, Koeller KM, Wong CH. J. Am. Chem. Soc. 2002;124:10773–10778. doi: 10.1021/ja0264605. [DOI] [PubMed] [Google Scholar]
- 28.(a) Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]; (b) Lin FL, Hoyt HM, van Halbeek H, Bergman RG, Bertozzi CR. J. Am. Chem. Soc. 2005;127:2686–2695. doi: 10.1021/ja044461m. [DOI] [PubMed] [Google Scholar]
- 29.(a) Breinbauer R, Kohn M. ChemBioChem. 2003;4:1147–1149. doi: 10.1002/cbic.200300705. [DOI] [PubMed] [Google Scholar]; (b) Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]; (c) Link AJ, Vink MK, Tirrell DA. J. Am. Chem. Soc. 2004;126:10598–10602. doi: 10.1021/ja047629c. [DOI] [PubMed] [Google Scholar]; (d) Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Naicker KP, Li H, Heredia A, Song H, Wang LX. Org. Biomol. Chem. 2004;2:660–664. doi: 10.1039/b313844e. [DOI] [PubMed] [Google Scholar]
- 31.Sen Gupta S, Kuzelka J, Singh P, Lewis WG, Manchester M, Finn MG. Bioconjugate Chem. 2005;16:1572–1579. doi: 10.1021/bc050147l. [DOI] [PubMed] [Google Scholar]
- 32.Deiters A, Cropp TA, Mukherji M, Chin JW, Anderson JC, Schultz PG. J. Am. Chem. Soc. 2003;125:11782–11783. doi: 10.1021/ja0370037. [DOI] [PubMed] [Google Scholar]
- 33.(a) Kalia J, Raines RT. ChemBioChem. 2006;7:1375–1383. doi: 10.1002/cbic.200600150. [DOI] [PubMed] [Google Scholar]; (b) Speers AE, Adam GC, Cravatt BF. J. Am. Chem. Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 34.(a) Galili U. Immunol. Today. 1993;14:480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]; (b) Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Proc. Natl. Acad. Sci. USA. 1993;90:11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Alwayn IP, Basker M, Buhler L, Cooper DK. Xenotransplantation. 1999;6:157–168. doi: 10.1034/j.1399-3089.1999.00030.x. [DOI] [PubMed] [Google Scholar]
- 35.Lis H, Sharon N. Chem. Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 36.Alper PB, Hung SC, Wong C-H. Tetrahedron Lett. 1996;37:6029–6032. [Google Scholar]
- 37.Salmain M, Vessieres A, Butler IS, Jaouen G. Bioconjug. Chem. 1991;2:13–15. doi: 10.1021/bc00007a002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 1H- and 13C-NMR spectra of key synthetic compounds. This material is available free of charge via the internet at http://pubs.acs.org.